Abstract
Zearalenone (ZEN) is an estrogenic mycotoxin produced by several Fusarium species, which can contaminate food and feed. These compounds elicit a wide spectrum of toxic effects, including the capacity to alter normal immune function. In this study, the in vitro effects of the treatment of ConA-stimulated splenic lymphocytes with ZEN (0–25 μg/mL) were examined. ZEN modulates the expression of IL-2, IL-6, and IFN-γ. The IL-2 levels were up to fourfold higher (P < 0.05) compared with the levels in the control at toxin concentrations of 25 μg/mL after 48 h of treatment. The IL-6 levels were critically suppressed at this concentration; these changes were very statistically significant (P < 0.05). At lower ZEN concentrations (0.1, 0.4 and 1.6 μg/mL), the IFN-γ levels changed slightly; however at 6.25 and 25 μg/mL, the IFN-γ results reached statistical significance compared with the control levels (P < 0.05). These data suggest that ZEN has potent effects on the expression of chicken splenic lymphocytes cytokines at the mRNA level.
1. Introduction
Zearalenone (ZEN) is a mycotoxin produced by several field fungi, including Fusarium graminearum (Gibberella zeae), F. culmorum, F. cerealis, F. equiseti, and F. semitectum [1, 2]. It exists widely in many cereal crops such as corn, barley, wheat, oats, sorghum, and sesame seeds, as well as in hay and corn silage. These are all ingredients in many food products for human or animal nutrition [3–5].
ZEN is a macrocyclic lactone with a high binding affinity for estrogen receptors. It is biologically potent, but it is hardly toxic. Rather, it has an estrogenic effect that causes alterations in the reproductive tract of laboratory animals (mice, rats, and guinea pigs) and farm animals [6, 7]. The mechanism of the estrogenic effects of ZEN appears to be mediated via binding of this mycotoxin or its metabolites to the cytoplasmic estrogen receptor [8–10]. This increases cell proliferation [11], resulting in uterine hyperplasia as well as cervical and vaginal metaplasia [12, 13].
The immune system is a potential target for estrogenic endocrine disruptors because various cells of the immune system have estrogen receptors [14]. However, only few studies have been carried out regarding the effects of ZEN on spleen immunity in chickens, specifically on lymphocytes. In the present study, we investigated the in vitro effects of ZEN at different concentrations on IL-2, IL-6, and IFN-γ mRNA expression levels in poultry spleen lymphocytes.
2. Materials and Methods
2.1. Reagents
All chemicals were of the highest grade of purity available. Fetal bovine serum (FBS) was purchased from Sijiqing Biological Engineering Material (Hangzhou, China). Zearalenone, RPMI 1640 medium, and Histopaque 1077 were purchased from Sigma-Aldrich, USA. Trizol reagent was purchased from Invitrogen Biotechnology Co. Ltd. (Shanghai, China). SYBR PremixScript RT-PCR Kit II was purchased from Takara, Shiga, Japan.
2.2. Cell Culture
All chickens used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. The spleens of two-month-old Isa Brown chicken were teased through a 200-mesh cell strainer into a Petri dish containing phosphate-buffered saline (PBS). The cell suspension was overlaid onto Histopaque 1077 and was centrifuged at 400 ×g for 15 min at room temperature. The lymphocytes at the interface were collected, washed twice with PBS at 250 g for 5 min at room temperature, and suspended in RPMI-1640 medium (without phenol red, a weak estrogen mimic) supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 U/mL streptomycin. More than 95% of cells were viable based on the trypan blue dye exclusion. The spleen cells were cultured in 6-well tissue culture plates (6 × 106 cell/mL) in triplicate and stimulated with concanavalin A (ConA, 12.5 μg/mL, to induce the proliferation of T cells) at 0, 0.1, 0.4, 1.6, 6.25, and 25 μg/mL ZEN at 41.5°C in a humidified 5% CO2 environment for 48 h [15]. The mycotoxin concentrations were selected based on preliminary dose-response experiments (data not shown). After 48 h of incubation, cells were collected for RNA isolation, then frozen at −80°C.
