Abstract
Background:
Endemic pemphigus foliaceus (EPF) is the only known autoimmune disease presenting in circumscribed geographic areas.
Aim:
We aim to provide information concerning the natural course of EPF, including systemic compromise in the presteroid era, which has been largely unavailable in the current medical literature.
Material & Methods:
By a retrospective review of the literature we aim to compile and compare the focus of EPF and the current knowledge about them. The main aim of this review is to summarize our current knowledge of EPF, including data described almost one century ago; and, to include several unindexed reports, which may have not been available to many current scientists and health care personnel.
Results:
Foci of EPF have been described in several Central American and South American countries, affecting predominately young people and Amerindians, with an additional female predilection. Although most cases have occurred in Brazil, some cases have been reported in Peru, Paraguay, El Salvador, and Venezuela. Another variant of EPF has been described in El Bagre, Colombia, affecting older men and a few post-menopausal females. Finally, another type of EPF was described in nomadic tribes affecting females of child bearing age in Tunisia, Africa.
Conclusion:
Our understanding of EPF has been hampered by a lack of government attention to these diseases, especially in some South and Central American countries. Other factors that have made past studies of EPF difficult include 1) that the disease foci are often located in rural areas bordering the rain forest of underdeveloped countries; and 2) military conflicts in some of these areas.
Keywords: Pemphigus foliaceus, endemic pemphigus foliaceus, fogo selvagem, autoimmunity
Introduction
General features of endemic pemphigus foliaceus (EPF):
Autoimmune diseases are presently divided as organ-specific or non-organ specific; these disorders may be simultaneously associated with other autoimmune diseases in the same patient[1]. Because of the chronic nature of this group of diseases, they cause significant decreases in the quality of life of affected patients, and their proper treatment often incurs significant cost. Autoimmune diseases are the result of a series of interactions within the immune system, which ultimately lead to the loss of self-tolerance to different autoantigens[1]. However, we do not fully understand how self-tolerance is initially lost, nor how the loss of tolerance is then amplified and maintained from acute “disease flares” to chronic autoimmune disease states. In general, it is currently accepted that 1) the interactions of B and T cells and 2) the loss of peripheral and central tolerance are needed to perpetuate autoimmunity, and to spread its effects among multiple autoantigen targets[1]. The aims of the current review are to summarize data concerning endemic pemphigus foliaceus (EPF) and its variants and to review relevant, extensive and largely unindexed literature. The unindexed literature was reported almost a century ago in languages other than English, and has therefore been unavailable to most current scientists and health care personnel. We utilized a retrospective review of the literature, both indexed and unindexed; in addition, we employed articles in multiple languages to correlate recent and older studies of EPF.
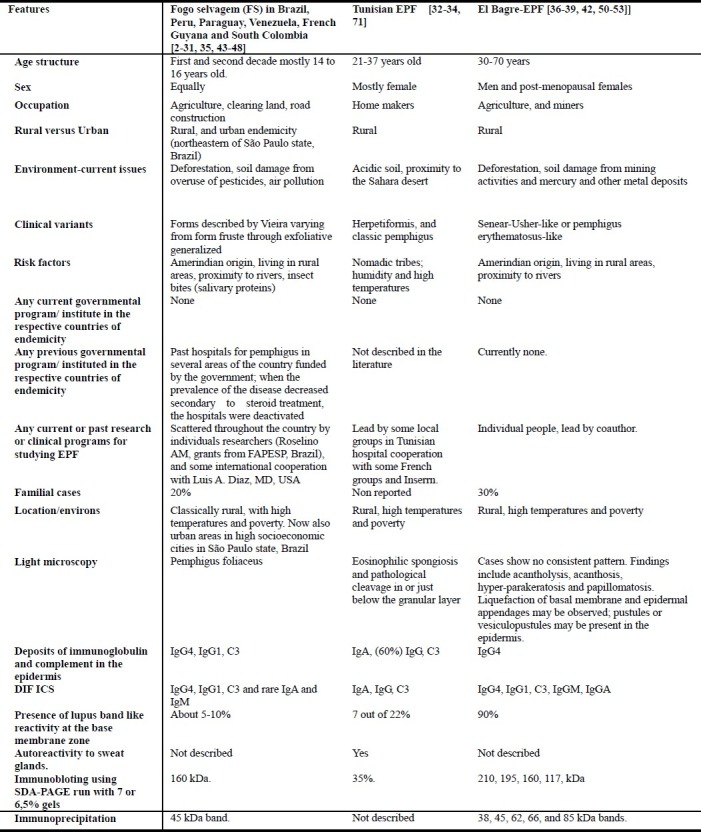
The most extensive and well-characterized variant of EPF is known as “fogo selvagem” (FS), or “wild fire” in Portuguese. The disease is present in some areas of Brazil, affects both sexes equally, and demonstrates its highest incidence of onset at 10-30 years of age[2–15]. In the older Brazilian literature, reported in Portuguese before the steroid era, multiple inflammatory and systemic abnormalities were found in patients suffering from FS. In the era of steroid therapy, such systemic findings have often been attributed to complications of the immunosuppressive therapy itself[2–15]. Several possible EPF foci in South and Central American countries and in Tunisia (Africa)[16–38], have been reported. Table 1 contains a summary of clinical, epidemiological, and immunological features documented in people affected by different types of EPF.
Table 1.
