Abstract
Background:
Our objective was to test the association between familial risk of type 2 diabetes mellitus (T2DM) and the prevalence of metabolic syndrome (MS) in adult Asian Indians.
Materials and Methods:
A total of 448 adult (>30 years) individuals (257 males and 191 females) participated in the study. Familial risk of T2DM was classified into three groups viz., 1=both parents affected; 2=parent and/or siblings affected and 3=none or no family history for T2DM. Anthropometric measures, blood pressures, fasting blood glucose and metabolic profiles were studied using standard techniques. MS was defined accordingly. The prevalence of MS phenotypes was estimated and compared among the three familial risk strata.
Results:
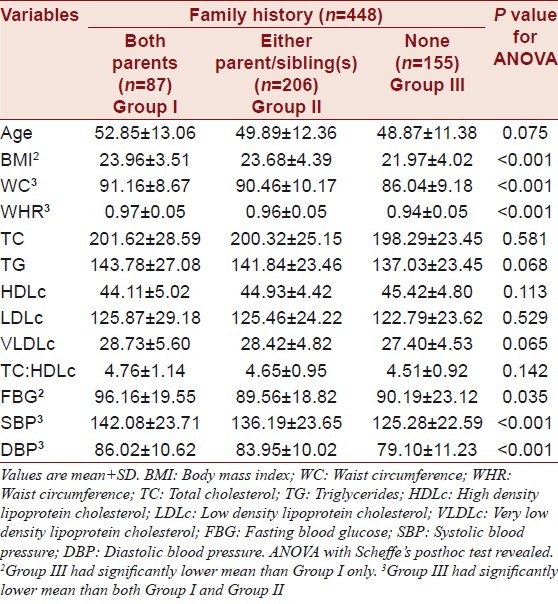
Individuals with a history of both parents affected from diabetes had significantly higher (P<0.001) body mass index (BMI), waist circumference (WC), waist-hip ratio (WHR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and fasting blood glucose (FBG; P=0.035) than individuals having no family history of T2DM. Significant difference was also noticed between individuals with and without MS according to the family history of diabetes (P<0.001). Differences were evident between individuals who fulfilled all the MS criteria (P=0.001) and individuals with only one or two criteria (phenotypes) according to family history of T2DM.
Conclusion:
Family history of T2DM had significant effect on individuals with MS as compared to their counterparts (individuals having no family history of T2DM). It therefore seems reasonable to argue that family history of T2DM could be useful as a predictive tool for early diagnosis and prevention of MS in Asian Indian population.
Keywords: Asian Indians, family history, metabolic syndrome, obesity, type 2 diabetes
INTRODUCTION
Most common chronic diseases are the result of interactions between multiple genetic variants and environmental factors. Despite significant advances in the last decade in the understanding of our genome, there are substantial limitations in epidemiological and analytic approaches to studying the effects of genetic determinants of common chronic diseases, e.g., metabolic syndrome (MS), type 2 diabetes mellitus (T2DM), etc. Family history has been shown to be a risk factor for a majority of chronic diseases of public health significance, including cardiovascular disease (CVD), T2DM, etc. Family history of specific diseases reflects the consequences of genetic susceptibility, shared environment and common behaviors.[1] Family history has been recognized in clinical medicine as an important, yet non-modifiable, disease risk factor that when present might influence the probability of a suspected diagnosis. However, collection and interpretation of family history has rarely been applied in the practice of preventive medicine to assess disease risk and influence early detection and prevention strategies.[2,3] Professional guidelines usually include family history to assess health risk, initiate interventions, and motivate behavioral changes. The advantages of family history over other genomic tools include a lower cost, greater acceptability, and a reflection of shared genetic and environmental factors. However, the utility of family history in public health has been poorly explored.[4]
Family history of diabetes is not only a risk factor for the disease but is also positively associated with risk awareness and risk-reducing behaviors. It may provide a useful screening tool for detection and prevention of diabetes.[5] In a recent study among the adult Chinese, it was found that sufficient physical activity and negative family history of diabetes might jointly reduce the risk of developing hyperglycemia and T2DM.[6] In the US population, family history of diabetes showed significant, independent, and graded association with the prevalence of diabetes. This association not only highlights the importance of shared genes and environment in diabetes but also opens the possibility of formally adding family history to public health strategies aimed at detecting and preventing the disease.[7]
In another study on a nationally representative sample of US adults without diabetes, family history of diabetes showed a significant, independent association with MS and its traits. This association supports the idea that shared genes and environment contribute to the expression of complex traits such as diabetes and MS.[8] MS is defined as a constellation of several CVD risk factors including genetic and environmental factors that is responsible for ultimate predisposition of the disease.[9] However, studies related to the effect of positive family history of diabetes on MS are sadly lacking in India. In one of the two recent studies from India, it was found that apparently healthy individuals with family history of T2DM had higher anthropometric values (body mass index [BMI], waist-hip ratio [WHR]) and lower physical fitness than their counterparts.[10] In the other study, multiple logistic regression analysis showed significant association of diabetes with family history of diabetes along with other risk factors.[11] But association between positive family history of diabetes and MS is not known to best of our knowledge from this part of the world.