2.3. Quantification of IL-2, IL-6, and IFN-γ mRNA
Total RNA was isolated from cells using Trizol reagent according to the instructions of the manufacturer. The RNA concentrations were determined using the GeneQuant 1300.
The 40 μL reverse transcription reaction mixture consisted of the following components: 10 μg of total RNA, 1 μL of Moloney murine leukemia virus reverse transcriptase, 1 μL of RNase inhibitor, 4 μL of deoxynucleoside triphosphate, 2 μL of Oligo dT, 4 μL of dithiothreitol, and 8 mL of 5x reverse transcriptase buffer. The reverse transcription was performed according to the instructions of the manufacturer (Invitrogen). The reverse transcription products (cDNA) were stored at −20°C for PCR.
To design primers, we used the chicken IL-2, IL-6, and IFN-γ mRNA GenBank sequence with accession numbers of NM204153.1, NM204628.1, and DQ470471.1, respectively. Chicken β-actin (GenBank accession number L08165.1), a housekeeping gene, was used as the internal reference. The primers (Table 1) were designed using the Prime 5 Software (Molecular Biology Insights, Cascade, CO) and were synthesized by Invitrogen Biotechnology Co. Ltd., Shanghai, China.
Table 1.
Real-time PCR primer sequences and products.
| Gene | Primer | Sequences (5′→3′) | Product size (bp) |
|---|---|---|---|
| β-actin | β-Actin Forward | CACCACAGCCGAGAGAGAAAT | 135 |
| β-Actin Reverse | TGACCATCAGGGAGTTCATAGC | ||
| IL-2 | IL-2 Forward | GCTAATGACTACAGCTTATGGAGCA | 138 |
| IL-2 Reverse | TGGGTCTCAGTTGGTGTGTAGAG | ||
| IL-6 | IL-6 Forward | AAATCCCTCCTCGCCAATCT | 106 |
| IL-6 Reverse | CCCTCACGGTCTTCTCCATAAA | ||
| IFN-γ | IFN-γ Forward | AAGTCATAGCGGCACATCAAAC | 132 |
| IFN-γ Reverse | CTGGAATCTCATGTCGTTCATCG |
Real-time PCR was performed to detect the expression of IL-2, IL-6, and IFN-γ genes in different cDNA sample using SYBR Premix Ex Taq (Takara, Shiga, Japan). Each sample was assayed in three replicates. The reaction mixtures were incubated in an ABI PRISM 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The program included 1 cycle at 95°C for 30 s, 40 cycles at 95°C for 5 s, and 60°C for 34 s. The dissociation curves were analyzed using Dissociation curve 1.0 Software (Applied Biosystems) for each PCR reaction to detect and eliminate the possible primer dimer and nonspecific amplification. Results (fold changes) are expressed using the Pfaffl method [16, 17] with the following formula:
| (1) |
where ΔCT, target (calibrator-test) = (CTtarget)control group − (CTtarget)treatment group, ΔCT, ref (calibrator-test) = (CTref)control group − (CTref)treatment group, Etarget is the real-time PCR efficiency of target gene transcript, and Eref is the real-time PCR efficiency of a reference gene transcript.
2.4. Statistical Analysis
Statistical analysis of all data was performed using SAS procedures (SAS Institute Inc, NC); differences in mean RT-PCR results were compared by one-way ANOVA. All values are expressed as mean ± SD, and P < 0.05 were considered as significant difference.
3. Results
To compare the effects of ZEN on cytokine expression in residual living cells among individuals, the results were calculated as mean fold changes in cytokine levels above the control (treated with only ConA) cultures at the same time points. Treatment of the ConA-stimulated cells with ZEN at concentrations ranging from 0 to 25 μg/mL consistently increased the IL-2 levels above those in the ConA-only stimulated cells (Figure 1). These results were statistically significant (P < 0.05) with toxin concentrations of 0.1, 0.4, 1.6, 6.25, and 25 μg/mL at 48 h (Figure 1). In contrast, the ZEN treatment moderately inhibited IL-6 levels in these cells; these changes were very statistically significant with toxin concentration in all treated groups (P < 0.05). The IL-6 levels were critically suppressed (Figure 2).