Summary of clinical, epidemiological and immunological features in distinct types of endemic pemphigus foliaceus (EPF)
In Colombia, South America, FS similar to the Brazilian type has been described in the southern areas of the Amazonian and Orinoquian forest regions, and it affects Indian tribes[26]. In addition, an EPF focus was described in El Bagre, Colombia, and initially documented as an FS focus[27]; however, years later this focus was identified as a new variant of EPF[28–30]. The new variant, El Bagre-EPF, occurs in a gold-mining region; it predominantly affects males between 30 and 60 years of age, as well as a few post-menopausal females[28–30]. In addition El Bagre-EPF patients possess autoantibodies to 1) desmogleins 1 and 3; 2) the bullous pemphigoid antigen of 230 kDa (BP230); and 3) several molecules belonging to the plakin family; thus, their disease autoreactivity resembles that seen in patients with paraneoplastic pemphigus (PNP)[31–32]. El Bagre-EPF patients and controls from the endemic area are exposed to mercury pollution in their environs[33], and high concentrations of this metal have been detected in affected individuals. The mercury findings differ from the FS cases, where high concentrations of copper have been reported[34,35]. Pemphigus foliaceus (PF) is endemic in the northeastern region of São Paulo state, Brazil; the water sources in this area are mainly the Mogi-Guaçu and Pardo rivers, where increased levels of mercury have been reported[36]. Nevertheless, a formal association between FS and mercury deposition in patient tissues has not been reported. No chronic changes in the incidence or prevalence of El Bagre-EPF have been noted, except for annual cyclic patterns observed during our 10-year epidemiological survey. In contrast, the epidemiology of FS has been studied for about a century, and has displayed cyclic patterns during a single year, as well as a decrease in disease incidence after complete development of the endemic areas[37].
El Bagre-EPF and FS have similar geographical distributions, and prevail in rural areas where the environment has been abruptly altered (as in colonization, road construction, agricultural and/or mining activities). The presence of 1) familial cases of EPF and 2) an increase in autoantibodies to EPF antigens are features commonly observed in relatives of the patients. The disease autoantibodies may also be identified in people unrelated to the patients, and living in the same region; however, these antibody titers are lower than those of the patient relatives[28,39–41]. Both El Bagre-EPF and FS affect people of lower socioeconomic status, in these patients, exposure to ultraviolet (UV) radiation[29,38], as well as pox and herpes viruses may produce a clinical eruption varicelliformis of Kaposi[41,43].
Historical and epidemiological aspects of EPF:
More than 15,000 cases of FS were reported to occur in Brazil in the past century, predominately in the states of Paraná, São Paulo, Minas Gerais, Goiás, and Mato Grosso do Sul. In Brazil, FS occurs in states located between 45o to 60o West longitude, and 5o to 25o South latitude, at altitudes between 500 and 800 meters and within a subtropical climate[2–15,37,43,44]. In 1730, the first possible case of FS was reported by missionaries, who described a syndrome in Brazilian Indians which they called “Roro.” The first reports on FS were done by Alexandre Cerqueira in Bahia before 1900, according to Flaviano Silva (1948), as cited by Anuar Auad in 1972. In his 1955 doctoral thesis, Caramuru Paes Leme described 1,903 cases of FS in Rio de Janeiro, which was then called “Tokelau”[2–15,43,44]. In 1906, Luciano Gualberto described a focus of EPF in the city of Franca. During a Medicine and Surgery Congress in Belo Horizonte in 1912, the first cases of FS in the State of Minas Gerais were reported by Antonio Aleixo. In the following years, João Paulo Vieira published several papers concerning different aspects of the disease. In the State of Paraná, the first case of FS was reported by Miranda in 1941. In 1942, Vieira reported the presence of EPF in the State of São Paulo, citing the case of a patient from Batatais who had acquired the disease over 50 years prior to 1942; the patient also remembered previous cases reported by elderly people who lived in Franca[2–15,43,44]. Between the middle of the 19th century and middle of the 20th century, tremendous colonization and immigration occurred in Brazil. Simultaneously, there was a marked increase in the number of EPF cases in these Brazilian states, prompting the government to create specialty hospitals for the sole treatment of FS patients in São Paulo, Goiás, and Parana. During the 1850-1950 periods, construction of new roads, increased human contact with native vegetation, increased human proximity to rivers and creeks, and deforestation occurred simultaneously. By 1946, 351 FS patients had been treated at the Pemphigus Hospital in São Paulo, where the mortality was 40.7%. In 1942 alone, Vieira registered 460 cases and designated Franca and Ribeirão Preto as cities with the highest indices of the disease, with a high incidence in almost all of the neighboring towns. He described the greatest incidence of the disease among people aged 14 to 16 years and among Caucasians (326 of the 460 cases) and pointed out that the disease occurred preferentially among poor people from rural areas, with exceptions arising occasionally in urban areas[2–15,43,44]. In the State of Paraná, Minelli registered 632 cases of FS in the period between 1941 and 1980, affecting mostly white people (68.67%) living in rural areas of the northern areas of the state, and aged 15 to 44 years[2–15,43,44]. In an epidemiological study conducted in 1973-74, Proença reported 41 cases of EPF in the state of São Paulo, with a higher occurrence in women, whites, and young people. He concluded that its occurrence was declining in the state of São Paulo, with a complete disappearance of endemic foci[2–15,43,44]. Disagreeing with his conclusion, Chiossi and Roselino have demonstrated the maintenance of PF endemicity in the Ribeirão Preto region[37]. In the state of Paraná, there was an abrupt decline in the incidence of FS with advancing urbanization and industrialization of endemic areas, to the extent that the need no longer exists for the pemphigus specialty hospital[37].
