Abstract
Background:
Nutmeg is commonly used as a spice in various dishes, as components of teas and soft drinks or mixed in milk and alcohol. The effect of chronic consumption of nutmeg on the medial geniculate body of adult Wistar rats was carefully studied.
Aim:
The objective is to observe any possible histological changes.
Materials and Methods:
Rats of both sexes (n = 24), with average weight of 200g were equally and randomly assigned into two treatment groups [A] and [B]; and untreated Control group [C] of (n = 8) per group. The rats in the treatment groups [A] and [B] were respectively given 1g and 2g of nutmeg thoroughly mixed with the feeds on a daily basis for thirty-two days. The control group received equal amount of feeds daily without nutmeg added for the thirty-two days period. All rats were fed with grower's mash and given water liberally. The rats were sacrificed by cervical dislocation method on day thirty-three of the experiment, medial geniculate body was carefully dissected out from the brain and quickly fixed in 10% formol-saline for histological study.
Results:
The findings indicate that rats in the treated groups (A & B) showed some cellular degenerative changes like hypertrophy, sparse cellular population, pyknotic nuclei with some microcystic changes, and vacuolation in the stroma of the treated medial geniculate body relative to those in the control group.
Conclusion:
Long term consumption of nutmeg may have adverse effect on microanatomy of medial geniculate body, which could negatively impact on the auditory sensibilities. Further research, including human observational studies, aimed at corroborating these observations is recommended.
Keywords: Medial geniculate body, microanatomy, nutmeg toxicity
Introduction
Nutmeg is widely used in a variety of ways and for various purposes. Dating back to the 16th century[1], nutmeg has been known for its psychoactive properties, which includes antidepressant, anxiogenic and hallucination[1–4]. Powdered nutmeg is rarely administered alone, but it is a constituent of numerous medicines such as aromatic adjuncts. It is accepted as flavoring agent in food[5–7]. It is used for its anticonvulsant, aphrodisiac and psychoactive properties in male rat[8–10].
Medicinally, nutmeg is known for its anti-inflammatory and anti-thrombotic[11], as well as anti-rheumatic, carminative and stimulant properties[12]. In pregnancy and lactation, nutmeg is used in traditional medicine practice for antenatal and postnatal treatment[13]. One of the active ingredients in nutmeg is called myristicine, which has neurotoxic effects on dopaminergic neurons and monoamine oxidase[2,14,15]. However, nutmeg is associated with toxicity and it is a cause of health concern when it comes to intoxication calls[3,16–18]. It has been reported to reduce cell viability in a dose dependent manner[19].
The medial geniculate body (MGB) is a metathalamic structure that serves as the last of a series of processing centers along the auditory relay pathway. It is a knob like protuberance in the lateral wall of caudal diencephalon, lateral to the brachium of inferior colliculus[20]. It represents the thalamic relay between the inferior colliculus and the auditory cortex.
The cerebral cortex strongly affects the MGB through descending projections, which were thought to consist primarily of small areas with slow conduction velocities[21,22]. It has been demonstrated that neurons of auditory cortex showed great physiological plasticity when rats were exposed to specific stimuli coupled with concurrent stimulation of a forebrain subcortical structure in the nucleus basalis[23]. It has also been postulated that besides the role in normal hearing, the auditory system could influence cognitive behaviors and dysfunctions such as sound-induced seizures[24].
Since the neurons of the central nervous system (CNS) are affected by nutmeg, it is relevant to investigate its effect on the MGB. It is probable that the adverse cytotoxic effects of myristicine on the CNS could be manifest in the microanatomy of MGB. Thus, the objective of this study is to investigate possible histological effects of chronic consumption of nutmeg on the MGB of adult Wistar rats.
Materials and Methods
Animal care ethics
The School of Basic Medical Sciences, University of Benin granted approval before the work began. The rats were obtained and maintained in the Animal Holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria. The animals were fed with grower's mash obtained from Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria.