Figure 1.
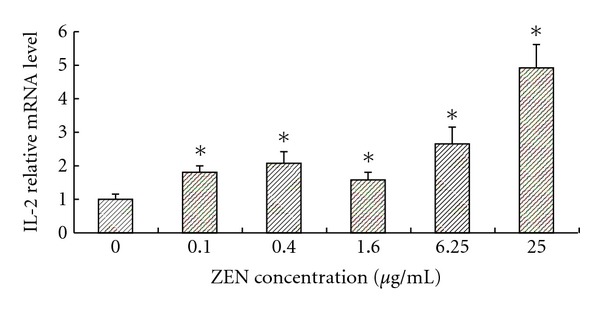
The effect of ZEN on the IL-2 relative mRNA level in ConA-stimulated lymphocytes at toxin concentration of 0, 0.1, 0.4, 1.6, 6.25, and 25 μg/mL at 48 h. The reporter activity in response to ConA alone is expressed as 100%. Values are the means ± SD from three independent experiments. Statistical analysis was performed using one-way ANOVA followed by Dunnett's post hoc test ( *P < 0.05 versus ConA treated control).
Figure 2.
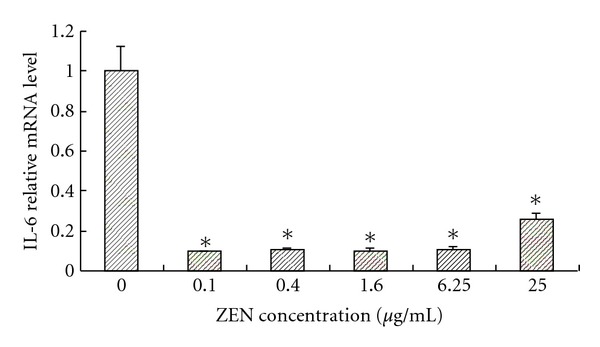
The effect of ZEN on the IL-6 relative mRNA level in ConA-stimulated lymphocytes at toxin concentrations of 0, 0.1, 0.4, 1.6, 6.25, and 25 μg/mL at 48 h. The reporter activity in response to ConA alone is expressed as 100%. Values are the means ± SD from three independent experiments. Statistical analysis was performed using one-way ANOVA followed by Dunnett's post hoc test ( *P < 0.05 versus ConA treated control).
To understand further the specific effects of ZEN on cytokine profiles, a characteristic TH 1 cytokine, IFN-γ, was measured in addition to IL-2 and TH 2, IL-6. A lower dose of ZEN (0.1, 0.4, and 1.6 μg/mL) was selected to eliminate the possibility of cytotoxic effects, which contributes to modulation; however, the two highest doses (6.25 and 25 μg/mL) reached statistical significance compared with the control (P < 0.05). The IFN-γ levels were slightly suppressed by the ZEN treatment (Figure 3).
Figure 3.
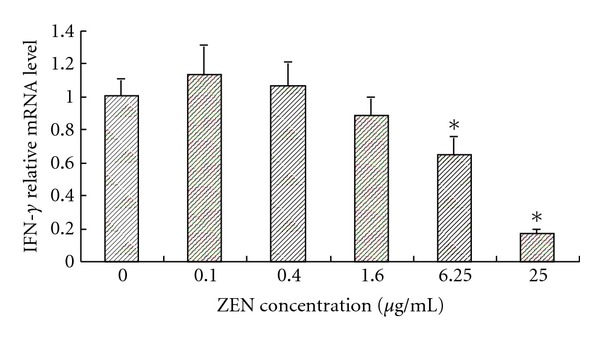
The effect of ZEN on the IFN-γ relative mRNA level in ConA-stimulated lymphocytes at toxin concentrations of 0, 0.1, 0.4, 1.6, 6.25, and 25 μg/mL at 48 h. The reporter activity in response to ConA alone is expressed as 100%. Values are the means ± SD from three independent experiments. Statistical analysis was performed using one-way ANOVA followed by Dunnett's post hoc test ( *P < 0.05 versus ConA treated control).