On the other hand, the first cases of El Bagre-EPF were seen in 1970, by a western dermatologist who visited the El Bagre area searching for patients with leprosy, but these were not reported (Abreu, personal interview). However, in 1983, a dermatologist in Medellin, Colombia, described the possible presence of a focus of EPF in patients after seeing an unusual presentation of patients admitted to a hospital in Medellin[49]. These patients came from the mining areas of rural El Bagre and Nechi. The number of patients seen in this same hospital increased, and a retrospective study between 1982 and 1986 reported the presence of 21 patients with pemphigus foliaceus (PF) of the FS type[27]. The majority of the patients were mestizo men, who worked as farmers, miners or both, with an average age of 44 years. However, the endemic nature of this focus was not confirmed at that time, since neither epidemiological nor field work studies were performed until 1992. Specifically, in order to confirm the skin disease in El Bagre as a possible new EPF focus, one of us began visiting the endemic area in 1992 and found epidemiological differences between this focus and FS[28–30]. We collected information from the most senior individuals affected by this disorder (now called El Bagre-EPF), many of whom descend from an Indian heritage. These patients described the disease in past decades, especially within some Indian families, claiming the Indian tribes used to “burn those patients” (Abreu et al., personal interviews). These reports were not corroborated, but were obtained from different senior community leaders. In 1993, the presence of EPF cases was also reported in the southern part of Colombia; this disease focus affected nomadic Indians that worked predominately as fishermen and farmers; the disease affected both sexes, with similar characteristics as those described in FS[26]. The El Bagre-EPF research team created a program that provided food, medicine, and medical treatment utilizing a tax exception for donors; several grants were also secured to continue the studies. Unfortunately, Colombian governmental institutions did not specifically support this pemphigus research, and thus grants for this program were not provided by Colombian public institutions.
Is EPF organ-specific?
In clinical descriptions before the therapeutic steroid era, FS patients displayed multiple alterations in almost multiple anatomic systems (e.g., endocrine, musculoskeletal, cardiac, pulmonary, reticuloendothelial, genital, immune, gastrointestinal, renal, and nervous)[2–15,43–48]. Some of the most common manifestations included: 1) dwarfism, 2) progressive weight loss, 3) ankyloses, 4) bone decalcification and pathological fractures, 5) pulmonary, cardiac, renal, and hepatic alterations, 6) yellowish pigmentation of the nails, 7) azoospermia (aspermia), 8) nanism, 9) atrophy of mammary glands, 10) amenorrhea, and 11) hypogonadism[2–15,43–48]. Some of these alterations could be attributed to a loss of proteins via the skin lesions, with consequent malnutrition. In the therapeutic steroid era, the most common disease manifestations include: effects on the pulmonary system (tuberculosis, pneumonia, and bronchial pneumonia), cardiac system (endocarditis and pericarditis), hepatic system (fulminant hepatitis and cirrhorris), renal system (diffuse acute glomeronephritis), nervous system (meningitis and meningoencephalitis), and intestinal system (intractable diarrhea), as well as general sepsis[2–15,43–48]. These disease manifestations are similar to those observed in the El Bagre-EPF patients, with the exception of gonadal compromise[2–15,43–48]. Further, in agreement with the Brazilian FS literature, we found that one third of El Bagre-EPF patients exhibited severe symptoms, and indeed were refractory to immunosuppressive therapy (i.e., steroids, cyclosporine, cyclophosphamide, azatioprine, or methotrexate)[2–15,43–48]. The El Bagre-EPF patients demonstrate complex dermatopathologic findings, and possess autoantibodies to sweat glands, meibomian glands, some nerves, and tarsal muscle; they also have a polyclonal immune response against the palms and soles, with some clinical hyperkeratoses observed, but without acral blisters[28–30,50–53]. Forty percent of El Bagre-EPF cases clinically resemble Senear-Usher syndrome (also known as seborrheic pemphigus) due to the presence of lupus-like skin lesions, localization to photoexposed areas of the skin, the existence of a lupus band by direct immunofluorescence (DIF), the presence of antinuclear antibodies (ANA) in 1/34 of the cases, and the characteristic EPF autoantibodies to the intercellular regions of the epidermis[28–30]. There are still controversies regarding the association of FS with other autoimmune diseases. While some case reports have showed positive associations between FS and other autoimmune diseases, other studies have demonstrated no relationship between relevant disease populations utilizing 1) nonspecific autoantibodies, (including ANA), or 2) when utilizing clinical disease manifestations[54].
In El Bagre-EPF, there seems to be a protective role vis-à-vis disease development due to female hormones, since only males and a few post-menopausal females are affected[28–30]. In this regard, El Bagre-EPF patients differ greatly from patients with systemic lupus (SLE), where most affected individuals are females[1], as well with FS, in which the incidence is unaffected by gender.
Additional systemic effects of FS are suggested by autopsies of FS patients before the therapeutic steroid era, which revealed multiple alterations in the sympathetic nerve chains (i.e., dissolution of tigroids corps in the spine), focal encephalitis, and cystic degeneration of the posterior spinal nerves[2–15,43–48]. Moreover, steatosis of the liver and adrenal glands, and intense adrenal function with later atrophy of adrenal glands (hypocorticoidism) has been observed[2–15,43–48]. Interstitial nephritis, medial pulmonary necrosis, capillary disturbances, venous thromboses, and reticuloendotheliopathy have also been reported in FS[2–15,43–48]. Other FS findings include disseminated septicemia, bronchial pneumonia, miliary tuberculosis, massive gastrointestinal hemorrhage, pulmonary infarcts and balantiasis with strongyloidiasis[43–48]. Finally, multiple comorbid infections with bacteria, fungi, and parasites have been found, indirectly demonstrating some dysregulation of the immune response.
Metabolic, nutritional and acute phase immunoreactants in EPF:
Hypovitaminosis, acidosis, hypocholesterolemia, increased copper[34,55–57] or mercury levels[33], hyperkalemia, hypoproteinemia, and hypoalbuminemia (both relative and absolute) have been previously described in EPF[55,56]. Patients with EPF consistently present signs and symptoms of low serum protein levels, but not hypocaloric malnutrition, which is noted in severely affected patients[55–57]. A slight increase in alpha and beta globulins, an increase of C-reactive protein, complement activation, an increased erythrocyte sedimentation rate, an increase of anti-streptolysin O (ASO) titers (ASO) and mannan binding lectin-associated serine protease-2 (MASP-2) titers have also been found[58–60]. Our 10-year field work experience studying El Bagre-EPF indicates that inflammatory aspects seem to play a major role in the severity of the disease, and its “flares” or “relapses.” For more than a century, FS disease severity has been consistently and directly associated with inflammation in the skin. At a systemic level, FS disease severity is manifested by increases in a series of inflammatory markers such as C-reactive protein, fibrinogen, plasma viscosity, albumin, and the peripheral blood white blood cell count (e.g., leukocytosis)[2–15,43–48]. In FS, significant changes in skin color (hyperpigmentation), described almost as a “change of race,” are one of the markers of improvement of the disease[2–15,43–48]. One very unusual alteration encountered In EPF patients is a yellowish line on the nails, i.e., - “Vieira sign”, the significance of which remains unknown[2–15,43,44].
