Abstract
Background:
Candida albicans infections of the cervix are not adequately diagnosed in Papanicolaou smears when compared with culture in Sabouraud dextrose agar.
Methods:
Cervical smears were collected from 1000, non-pregnant, asymptomatic women. The specimens were prepared using the Papanicolaou (Pap) smear method examined by microscopy and subsequently cultured in Sabouraud dextrose agar.
Results:
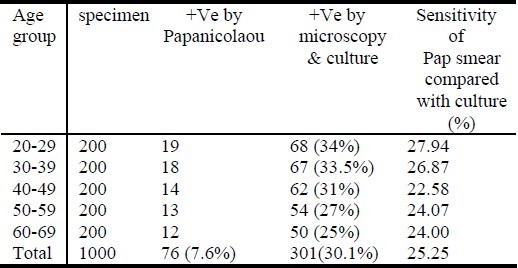
The overall incidences of C. albicans in cervical smears cultured in Sabouraud dextrose agar was 30.10%, while the incidence in the Pap smears was 7.60%, making the sensitivity of Pap smears for the diagnosis of C. albicans to be 25.25%. Mild, and some moderate, infections detectable in Sabouraud dextrose agar could not be detected in the Pap smear specimens. Vulnerability to C. albicans infection decreased with age, the highest infection rate being between 20 and 39 years of age while the least was between 60 and 69 years of age. Papanicolaou's method stained the nuclei, chromatin and nucleoli very well and enhanced differentiation between malignant and non-malignant cells. It also stained the cytoplasm and its contents such as keratin, vacuoles and granules and differentiated between acidophilic and basophilic materials. Non-cellular substances such as fibrin, crystals and pigments were also stained using this method. In addition, Papanicolaou's method also stained some fungal and bacterial species, but did not provide differential staining characteristics seen in Gram staining technique.
Conclusions:
Papanicolaou stain should not be used as an alternative to culture in the diagnosis of Candida albicans.
Keywords: Pap smear, culture, Candida albicans infections, cervix, Sabouraud dextrose agar
Introduction
In most healthy people, Candida albicans is a harmless microflora but, in immune compromised patients, C. albicans develops into an opportunistic pathogen that can cause life-threatening disseminated infections[1]. Although the immune status of the host is the major factor that determines whether C. albicans can become a pathogen and cause infection, the fact that C. albicans is by far the most frequent cause of fungal infections in such debilitated patients, indicates that it must possess traits that make it a more successful colonizer and pathogen than other medically important Candida species[2].
The relationship between inflammatory cervical smears and the diagnosis of malignancies has been examined and it was reported that underlying infections could mask cancerous or precancerous changes and that certain invasive cervical squamous cell carcinomas could not be detected until the infections were treated[3]. C. albicans is a major cause of the disease called vulvovaginal candidiasis and it possesses a gene family which encodes secreted aspartic proteinases[4] which have been linked with the virulence of the fungus[5]. During infections, the proteinases digest the host's proteins for nutrient supply and evade the host defenses by degrading immunoglobulins and complement proteins[6]. Individual members of the gene family might have their own special role in infection, and this might be reflected by a differential expression pattern at various stages of the infection process[3].
It is a general view that the pathogenicity of C. albicans is not caused by single dominant virulence factors[7], rather, it seems to be the high adaptability of C. albicans to many different host niches, as illustrated by the possession of many different adhesins that mediate binding to a variety of tissues, which allows the fungus to colonize and infect virtually all body locations[8]. A prerequisite for this adaptability is the capacity to respond to complex environmental signals, representing the different host niches, by the expression of an appropriate set of virulence-related genes. Intra-amniotic and placental infections with Candida albicans are rare during pregnancy, but these have been described[9] and have also been found in association with the intrauterine device (IUD)[10,11]. The IUD, being the facilitator of the infection.
The Pap smear is a routine screening test used for the detection of cervical abnormalities and precancerous dysplastic changes of the uterine cervix[12]. It also detects certain viral, bacterial, and fungal infections of the cervix and vagina[13]. There is also epidemiological and experimental evidence that Pap smears are beneficial in detecting infections that are risk factors associated with cervical cancer, such as human papilloma virus[14]. The aim of this study was to determine the suitability of Papanicolaou stain for the detection of C. albicans in cervical specimens.
Patients and Methods
From 2005 to 2007, twin cervical specimens were collected from 1000, asymptomatic, non-pregnant women between the ages of 20 and 70 years in South Western Nigeria. Individuals who presented with heavy vaginal discharge were excluded from the study because this often leads to false positive Pap smear results. The specimens were collected with swab sticks and an Ayre's spatula with the support of a speculum. Smears from the Ayre's spatula were made on clean dry slides. The smears were fixed immediately in equal volumes alcohol and ether while they were still wet for 1 hour.