4. Discussion
There is major concern over the potential for mycotoxins to influence poultry negatively. In the present study, the use of cell cultures offers several advantages over other methods, particularly in terms of the quantification of toxic effects and for defining the immune organ specificity related to a preferential action of a particular cell type. We addressed the issue by evaluating the effects of ZEN on the immune function through in vitro assessment of its activity on the splenic lymphocytes of chickens. The splenic lymphocytes of chickens have been a very useful model for different immunologic studies. ConA is a polyclonal T-cell mitogen, and we determined the effects of ZEN on ConA-stimulated lymphocytes on certain T-helper cell (TH), cytokine profiles (IL-2, IL-6, and IFN-γ) were assessed.
To date, there have been no studies regarding the effects of ZEN on the cytokine profiles of chicken splenic lymphocyte. The addition of ZEN in vitro to ConA-stimulated lymphocytes caused a fivefold increase in the expression of IL-2 cytokines at the mRNA level. As the chemical structure of ZEN enables its binding to the estrogenic receptors, our data are not in line with previous results, which indicate that 17-beta-estradiol reduced IL-2 gene transcription in the Jurkat T-cell line [18]. Considering that T-lymphocyte proliferation is essentially mediated by IL-2, the observed inhibition could be considered a primary mechanism of ZEN-induced immunosuppression also acting in vivo. Accordingly, inhibition of the mRNA expression of IL-2 cytokines by α-ZEN remain unclear assuming an estrogen-like activity [18]. Our results on IL-2 are not broadly in agreement with studies on cytokine protein expression in humans [19]. However, several alterations in immunologic parameters have been associated with ZEN concentrations in humans [20]and mice [21] including in vitro inhibition of mitogen-stimulated lymphocyte proliferation, increase of IL-2 and IL-5, and induction of immunosuppressive effects [22, 23]. Alterations in immunologic parameters such as the inhibition of mitogenstimulated lymphocyte proliferation and the increase in IL-2 production were found at high ZEA concentrations in vitro [22]. In addition, populations that are continuously exposed to ZEN may chronically produce IL-2; therefore, IL-6 is decreased. There are only few reports regarding the effects of ZEN on IL-6. Aside from ZEN, Fusarium can also produce other mycotoxins such as deoxynivalenol (DON), fumonisin B1 (FB1), and T-2 toxin. The effect of these mycotoxins on IL-6 has been studied. A study [24] quantified IL-6 in the spleen, ileum, and mesenteric lymph nodes of 24 pigs which received either control feeds or feeds naturally contaminated with 2.2–2.5 mg/kg of DON feed for 9 weeks using RT-PCR. FB1 inhibited the LPS-induced expression of IL-6 in human dendritic cells (DCs) [25], whereas the modulation of FB1 toxicity in the brain of female BALB/c mice; FB1 augments the LPS-induced IL-6 expression in the brain [26]. The effects of in vivo exposure to T-2 toxin on the alteration of IL-6 in lipopolysaccharide-stimulated peritoneal macrophages [27]; IL-6 mRNA from activated peritoneal macrophages showed no significant differences between the control and the treatment groups. The effects of different Fusarium toxins on IL-6 varied because of differences in toxicities and animal models.
IFN-γ is a master cytokine that affects the functioning of all cells of the immune system and plays a major role in host defense against intracellular infections [28]. This cytokine has also been implicated in autoimmune and inflammatory diseases [29]. Importantly, the IFN-γ levels in ConA-activated splenic lymphocytes that were exposed to zearalenone decreased after 48 h of culture, consistent with previous findings. Studies revealed that zearalenone causes a decrease in IFN-γ in geriatric mice [30], and a slight reduction in both the mitotic index and the cell survival of bovine lymphocytes [31].
To our knowledge, this is the first study that investigates the effects of ZEN on the cellular immune response of chickens. Although the exact mechanism of action of these toxins is still unknown, the results of the current study suggest that ZEN may have divergent effects on chicken lymphocyte cell viability, and production of IL-2, IL-6 and IFN-γ. Further studies are needed to elucidate the specific mechanisms by which ZEN affects immune functions.