Risk factors for EPF: black flies, minerals, metals, metalloids, leishmania, hydathidosis and thiol-containing drugs:
Exposure to hematophagous black flies, especially Simulium pruinosum, has been associated with the development of FS disease for many years[2–15,61,62]. The presence and density of simulid species, as well as the biting activity of adult females were all described to be greater within a focus of FS than within neighboring regions with no reported FS cases[61,62]. Simulium nigrimanum was described as the most common black fly collected in human bait experiments in FS areas[61,62]. Further, this species was either absent (or present in very low numbers) in neighboring valleys and villages where cases of FS were not described[61,62]. Also, specific serum autoreactivity from EPF patients to extracts of S. nigrimanum has now been reported[61,62]. Since cutaneous leishmaniasis as well as PF is endemic in the northeastern region of São Paulo state, we searched for antibodies against maxadilan in sera of PF patients; we found increased antibodies compared to control sera[63]. Maxadilan represents a potent salivary vasodilator of Lu. longipalpis, a vector involved in systemic leishmaniasis. We have also demonstrated the similarity of some DNA and cDNA maxadilan sequences in Simulidae black fly species (Kim OM, Roselino AM et al, manuscript submitted), confirming some phylogenetic similarity among insect salivary peptides[63–65]. The presence of bed bugs also has been recently associated with FS[64]. In the El Bagre-EPF focus, no Simulium pruinosum has been demonstrated, and only a small number of S. exiguim and S. Mexican flies have been demonstrated in the endemic area (unpublished data). An increased ASO titer was also described in association with FS, but it was not reported whether or not Streptococcus superinfected the lesional skin of the patients, or, alternatively, was present preceding disease onset[56]. We also tested for ASO titers in El-Bagre-EPF patients in comparison with controls, but no significant differences were detected[28,29].
Other possible environmental risk factors associated with EPF have been reported in the area of xenobiotic exposure[33–36]. While the existing correlation between autoimmunity and environmental agents has not yet been well established, some possible predisposing environmental agents have been implicated in EPF development, including mercury, iodine, vinyl chloride, canavanine, organic solvents, silica, L-tryptophan, particulates, trace elements, ultraviolet radiation, and ozone. Mercury is commonly utilized to amalgamate gold, thereby facilitating its extraction during mining activities[34–36]. Mercury also amalgamates multiple trace elements, and can compete in an allosteric manner with calcium in binding to calcium-dependent proteins[39]. Desmogleins are also calcium-dependent proteins, and thus can be affected by mercury. Mercury, as well as other minerals, metalloids, and trace elements, is present in many products, such as fertilizers, pesticides and herbicides, employed the in farming activities present in both the Brazilian and El Bagre areas of EPF. The El Bagre area also has a unique constellation of environmental conditions, including acidic soil, ash products from forest fires, widespread jungle deforestation, extremely high temperatures, high humidity, thunderstorms and extensive microbe-mineral-metalloid interactions[33]. These factors may increase the bioavailability of environmental levels of mercury and other heavy metals, metalloids, minerals and trace elements. The population living in the El Bagre-EPF focus is exposed to high environmental levels of mercury due to gold extraction in mining activities, as well as from agricultural products; the population is also exposed to other minerals, metalloids and trace elements (e.g., quartz, rutile, granite, magnetite, and almenite), and to significant UV radiation due to the deforestation and their occupational activities. We have reported the presence of mercuric sulfides and selenides in skin biopsies from people living in a focus of EPF; we suggested that these compounds may play a role in the pathogenesis of this disorder[33–36]. In Brazil, most current FS cases are reported in the states of Goiás, Distrito Federal, Mato Grosso do Sul, Tocantins, and Minas Gerais. Brazil is known for its vast mineral resources, as a result of the geological diversity of its land[33–36]. Further, FS patients have documented increased copper levels, in comparison with matched controls living in the same areas[35]. Pemphigus can also be induced by thiol-containing drugs (e.g., D-penicillamine (DP)] and ACE inhibitors (e.g., captopril and enalapril)[35]. Hailey-Hailey disease (HHD), a genodermatosis, is histologically characterized by acantholysis similar to that seen in pemphigus and caused by a mutation in the ATP2C1 gene, which encodes a novel P-type Ca++ transport ATPase[66]. Coincidentally, Wilson's disease (WD) (caused by a mutation in a different P-type Ca++ transporter)[67], and SLE can also be triggered following exposure to DP[68]. Moreover, the El Bagre-EPF patients seem to share some clinical features with lupus erythematosus. We have identified mercuric selenides and sulfides, gold and trace elements as possible triggers of El Bagre-EPF. We hypothesize that in EPF, lupus, pemphigus and WD, as well as in rodents with autoimmunity induced by DP, HgCl2, copper, and/or gold compound, physiological ion exchanges may be altered.