Nutmeg administration
Twenty-four adult Wistar rats of both sexes with average weight of 200g were equally and randomly assigned into two treatment groups [A] and [B]; and untreated Control group [C] of (n = 8) per group. Unbranded nutmeg was purchased from a local retailer at Oba market in Benin. They were dried and graded into powder at the Department of Pharmacognosy, Faculty of Pharmacy University of Benin, Benin City Edo State.
The rats in the treatment groups [A] and [B] were respectively given 1g and 2g of nutmeg thoroughly mixed with the feeds on a daily basis for thirty-two days. The control group received equal amount of feeds daily without nutmeg added for the thirty-two days period. All rats were fed with grower's mash and given water liberally. The rats were sacrificed by cervical dislocation on the thirty-third day of the experiment. The skulls were opened using bone forceps. The MGB was quickly dissected out and fixed in 10% formol-saline for routine histological techniques.
Histological study
The tissues were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7μm thick were obtained using a rotatory microtome. The deparaffinized sections were stained routinely with haematoxyline and eosin (H & E). Photomicrographs of the results were obtained using research photographic microscope in the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria.
Results
The sections of MGB from the control group showed normal histological features with the neurons appearing distinct and of various sizes. The neuron and glial cells appeared normal and no vacuolation in the stroma of the sections (Fig. 1).
Fig. 1.
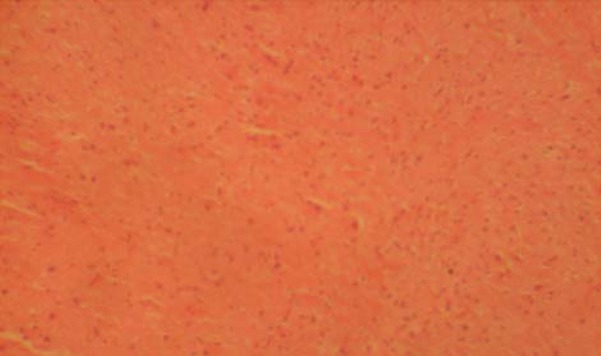
Control section of the medial geniculate body (H & E ×100).
Sections of the MGB from the treated groups revealed some changes like hypertrophy, relatively sparse cellular population, pyknotic nuclei with some microcystic changes, and vacuolation, when compared to the control group. There was no remarkable difference between the treated sections (Figs. 2 & 3).
Fig. 2.
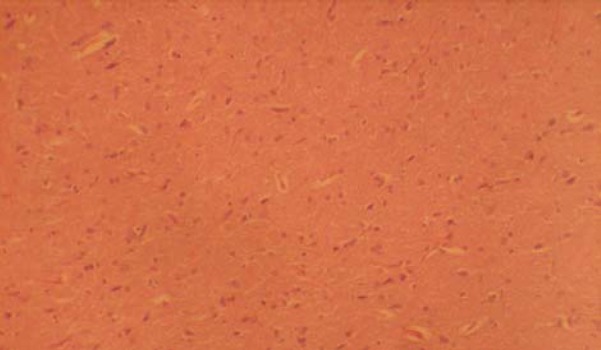
Section of medial geniculate body treated with nutmeg 1g/day (H & E ×100).
Fig. 3.
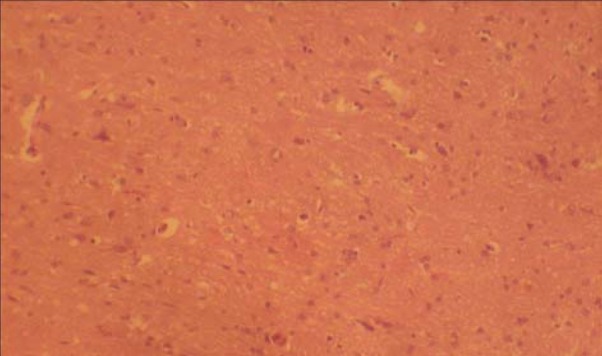
Section of medial geniculate body treated with nutmeg 2g/day (H & E ×100).