Disclosure
All other authors have read the paper and have agreed to submit it in its current form for consideration for publication in The Scientific World Journal.
Acknowledgments
The present work was supported by the National Natural Science Foundation of China Funds (grant no. 31072182) and the Changjing Scholars & Innovative Reasearch Team of Ministry of Education of China Funds (Grant no. IRTO848). The authors gratefully acknowledge the members of the Veterinary Internal Medicine Laboratory in the College of Veterinary Medicine, Sichuan Agricultural University, and in College of Veterinary Medicine, Northeast Agricultural University for their help in feeding the laboratory animals and analyzing the data.
References
- 1.Richard JL. Some major mycotoxins and their mycotoxicoses-An overview. International Journal of Food Microbiology. 2007;119(1-2):3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JW, Klich M. Mycotoxins. Clinical Microbiology Reviews. 2003;16(3):497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald SJ, Anderson S, Brereton P, et al. Determination of zearalenone in barley, maize and wheat flour, polenta, and maize-based baby food by immunoaffinity column cleanup with liquid chromatography: interlaboratory study. Journal of AOAC International. 2005;88(6):1733–1740. [PubMed] [Google Scholar]
- 4.Engelhardt G, Barthel J, Sparrer D. Fusarium mycotoxins and ochratoxin A in cereals and cereal products: results from the Bavarian Health and Food Safety Authority in 2004. Molecular Nutrition and Food Research. 2006;50(4-5):401–405. doi: 10.1002/mnfr.200500191. [DOI] [PubMed] [Google Scholar]
- 5.Tabuc C, Marin D, Guerre P, Sesan T, Bailly JD. Molds and mycotoxin content of cereals in southeastern romania. Journal of Food Protection. 2009;72(3):662–665. doi: 10.4315/0362-028x-72.3.662. [DOI] [PubMed] [Google Scholar]
- 6.Tiemann U, Dänicke S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: a review. Food Additives and Contaminants. 2007;24(3):306–314. doi: 10.1080/02652030601053626. [DOI] [PubMed] [Google Scholar]
- 7.Minervini F, Aquila MED. Zearalenone and reproductive function in farm animals. International Journal of Molecular Sciences. 2008;9(12):2570–2584. doi: 10.3390/ijms9122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzenellenbogen BS, Katzenellenbogen JA, Mordecai D. Zearalenones: characterization of the estrogenic potencies and receptor interactions of a series of fungal β-resorcylic acid lactones. Endocrinology. 1979;105(1):33–40. doi: 10.1210/endo-105-1-33. [DOI] [PubMed] [Google Scholar]
- 9.Mueller SO. Overview of in vitro tools to assess the oestrogenic and antioestrogenic activity of phytooestrogens. Journal of Chromatography B. 2002;777(1-2):155–156. doi: 10.1016/s1570-0232(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 10.Tiemann U, Viergutz T, Jonas L, Schneider F. Influence of the mycotoxins α- and β-zearalenol and deoxynivalenol on the cell cycle of cultured porcine endometrial cells. Reproductive Toxicology. 2003;17(2):209–218. doi: 10.1016/s0890-6238(02)00141-7. [DOI] [PubMed] [Google Scholar]
- 11.Coffey DS. Similarities of prostate and breast cancer: evolution, diet, and estrogens. Urology. 2001;57(4, supplement 1):31–38. [Google Scholar]
- 12.Gajecki M. Zearalenone—undesirable substances in feed. Polish Journal of Veterinary Sciences. 2002;5(2):117–122. [PubMed] [Google Scholar]
- 13.Zwierzchowski W, Przybyłowicz M, Obremski K, et al. Level of zearalenone in blood serum and lesions in ovarian follicles of sexually immature gilts in the course of zearalenone micotoxicosis. Polish Journal of Veterinary Sciences. 2005;8(3):209–218. [PubMed] [Google Scholar]