Multiple other triggering factors have been implicated in the etiology of pemphigus. To attempt to confirm them, we utilized new tools to search for environmental antigenic molecules. One of these tools utilizes in vitro proliferative assays, and some hypothetic modeled antigens. For instance, we isolated and identified an “EXIKFAAAXREGED” sequence that encodes the N-terminal ectodomain of the mature form of desmoglein 1 (Dsg1), which represents the most-common linear and conformational epitope in both EPF variants[69]. Following protein blasting of this sequence, we determined that one of the molecules that shares partial homology with this sequence is the putative orotate phosphoribosyl molecule (OPM) in Streptococcus pyogenes M1 (SPM1). Cross-reactivity of antibodies against OPM to autoantigens in SPM1-induced acute rheumatic fever is one of the best examples of post-infectious autoimmunity due to molecular mimicry between host and pathogen. Other molecules with partially homologous sequences to the previously described EPF antigen include the ATP-dependent hsl protease from the Caulobacter vibrioides. The hsl protease functions as a chaperone subunit of a proteasome-like degradation complex. We thus speculate that other molecules involved in detoxification/ion exchange and/or catalytic mechanisms may also show some homology with the EPF antigen. In summary, we suggest that the metals, metalloids and trace elements that prevail in either or both of the EPF foci could act as “sensitizers to prevalent environmental antigenic molecules” in people who carry an appropriate genetic predisposition, and thus induce EPF development.
In Tunisian EPF, researchers are also searching for definitive environmental trigger factors[70]. A multicenter, case-control study was conducted prospectively from 1992 to 1996 in Tunisia. Sixty-eight incident female cases of pemphigus and 166 controls matched by age, hospital, and geographic living area were included. Data were collected concerning socioeconomic status, medical history, drug intake, lifestyle, and environment. Several risk factors were significantly associated with pemphigus in multivariate logistic regression analyses: 1) traditional cosmetics (odds ratio (OR) = 4.0, 95% confidence interval (CI): 1.1, 14.8); 2) Turkish baths (OR = 3.2, 95% CI: 1.4, 7.3); 3) cutting up raw poultry (OR = 5.1, 95% CI: 1.3, 19.4); 4) contact with ruminants (OR = 2.7, 95% CI: 1.3, 5.8); and 5) wasp, bee, and spider stings (OR = 3.1, 95% CI: 1.5, 6.4). A dose-dependent relationship was observed with traditional cosmetics. All risk factors (except insect bites) were greater when the analysis was restricted to younger women, a demographic group with higher disease incidence. The strength of the associations, the dose-dependent relationship with traditional cosmetics, and the increase of risk estimates in younger women support causal disease relationships with these risk factors[70].
Further, another study was reported using Tunisian EPF sera[70]. These authors utilized the rationale that PF and EPF sera react strongly against one of the ectodomains of the mature form of Desmoglein 1. Based on geographic clustering of cases, FS is thought to have at least one (as yet unidentified) environmental trigger. In this study, the authors searched for Dsg1 antibodies in sera from parasitic disease patients (leishmaniasis, Chagas disease and onchocerciasis), and infectious disease patients (leprosy and South American (SA) blastomycosis), which are prevalent in the same geographic regions of Brazil as FS. A specific and sensitive Dsg1 ELISA detected antibodies in 34 of 41 onchocerciasis sera (83%), 38 of 88 leishmaniasis sera (43%), 18 of 31 Chagas disease sera (58%), 7 of 28 SA blastomycosis sera (25%) and in 14 of 83 leprosy sera (17%). These sera recognized epitopes restricted to the Dsg1 EC5 domain. Thus, these findings identify several potential etiological factors for FS. We further hypothesize that a component of insect vector saliva, rather than the parasite itself, may trigger an antibody response to Dsg1 EC-5. The authors suggested that in persons with the known HLA susceptibility alleles (and living in endemic areas), an additional response to the Dsg1 EC1-2 domains may subsequently develop (by epitope spreading from the EC5 domain), resulting in a classic presentation of clinical FS. Finally the complexity of this disease based on current and past studies demonstrating several abnormalities in patients necropsies affected by EPF[72] demonstrated that is a very complex disease.
Conclusion
EPF diseases seem to be more complex than originally thought. The description of three endemic variants, including classical Brazilian FS, Tunisian and El Bagre-EPF support further research to identify disease trigger associations, and thus further explain the endemic nature of these disorders.
References
- 1.Theofilopoulos AN. The basis of autoimmunity: Part I: Mechanisms of aberrant self-recognition. Immunol Today. 1995;16:90–97. doi: 10.1016/0167-5699(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 2.Diaz LA, Sampaio SA, Rivitti EA, et al. Endemic pemphigus foliaceus (fogo selvagem). II. Current and historical, epidemiologic studies. J Invest Dermatol. 1989;12:92–94. doi: 10.1111/1523-1747.ep13070394. [DOI] [PubMed] [Google Scholar]