They were then stained by the Papanicolaou method as follows: Harris's haematoxylin without acetic acid for 5 minutes, rinsed in tap water and differentiated in 1% acid-alcohol for 30 seconds and blued in Scott's water for 2 minutes. Smears were taken to 95% alcohol and stained in OG6 for 2 minutes, rinsed in 95% alcohol and stained in EA 35 for 2 minutes. Smears were then taken to two changes of absolute alcohol, xylene and mounted in DPX. The stained smears were examined under the light microscope at low and high power objectives for the presence of Candida albicans.
Specimens from the swab sticks were cultured on Sabouraud dextrose agar (SDA) with 0.05 mg/mL chloramphenicol and incubated at 37 °C for 48 hours and examined for the growth of yeasts. Pasty, creamy and smooth colonies were considered as yeasts and then further identified by germ tube test and by chlamydospore production on corn meal agar. Colonies were smeared on slides for Gram's standard staining technique. Wet preparations of the swabs in sterile normal saline were also examined with low and high power objectives.
Results
A total of 1000 cervical specimens were examined. These were arranged into 5 age groups of 200 each. Incidence of Candida albicans in Papanicolaou smears were compared with incidence of Candida albicans in microscopy and culture. The over all incidences of C. albicans in cervical smears cultured in SDA was 30.10%, while the incidence in the Pap smears was 7.60%, making the sensitivity of Pap smear for the diagnosis of C. albicans to be 25.25%. Culture growths on Sabouraud dextrose agar plates ranged from mild, moderate to profuse growths. Mild, and some moderate, infections detectable in culture could not be detected in the Pap smears. Vulnerability to C. albicans infection decreased with age, the highest infection rate being between 20 and 39 years while the lowest was between 60 and 69 years (Table 1).
Table 1.
Comparative study of C. albicans in Papanicolaou smears with microscopy and culture
Papanicolaou's method stained the nucleus, chromatin and nucleolus very well and enhanced differentiation between malignant and non-malignant cells. It also stained the cytoplasm and its contents such as keratin, vacuoles and granules and differentiated between acidophilic and basophilic materials. Non cellular substances such as fibrin, crystals and pigments were also stained using this method. In addition, Papanicolaou's method also stained some fungal and bacterial species but it did not provide the unique differential staining characteristics seen in Gram staining technique.
Discussion
Papanicolaou's is the most important staining method in cytology, because it enhances differentiation between malignant and non-malignant cells and also stains the cytoplasm and its contents. Being able to differentiate between acidophilic and basophilic materials as well as its ability to stain non-cellular substances such as fibrin, crystals and pigments, make it an essential stain in cytology. Papanicolaou's method also stains fungi and bacteria but it does not provide differential staining characteristics seen in Gram staining technique[13].
This study confirms that C. albicans is detectable by the Pap technique, but the major disadvantage is in its inability to detect mild infections as it is only 25.25% sensitive when compared with microscopy and culture results in Sabouraud dextrose agar. Women below the age of 20 years were excluded from the study, because they are often excluded from routine Pap smear tests and it was difficult to get volunteers who were below 20 years of age. Of the 1000 cases studied, 301(30.10%), had C. albicans in their cervix by the culture method while only 76 (7.60%) were positive by the Pap smear technique. This result is slightly lower than a previous study[15] where, in samples taken from pregnant women, 40% were positive in culture versus 20% in the Pap smears. The authors concluded that positive cultures were strongly related to a number of clinical signs and symptoms, but Pap smears were not sensitive for diagnosing symptomatic fungal infections.
In a separate study, C. albicans was detected in 42.3% of samples when an immunologic latex agglutination test method was used for the detection of C albicans in the vagina[16] but this was not confirmed by culture. A low value of 1.2% C. albicans was detected in Pap smears in a study conducted in Jordan[17] although they also did not compare their results with culture. Yet another study reported a striking low value of 14% observed in the cultures of vaginal specimens of pregnant women[18].
In some countries, people have unlimited access to drugs including antifungal drugs and use them at will. This may account for the wide variation in the presence of the organisms in the cervix from one country to another. The immune status, hygiene and level of infection of patients may also have a profound influence on the ability of C. albicans to cause an infection in the cervix as a 50% incidence of C. albicans in the vagina of patients that presented with vaginal itching and discharge were observed[19].