Discussion
The results of the H & E stain from this experiment revealed some cellular degenerative changes like hypertrophy, sparse cellular population, pyknotic nuclei with some microcystic changes, and vacuolation in the stroma of MGB of the treated groups. A more prominent feature in Fig. 2 and Fig. 3 is the fewer cell population, when compared to the control section of Fig. 1. It could be inferred that prolonged consumption of nutmeg resulted in toxic effect that has manifested as sparse cellular population of the MGB. These observations are similar to histological effects of some other substances from our laboratory[25–27].
Neuronal degeneration has been reported to result in cell death[28]. It is known that pathological or accidental cell death can result from extrinsic insults to the cell[29]. The prime candidates for inducing the massive cell destruction observed in neurodegeneration are neurotoxins. These may be substances present in small amounts in the environment, or even naturally occurring chemicals. It is known that failure in the regulation of the release or storage mechanism of dopamine can trigger neurodegenerative process[30]. It also known that nutmeg has neurotoxic effects on dopaminergic neurons[2]. What this report contributes is that the toxic effect of nutmeg is observable at the microanatomical level vis-à-vis by histopathological procedure.
Furthermore, cytotoxic edema usually involves intracellular swelling and this could involve neuronal cells. Typical brain edema is associated with endothelial cell hypertrophy and neuronal cell vacuolation[31]. Brain edema is also generally associated with electrolyte imbalance. The pharmacologic disruption of the MGB caused by nutmeg is a cardinal feature of the results of this experiment. Therefore, it is thinkable that nutmeg intoxication may be associated with electrolyte imbalance.
This study revealed that high doses and long term consumption of nutmeg causes some cellular degenerative changes in MGB of adult Wistar rats. Nutmeg is a common household spice sometimes abused for its hallucinogenic properties. This abuse is well reported in the medical literature[3,16–18], but histological perspective is lacking. Thus, the importance of this report is the presentation of microanatomical perspective of nutmeg toxicity.
Conclusion
Nutmeg abuse often presents with clinical symptoms that the pathology tests do turn up negative. This report provides a stepping stone to investigate feasible novel laboratory tests concordant with MGB pathology and/or auditory dysfunction that could serve for better assessment of nutmeg toxicity. It is therefore recommended that further studies aimed at corroborating these observations be carried out.
Acknowledgement
Mr. Josiah Adjene performed the experiment and drafted the manuscript. Dr Uba Nwose contributed in revising it critically for important intellectual content and final approval of the version to be published.
References
- 1.Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86:179–180. doi: 10.1177/014107689308600326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9:84–89. doi: 10.1089/jmf.2006.9.84. [DOI] [PubMed] [Google Scholar]
- 3.Sonavane GS, Sarveiya VP, Kasture VS, Kasture SB. Anxiogenic activity of Myristica fragrans seeds. Pharmacol Biochem Behav. 2002;71:239–244. doi: 10.1016/s0091-3057(01)00660-8. [DOI] [PubMed] [Google Scholar]
- 4.Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24:563–566. doi: 10.1191/0960327105ht567oa. [DOI] [PubMed] [Google Scholar]
- 5.Burdock GA, Carabin IG. Safety assessment of myristic acid as a food ingredient. Food Chem Toxicol. 2007;45:517–529. doi: 10.1016/j.fct.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Beyer J, Ehlers D, Maurer HH. Abuse of nutmeg (Myristica fragrans Houtt.): studies on the metabolism and the toxicologic detection of its ingredients elemicin, myristicin, and safrole in rat and human urine using gas chromatography/mass spectrometry. Ther Drug Monit. 2006;28:568–575. doi: 10.1097/00007691-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55:373–379. doi: 10.1016/j.disamonth.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Wahab A, Ul Haq R, Ahmed A, Khan RA, Raza M. Anticonvulsant activities of nutmeg oil of Myristica fragrans. Phytother Res. 2009 Feb;23(2):153–158. doi: 10.1002/ptr.2548. [DOI] [PubMed] [Google Scholar]