- 3.Diaz LA, Sampaio SA, Rivitti EA, et al. Endemic pemphigus foliaceus (fogo selvagem). I. Clinical features and immunopathology. J Amer Acad Dermatol. 1989;20:657–669. doi: 10.1016/s0190-9622(89)70079-7. [DOI] [PubMed] [Google Scholar]
- 4.Vieira JP. São Paulo, Brazil: Empresa Gráfica da Revista dos Tribunais (Publisher); 1940. Novas contribuições ao estudo do pênfigo foliáceo (fogo- selvagem) no Estado de São Paulo. [Google Scholar]
- 5.Silva F. Contribuição ao estudo do pênfigo foliáceo. Brasil Medico. 1938;87:1–7. [Google Scholar]
- 6.Proença NG, Ribeira AG. Aspectos epidemiológicos do pênfigo foliáceo no Brazil. Rev Assoc Med Brasil. 1976;22:281–284. [PubMed] [Google Scholar]
- 7.Azulay RD. Brazilian pemphigus foliaceus. Inter J Dermatol. 1982;21:121–124. doi: 10.1111/j.1365-4362.1982.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 8.Castro RM, Proenca NG. Semelhanças e diferenças entre o fogo selvagem e o pênfigo foliáceo de Cazanave.Similarities and differences between South American pemphigus foliaceus and Cazanave pemphigus foliaceus. An Bras Dermatol. 1983;53:137–139. [Google Scholar]
- 9.Aranha Campos J. São Paulo, Brasil: Comp Melhoramentos; 1942. Pênfigo foliáceo: (fogo selvagem). Aspectos clinicos e epidemiológicos. [Google Scholar]
- 10.Minelli L, Bostelman CR, Minelli HJ. Pênfigo e penfigóides.Casuística de 194 casos internados no Hospital São Roque (Paraná) An Bras Dermatol. 1990;65:67–69. [Google Scholar]
- 11.Castro RM, Roscoe JT, Sampaio SAP. Brazilian pemphigus foliaceus. Clin Dermatol. 1983;1:22–41. doi: 10.1016/0738-081x(83)90021-4. [DOI] [PubMed] [Google Scholar]
- 12.Pupo JA. Aspectos originais do pênfigo foliáceo no Brasil. An Bras Dermatol. 1971;46:53–60. [PubMed] [Google Scholar]
- 13.Brown MV. Fogo selvagem (pemphigus foliaceus).Review of the Brazilian literature. Arch Derm Syphilo. 1954;69:589–599. doi: 10.1001/archderm.1954.01540170059008. [DOI] [PubMed] [Google Scholar]
- 14.Rezende ST. Pênfigo foliáceo. Rev Bras Med. 1967;24:61–62. [PubMed] [Google Scholar]
- 15.Auad A. Pênfigo Foliáceo Sul-Americano no Estado de Goiás. Rev Patol Trop. 1972;1:293–346. [Google Scholar]
- 16.Leme CLP. Contribuíção ao estudo do tokelau. Tese da Faculdade de Medicina do Rio de Janeiro, 7 outubro de 1903. Thesis of the Medicine Faculty of Rio de Janeiro. 1903 Oct 3rd; [Google Scholar]
- 17.González F, Sáenz AM, Cirocco A, Tacaronte IM, Fajardo JE, Calebotta A. Endemic pemphigus foliaceus in Venezuela: report of two children. Pediatr Dermatol. 2006;23:132–135. doi: 10.1111/j.1525-1470.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Heimgartner E, De Heimagartner V. Experience with endemic dermatological diseases in the Peruvian wilderness: mucocutaneous leishmaniasis and Brazilian foliaceous pemphigus] Med Cutan Ibero Lat Am. 1976;4:1–6. [PubMed] [Google Scholar]
- 19.Ortega Loayza AG, Ramos W, Elgart G, et al. Antibodies against desmoglein 1 in healthy subjects in endemic and nonendemic areas of pemphigus foliaceus (fogo selvagem) in Peru. Int J Dermatol. 2006;45:538–542. doi: 10.1111/j.1365-4632.2006.02823.x. [DOI] [PubMed] [Google Scholar]
- 20.Guillet G, Strobel M, Pradinaud R. Brazilian pemphigus in a child in French Guyana.Discussion on the clinical polymorphism and epidemiology of the disease. Ann Dermatol Venereol. 1984;111:1087–1092. [PubMed] [Google Scholar]
- 21.Aldama A, Correu J, Rivelli V, et al. Tipos y variantes de pénfigo en el Hospital Nacional de Paraguay. Revisión de 70 casos. Med Cut ILA. 2000;28:242–247. [Google Scholar]
- 22.Castillo A, Maguiña C, Caciano I, Chacón P, Mansilla T. Pénfigo foliáceo variedad fuego salvaje en la selva peruana.Provincias de Requena y Ucayali. Bol Soc Per Med Int. 1993;6:65–67. [Google Scholar]
- 23.Ayed MB, Martel P, Zitouni M, et al. Tunisian endemic pemphigus foliaceus is associated with desmoglein 1 gene polymorphism. Genes Immun. 2002;3:378–379. doi: 10.1038/sj.gene.6363868. [DOI] [PubMed] [Google Scholar]
- 24.Bastuji-Garin S, Souissi R, Blum L, et al. Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol. 1995;104:302–305. doi: 10.1111/1523-1747.ep12612836. [DOI] [PubMed] [Google Scholar]
- 25.Bastuji-Garin S, Turki H, Mokhtar I, et al. Possible relation of Tunisian pemphigus with traditional cosmetics: a multicenter case-control study. Am J Epidemiol. 2002;155:249–256. doi: 10.1093/aje/155.3.249. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez G, Sarmiento L, Silva A. Penfigo foliaceo en indigenas colombianos.[ Pemphigus foliaceus in Colombian Indians] Rev Soc Col Dermatol. 1993;3:91–94. [Google Scholar]
- 27.Robledo MA, Prada SC, Jaramillo D, et al. South-American pemphigus foliaceus: Study of an epidemic in El Bagre and Nechi, Colombia 1982 to 1986. Br J Dermatol. 1988;118:737–744. doi: 10.1111/j.1365-2133.1988.tb02590.x. [DOI] [PubMed] [Google Scholar]
- 28.Abrèu-Velez AM, Beutner EH, Montoya F, Bollag WB, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Ac Dermatol. 2003;4:609–614. doi: 10.1067/s0190-9622(03)00852-1. [DOI] [PubMed] [Google Scholar]
- 29.Abrèu-Velez AM, Hashimoto T, Bollag WB, et al. A unique form of endemic pemphigus in Northern Colombia. J Am Ac Dermatol. 2003;4:609–614. doi: 10.1067/s0190-9622(03)00851-x. [DOI] [PubMed] [Google Scholar]