- 14.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamura M, Lillehoj HS, Raybourne RB, Babu US, Heckert RA. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-γ production. Comparative Immunology, Microbiology and Infectious Diseases. 2004;27(4):255–272. doi: 10.1016/j.cimid.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Michael WP. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(900):2004–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Čikoš S, Bukovská A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Molecular Biology. 2007;8, article 113 doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurray RW, Ndebele K, Hardy KJ, Jenkins JK. 17-β-estradiol suppresses IL-2 and IL-2 receptor. Cytokine. 2001;14(6):324–333. doi: 10.1006/cyto.2001.0900. [DOI] [PubMed] [Google Scholar]
- 19.Luongo D, Severino L, Bergamo P, De Luna R, Lucisano A, Rossi M. Interactive effects of fumonisin B1 and α-zearalenol on proliferation and cytokine expression in Jurkat T cells. Toxicology in Vitro. 2006;20(8):1403–1410. doi: 10.1016/j.tiv.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Berek L, Petri IB, Mesterházy Á, Téren J, Molnár J. Effects of mycotoxins on human immune functions in vitro. Toxicology in Vitro. 2001;15(1):25–30. doi: 10.1016/s0887-2333(00)00055-2. [DOI] [PubMed] [Google Scholar]
- 21.Marin ML, Murtha J, Dong W, Pestka JJ. Effects of mycotoxins on cytokine production and proliferation in EL-4 thymoma cells. Journal of Toxicology and Environmental Health. 1996;48(4):379–396. doi: 10.1080/009841096161267. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen GS, Alexander J, editors. Fusarium Toxins in Cereals—A Risk Assessment. Vol. 502. Copenhagen, Denmark: Nordic Council of Ministers; 1998. [Google Scholar]
- 23.Murata H, Sultana P, Shimada N, Yoshioka M. Structure-activity relationships among zearalenone and its derivatives based on bovine neutrophil chemiluminescence. Veterinary and Human Toxicology. 2003;45(1):18–20. [PubMed] [Google Scholar]
- 24.Pinton P, Accensi F, Beauchamp E, et al. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicology Letters. 2008;177(3):215–222. doi: 10.1016/j.toxlet.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Stockmann-Juvala H, Alenius H, Savolainen K. Effects of fumonisin B1 on the expression of cytokines and chemokines in human dendritic cells. Food and Chemical Toxicology. 2008;46(5):1444–1451. doi: 10.1016/j.fct.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Osuchowski MF, He Q, Sharma RP. Endotoxin exposure alters brain and liver effects of fumonisin B 1 in BALB/c mice: implication of blood brain barrier. Food and Chemical Toxicology. 2005;43(9):1389–1397. doi: 10.1016/j.fct.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Dugyala RR, Sharma RP. Alteration of major cytokines produced by mitogen-activated peritoneal macrophages and splenocytes in T-2 toxin-treated male CD-1 mice. Environmental Toxicology and Pharmacology. 1997;3(1):73–81. doi: 10.1016/s1382-6689(96)00142-1. [DOI] [PubMed] [Google Scholar]
- 28.Durbin JE, Johnson TR, Durbin RK, et al. The role of IFN in respiratory syncytial virus pathogenesis. The Journal of Immunology. 2002;168(6):2944–2951. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton NHR, Banyer JL, Hapel AJ, et al. IFN-γ regulates murine interferon-inducible T cell alpha chemokine (I-TAC) expression in dendritic cell lines and during experimental autoimmune encephalomyelitis (EAE) Scandinavian Journal of Immunology. 2002;55(2):171–177. [PubMed] [Google Scholar]
- 30.Calemine J, Zalenka J, Karpuzoglu-Sahin E, Ward DL, Lengi A, Ahmed SA. The immune system of geriatric mice is modulated by estrogenic endocrine disruptors (diethylstilbestrol, α-zearalanol, and genistein): effects on interferon-γ . Toxicology. 2003;194(1-2):115–128. doi: 10.1016/s0300-483x(03)00286-5. [DOI] [PubMed] [Google Scholar]
- 31.Lioi MB, Santoro A, Barbieri R, Salzano S, Ursini MV. Ochratoxin A and zearalenone: a comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutation Research. 2004;557(1):19–27. doi: 10.1016/j.mrgentox.2003.09.009. [DOI] [PubMed] [Google Scholar]