- 9.Parle M, Dhingra D, Kulkarni SK. Improvement of mouse memory by Myristica fragrans seeds. J Med Food. 2004;7:157–161. doi: 10.1089/1096620041224193. summer. [DOI] [PubMed] [Google Scholar]
- 10.Tajuddin, Ahmad S, Latif A, Qasmi IA, Amin KM. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (Nutmeg) BMC Complement Altern Med. 2005;5:16. doi: 10.1186/1472-6882-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olajide OA, Ajayi FF, Ekhelar AI, Awe SO, Makinde JM, Alada AR. Biological effects of Myristica fragrans (nutmeg) extract. Phytother Res. 1999;13:344–345. doi: 10.1002/(SICI)1099-1573(199906)13:4<344::AID-PTR436>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavy G. Nutmeg intoxication in pregnancy. A case report. J Reprod Med. 1987;32:63–64. [PubMed] [Google Scholar]
- 14.Truitt EB, Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647–650. doi: 10.3181/00379727-112-28128. [DOI] [PubMed] [Google Scholar]
- 15.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 16.Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10:429–430. doi: 10.1016/0735-6757(92)90069-a. [DOI] [PubMed] [Google Scholar]
- 17.Demetriades AK, Wallman PD, McGuiness A, Gavalas MC. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22:223–225. doi: 10.1136/emj.2002.004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11:240–241. doi: 10.1097/01.mej.0000127649.69328.a5. [DOI] [PubMed] [Google Scholar]
- 19.Lee BK, Kim JH, Jung JW, Choi JW, Han ES, Lee SH, et al. Myristicin-induced Neurotoxicity in Human neuroblastoma SK-N-SH Cells. Toxicol Lett. 2005;157:49–56. doi: 10.1016/j.toxlet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Dada R, Khanna J, Prakash R. Morphometric analysis of rat medial geniculate body. J Anat Soc India. 2001;50:145–147. [Google Scholar]
- 21.Suga N. The neural circuit for tone-specific plasticity in the auditory system elicited by conditioning. Learn Mem. 2008;15:198–201. doi: 10.1101/lm.791408. [DOI] [PubMed] [Google Scholar]
- 22.Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc Natl Acad Sci U S A. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winer JA, Lee CC. The distributed auditory cortex. Hear Res. 2007;229:3–13. doi: 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winer JA, Chernock ML, Larue DT, Cheung SW. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hear Res. 2002;168:181–195. doi: 10.1016/s0378-5955(02)00489-6. [DOI] [PubMed] [Google Scholar]
- 25.Adjene JO, Igbigbi PS, Nwose EU. Histological effects of chronic administration of efavirenz lateral geniculate body of adult Wistar rats. North Am J Med Sci. 2010;2:381–384. doi: 10.4297/najms.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adjene JO, Caxton-Martins AE. Some histological effect of chronic administration of Chloroquine on the medial geniculate body of adult Wistar rat. Afri J Med Med Sci. 2006;35:131–135. [PubMed] [Google Scholar]
- 27.Adjene JO, Adenowo TK. Histological studies of the effect of chronic administration of Chloroquine on the inferior colliculus of adult Wistar rat JMBR. 2005;4:83–87. [Google Scholar]
- 28.Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson's disease. BMC Neurosci. 2009;10:109. doi: 10.1186/1471-2202-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natale JE, Knight JB, Cheng Y, Rome JE, Gallo V. Metallothionein I and II mitigate age-dependent secondary brain injury. J Neurosci Res. 2004;78:303–314. doi: 10.1002/jnr.20265. [DOI] [PubMed] [Google Scholar]
- 30.Boada J, Cutillas B, Roig T, Bermúdez J, Ambrosio S. MPP (+)-induced mitochondrial dysfunction is potentiated by dopamine. Biochem Biophys Res Commun. 2000;268:916–920. doi: 10.1006/bbrc.2000.2232. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Ying D, Sun J, Bian X, Zhang Y, He B. Comparative observation with MRI and pathology of brain edema at the early stage of severe burn. Chin J Traumatol. 2001;4:226–230. [PubMed] [Google Scholar]