- 30.Hisamatsu Y, Abreu-Velez AM, Amagai M, Ogawa MM, Kanzaki T, Hashimoto T. Comparative study of autoantigen profile between Colombian and Brazilian types of endemic pemphigus foliaceus by various biochemical and molecular biological techniques. J Dermatol Sci. 2003;32:33–41. doi: 10.1016/s0923-1811(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 31.Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol. 1997;12:77–96. [PubMed] [Google Scholar]
- 32.Nagata Y, Karashima T, Watt FM, Salmhofer W, Kanzaki T, Hashimoto T. Paraneoplastic pemphigus sera react strongly with multiple epitopes on the various regions of envoplakin and periplakin, except for C-terminal homologous domain of periplakin. J Invest Dermatol. 2001;116:556–563. doi: 10.1046/j.1523-1747.2001.01263.x. [DOI] [PubMed] [Google Scholar]
- 33.Abréu Vélez AM, Warfvinge G, Herrera WL, et al. Detection of mercury and other undetermined materials in skin biopsies of endemic pemphigus foliaceus. Am J Dermatopathology. 2003;25:384–391. doi: 10.1097/00000372-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Figueira MV, Ferreira de Souza L. Estudo sobre cobre sėrico. [Tese apresentada ao Departamento de Patologia de Apoio clinico da Universidade Federal Fluminense, Rio de Janeiro, para habilitacão à docência livre de Bioquímica Clínica]. (Studies on serologic levels of cooper, thesis of the Department of Clinical Pathology, Federal University, Rio de Janeiro) Rio de Janeiro, Brazil. 1976 [Google Scholar]
- 35.Gamarski J, Auad T. D-Penicilamina no pênfigo foliáceo Sul-Amerricano. (D-penicillamine and South American pemphigus foliaceus) Rev Inst Med Trop São Paulo. 1977;19:138–139. [PubMed] [Google Scholar]
- 36.Tavares GA, Ferreira JR, Magalhaes CE, da Silva NC, Taddei MH. Mercury in the Moji-Guacu river basin, S.P-Brazil: the link between marginal lagoons and river contribution, assessed by 210Pb dating profiles. Ambio. 2003;32:47–51. [PubMed] [Google Scholar]
- 37.Chiossi MPV, Roselino AM. Endemic pemphigus (“fogo selvagem”): A series from the Northeastern region of the State of Sao Paulo, Brazil, 1973-1998. Rev Inst Med Trop São Paulo. 2001;43:59–62. doi: 10.1590/s0036-46652001000200001. [DOI] [PubMed] [Google Scholar]
- 38.Reis VM, Toledo RP, Lopez A, Diaz LA, Martins JE. UVB-induced acantholysis in endemic Pemphigus foliaceus (Fogo selvagem) and Pemphigus vulgaris. J Am Acad Dermatol. 2000;42:571–576. [PubMed] [Google Scholar]
- 39.Warren SJ, Lin MS, Giudice GJ, et al. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil.Cooperative Group on Fogo Selvagem Research. N Engl J Med. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 40.Ortega Loayza AG, Ramos W, Elgart G, et al. Antibodies against desmoglein 1 in healthy subjects in endemic and nonendemic areas of pemphigus foliaceus (fogo selvagem) in Peru. Int J Dermatol. 2006;45:538–542. doi: 10.1111/j.1365-4632.2006.02823.x. [DOI] [PubMed] [Google Scholar]
- 41.Kallel Sellami M, Ben Ayed M, Mouquet H, et al. Anti-desmoglein 1 antibodies in Tunisian healthy subjects: arguments for the role of environmental factors in the occurrence of Tunisian pemphigus foliaceus. Clin Exp Immunol. 2004;137:195–200. doi: 10.1111/j.1365-2249.2004.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins-Castro R, Proença N, de Salles-Gomes LF. On the association of some dermatoses with South American pemphigus foliaceus. Int J Dermatol. 1974;13:271–275. doi: 10.1111/j.1365-4362.1974.tb05080.x. [DOI] [PubMed] [Google Scholar]
- 43.Viera JP. Algumas modalidades raras do pênfigo foliáceo sul americano. Arq Dermat Siphil de São Paulo. 1937;1:22–26. [Google Scholar]
- 44.Viera JP. Pênfigo foliáceo e Síndrome de Senear-Usher. São Paulo; Empresa Gráfica da Revista dos Tribunais. (Pemphigus foliaceus and Senear-Usher Syndrome) 1942 [Google Scholar]
- 45.Proença Guimares N, de Moraes Neto MP, de Próspero JD. Azoospermia caused by South American pemphigus foliaceus. Rev Inst Med Trop São Paulo. 1978;20:307–311. [PubMed] [Google Scholar]
- 46.Arneondola R. Lesiones oculars do pênfigo foliáceo. Bol Soc Bras Dermatol Sif. 1944;19:329. [Google Scholar]
- 47.Da Matta A. Fogo Selvagem ou Pemphigo Foliáceo (endemic pemphigus foliaceus or fogo selvagem) Amazonas Med. 1941;5:5–22. [Google Scholar]
- 48.de Abreu-Leme C. Considerações sobre a etipathogenia do penfigo foliaceus.Esquema do tratamento. Trabalho apresentado no Departamento de Dermatologia e Sifilografia da Associação Paulista de Medicina emjunho(Work presented in the Department of Dermatology of the Association of Medicine of São Paulo, June 13 1955) Rev Paul Med. 1955:88–96. [Google Scholar]
- 49.Yepes A. Brote de penfigo foliaceo en el municipio de El Bagre. [A focus of endemic pemphigus in El Bagre municipaltite] Bol Epidemiol Antioquia. 1983;2:87. [Google Scholar]
- 50.Howard MS, Yepes MM, Maldonado-Estrada JG, et al. Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus-part 1. J Cutan PatholJul. 2009 Jul 13; doi: 10.1111/j.1600-0560.2009.01315.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Abreu-Velez AM, Howard MS, Hashimoto K, Hashimoto T. Autoantibodies to sweat glands detected by different methods in serum and in tissue from patients affected by a new variant of endemic pemphigus foliaceus. Arch Dermatol Res. 2009;301:711–718. doi: 10.1007/s00403-009-0972-4. [DOI] [PubMed] [Google Scholar]