Conclusion
Culture is therefore, the gold standard for the diagnosis of Candida albicans and Pap smears should not be used as an alternative for the diagnosis of C. albicans because it cannot detect mild and some moderate infections.
References
- 1.Odds F. C. Candida and Candidosis (Baillie´re Tindall, London) 1988 [Google Scholar]
- 2.Staib P, Kretschmar M, Nichterlein T, Hof H, Morschha J. Differential activation of a Candida albicans virulence gene family during infection. PNAS. 2000;97(11):6102–6107. doi: 10.1073/pnas.110031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly BA, Black SA. The inflammatory cervical smear: a study in general practice. Br J Gen Pract. 1990;40:238–240. [PMC free article] [PubMed] [Google Scholar]
- 4.Monod M, Hube B, Hess D, Sanglard D. Differential regulation of SAP8 and SAP9, which encode two new members of the secreted aspartic proteinase family in Candida albicans. Microbiology. 1998;144:2731–2737. doi: 10.1099/00221287-144-10-2731. [DOI] [PubMed] [Google Scholar]
- 5.Cassone A, De Bernardis F, Mondello F, Ceddia T, Agatensi L. Evidence for a correlation between proteinase secretion and vulvovaginal candidosis. J Infect Dis. 1987;156(5):777–783. doi: 10.1093/infdis/156.5.777. [DOI] [PubMed] [Google Scholar]
- 6.Hube B. Possible role of secreted proteinases in Candida albicans infection. Rev. Iberoam. Micol. 1998;15:65–68. [PubMed] [Google Scholar]
- 7.Odds F. C. Candida species and virulence. ASM News. 1994;60:313–318. [Google Scholar]
- 8.Calderone RA. Recognition between Candida albicans and host cells. Trends Microbiol. 1993;1(2):55–58. doi: 10.1016/0966-842x(93)90033-n. [DOI] [PubMed] [Google Scholar]
- 9.Smith CV, Horenstein J, Platt LD. Intra amniotic infection with Candida albicans associated with a retained intrauterine contraceptive device: a case report. Am J Obstet Gynecol. 1988;159(1):123–124. doi: 10.1016/0002-9378(88)90505-4. [DOI] [PubMed] [Google Scholar]
- 10.Michaud P, Lemaire B, Tescher M. Spontaneous abortion with an IUD and Candida chorioamnionitis. Gynecol Obstet. 1989;84(1):45–46. [PubMed] [Google Scholar]
- 11.Meizoso T, Rivera T, Fernández-Aceñero M. J, Mestre M. J, Garrido M, Garaulet C. Intrauterine candidiasis: report of four cases. Arch Gynecol Obstet. 2008;278(2):173–176. doi: 10.1007/s00404-007-0554-7. [DOI] [PubMed] [Google Scholar]
- 12.Papanicolaou G. N. A new procedure for staining vaginal smears. Science. 1942;95:438–439. doi: 10.1126/science.95.2469.438. [DOI] [PubMed] [Google Scholar]
- 13.Avwioro OG. 1st ed. Claverianun press Nigeria; 2002. Histochemistry and tissue pathology. [Google Scholar]
- 14.Schiffman MH, Bauer HM, Hoover RN. Epidemiologic evidence that human papillomavirus infection causes most cervical intraepithelial neoplasia. JNCI. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 15.Donders GG, van Straeten D, Hooft P, De Wet GH. Detection of Candida cell forms in Pap smears during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;43(1):13–18. doi: 10.1016/0028-2243(92)90237-s. 9. [DOI] [PubMed] [Google Scholar]
- 16.Limia OF, Lantero DMI. Prevalence of Candida albicans and Trichomonas vaginalis in Pregnant Women in Havana City by an Immunologic Latex Agglutination Test Med Gen Med. 2004;6:50. [PMC free article] [PubMed] [Google Scholar]
- 17.Malkawi SR, Abu Hazeem RM, Hajjat BM, Hajjiri FK. Evaluation of cervical smears at King Hussein Medical Centre, Jordan, over three and a half years. Eastern Mediterranean Health Journal Report. 2004;10:676–679. [PubMed] [Google Scholar]
- 18.Alteras I, Aryeli J. The incidence of Candida albicans in the last day of pregnancy and the first days of the new born. Mycopathologia. 1980;72:85–87. doi: 10.1007/BF00493816. [DOI] [PubMed] [Google Scholar]
- 19.Umeh SO, Emelugo BM. Incidence of Candida albicans infection among women having cases of vaginal itching and discharge in Awka Anambra State, Nigeria. Trop J Med Res. 2007;11:9–11. [Google Scholar]