- 52.Abreu-Velez AM, Howard MS, Hashimoto T. Palm tissue displaying a polyclonal autoimmune response in patients affected by a new variant of endemic pemphigus foliaceus in Colombia, South America. Eur J Dermatol. 2009 Nov 4; doi: 10.1684/ejd.2010.0834. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Abreu-Velez AM, Howard MS, Hashimoto T, Grossniklaus HE. Human eyelid meibomian glands and tarsal muscle are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in El-Bagre, Colombia, South America. J Am Acad Dermatol. 2010 Jan 8; doi: 10.1016/j.jaad.2009.06.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nisihara RM, Schmidt de Bem R, Hausberger R, et al. Prevalence of autoantibodies in patients with endemic pemphigus foliaceus (fogo selvagem) Arch Dermatol Res. 2003;295:133–137. doi: 10.1007/s00403-003-0412-9. [DOI] [PubMed] [Google Scholar]
- 55.Ferri RG, Castro RM, Rivitti E, Sampaio SA. Electrophoretic and immunoelectrophoretic study of the South American pemphigus foliaceus. Rev Inst Med Trop São Paulo. 1970;12:388–394. [PubMed] [Google Scholar]
- 56.Rivitti EA. Electroforese, imunoelectroforese, antiestreptolosina-O e anticorpos antiepitélio no pênfigo foliáceo Sul Americano. Estado Evolutivo. Tese de Doutoramento apresentada ao Departamento de Medicina Tropical e Dermatologia da Faculdade de Medicina da Universidade de São Paulo. 1972:1–105. [Google Scholar]
- 57.Barraviera SR, Dillon NL, Curi PR, Pereira PC, de Almeida DB. Evaluation of nutritional status in patients with endemic pemphigus foliaceus. Rev Inst Med Trop São Paulo. 1995;37:51–58. doi: 10.1590/s0036-46651995000100008. [DOI] [PubMed] [Google Scholar]
- 58.de Messias IT, Santamaria J, Ragiotto R, Doi EM, Kajdacsy-Balla A.Complement activation in Brazilian Pemphigus foliaceus. Clin Exp Dermatol. 1989;4:51–55. doi: 10.1111/j.1365-2230.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 59.de Messias IT, Von Kuster LC, Santamaría J, Kajdacsy-Balla A. Complement and antibodies deposition in Brazilian Pemphigus Foliaceus: correlation of circulating antibodies with clinical activity. Arch Dermatol. 1988;124:1664–1668. doi: 10.1001/archderm.124.11.1664. [DOI] [PubMed] [Google Scholar]
- 60.Messias-Reason IJ, Bosco DG, Nisihara RM, Erler MLP, Jensenius JC. Mannan-binding lectin (MBL) and MBL- associated serine protease 2 (MASP-2) circulating levels in endemic pemphigus foliaceus. Clin Exp Dermatol. 2008;33:495–497. doi: 10.1111/j.1365-2230.2008.02743.x. [DOI] [PubMed] [Google Scholar]
- 61.Lombardi C, Barges PC, Chaul A, et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 1992;95:847–850. doi: 10.1111/1523-1747.ep12456932. [DOI] [PubMed] [Google Scholar]
- 62.Eaton DP, Diaz LA, Hans-Filho G, et al. Comparison of black fly species (Diptera: Simuliidae) on an Amerindian reservation with a high prevalence of fogo selvagem to neighboring disease-free sites in the State of Mato Grosso do Sul, Brazil.The Cooperative Group on Fogo Selvagem Research. J Med Entomol. 1988;35:120–131. doi: 10.1093/jmedent/35.2.120. [DOI] [PubMed] [Google Scholar]
- 63.Kim OM, Pepinelli M, Passeri M, Figueiredo JFC, Roselino AM. Soro de pacientes com pênfigo foliáceo (fogo selvagem) reage contra proteínas de insetos hematófagos (Simulium nigrimanum) An Bras Dermatol. 2008;83:S30–S30. [Google Scholar]
- 64.Aoki V, Millikan RC, Rivitti EA, et al. Cooperative Group for Fogo Selvagem Research.Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem) J Investig Dermatol Symp Proc. 2004;9:34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 65.Roselino AM, Figueiredo JFC, Kounga K, Reddy V, EA Lerner. Serum IgG from pemphigus foliaceus patients reacts against maxadilan. J Investig Dermatol. 2001;117:460. [Google Scholar]
- 66.Zhoujiang L, Tao Y, Xiuchao L, Mugen L, Wang-Qing K, Jing YL. A novel mutation in the ATP2C1 gene is associated with Hailey-Hailey disease in a Chinese family. Intern J Dermatol. 2009;48:47–51. doi: 10.1111/j.1365-4632.2009.03878.x. [DOI] [PubMed] [Google Scholar]
- 67.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 68.Tsay GT, Zouali M. Toxicogenomics — A novel opportunity to probe lupus susceptibility and pathogenesis. Internal Immunopharmacol. 2008;8:1330–1337. doi: 10.1016/j.intimp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Abréu-Vélez AM, Javier Patiño P, Montoya F, Bollag WB. The tryptic cleavage product of the mature form of the bovine desmoglein 1 ectodomain is one of the antigen moieties immunoprecipitated by all sera from symptomatic patients affected by a new variant of endemic pemphigus. Eur J Dermatol. 2003;4:359–366. [PubMed] [Google Scholar]
- 70.Bastuji-Garin S, Turki H, Mokhtar I, et al. Possible relation of Tunisian pemphigus with traditional cosmetics: a multicenter case-control study. Am J Epidemiol. 2002;155:249–256. doi: 10.1093/aje/155.3.249. [DOI] [PubMed] [Google Scholar]
- 71.Diaz LA, Arteaga LA, Hilario-Vargas J, et al. Anti-desmoglein-1 antibodies in onchocerciasis, leishmaniasis and Chagas disease suggest a possible etiological link to Fogo selvagem.Cooperative Group on Fogo Selvagem Research. J Invest Dermatol. 2004;123:1045–1051. doi: 10.1111/j.0022-202X.2004.23438.x. [DOI] [PubMed] [Google Scholar]
- 72.Aki HE, Sotto MN, Sampaio SA. [ Post-mortem evaluation in endemic pemphigus foliaceus and pemphigus vulgaris] Med Cutan Ibero Lat Am. 1984;12:151–157. [PubMed] [Google Scholar]