The present study was therefore undertaken to find out whether there exists any significant association between family history of T2DM and prevalence of MS among the adult Asian Indians.
MATERIALS AND METHODS
Study population
The present community based cross-sectional study comprised adults (≥30 years) living in and around Kolkata (erstwhile Calcutta), India. A total of 448 individuals (257 males and 191 females) participated in the study. Demographic profiles including name, date of birth, occupation, etc., were obtained from participants using a schedule. Subjects’ age was ascertained subsequently from date of birth to the nearest month. Family history of T2DM was obtained from each subject and classified into three groups, viz. 1=both parents affected, 2=parent and/or siblings affected and 3=none or no family history for T2DM. The institutional ethics committee (IEC) of the “Human Genetic Engineering Research Center” (HGERC), Kolkata, India, has approved the study. Written consent from participants was also obtained prior to actual commencement of the study.
Anthropometric measures
Anthropometric measures namely height, weight, circumferences of waist (WC) and hip were obtained using standard techniques.[12] Height and weight (in light clothing) was measured to the nearest 0.1 cm and 0.5 kg, respectively. Waist and hip circumferences were measured to the nearest 0.1 cm using an inelastic tape. The minimum WC was measured at the level of natural waist, which was the narrowest part of the torso. The body mass index (BMI in kg/m2); waist-hip ratio (WHR) and TC:HDLc ratio was computed subsequently.
Blood pressures
Left arm systolic (SBP) and diastolic (DBP) blood pressure measurements were twice taken using sphygmomanometer and stethoscope and were averaged for analyses. A third measurement was taken only when the differences between the two measurements were >5 mmHg. Prior medical records for blood pressure were also taken into consideration. To obtain blood pressure, each participant was requested to seat at least 5 minutes in complete relaxed mood on a chair and was also requested not to change his posture during that relaxation period.
Metabolic profiles
A fasting blood sample (~7 mL) was collected from each subject for the determination of metabolic profiles. All subjects maintained an overnight fast of ~12 h prior to blood collection. Estimation of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDLc) and fasting blood glucose (FBG) was carried out on separated plasma using a semi auto-analyzer (ErbaChem-5V2, Transasia Biomedical Ltd., Mumbai, India). Very low-density lipoprotein cholesterol (VLDLc) was calculated as TG/5. However, in case of TG > 400 mg/dL VLDLc was estimated separately using the semi auto-analyzer. Low-density lipoprotein cholesterol (LDLc) was estimated using standard formula LDLc=TC – (HDLc+VLDLc). TC:HDL ratio was subsequently calculated. All biochemical analyses were measured in mg/dL (mg %) unit.
Metabolic syndrome
Individuals with three or more of the following conditions were considered as MS.[13]
WC (cm): Male>90, female>80;
TG (mg/dL): >200;
HDLc (mg/dL): Male<40, female <50;
Blood pressures (mmHg): SBP>130 and/or DBP>85;
Fasting blood glucose (mg/dL): >100.
Statistical analysis
Differences in means of the studied variables between the three groups were undertaken using analysis of variance (ANOVA) with Scheffe's posthoc test to determine which two family history groups were different from each other. Contingency chi-square test was performed to find out the differences in accordance with family history on prevalence of MS as well as its confounding factors and illustrated by means of bar diagram. All statistical analyses were performed using SPSS (PC + version 10.0). A statistical significance (two tailed) was set at P<0.05.
RESULTS
The descriptive statistics of the study population is presented in Table 1. In the present study, it was found that the prevalence of MS was 29.2%. ANOVA with Scheffe's posthoc test revealed that individuals with either or both of their parents affected with T2DM had significantly (P<0.001) higher BMI, WC, WHR, SBP, DBP and FBG (P=0.035) than individuals having no family history of T2DM.
Table 1.
Descriptive statistics of the study population
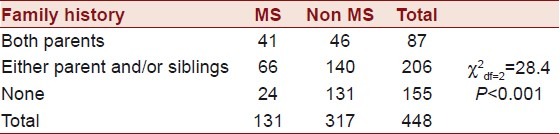
The prevalence of MS by family history of diabetes is presented in Table 2. It was observed that there was a highly significant difference (P<0.0001) between individuals having MS with and without family history.
Table 2.
Prevalence of metabolic syndrome by family history of type 2 diabetes mellitus
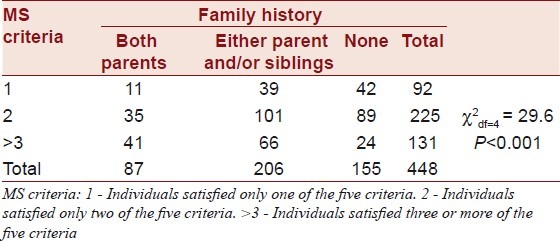
The difference in family history of diabetes by confounding factors satisfying the criteria for MS is presented in Table 3. It was found that individuals with positive family history had significantly higher (P<0.001) prevalence of satisfying more MS criteria than their counterparts.
Table 3.
Family history of T2DM and metabolic syndrome phenotypes
It was also found that females with positive family history had relatively higher prevalence of MS than males, whereas the prevalence was similar among males and females with no family history of T2DM as illustrated in Figure 1.
Figure 1.
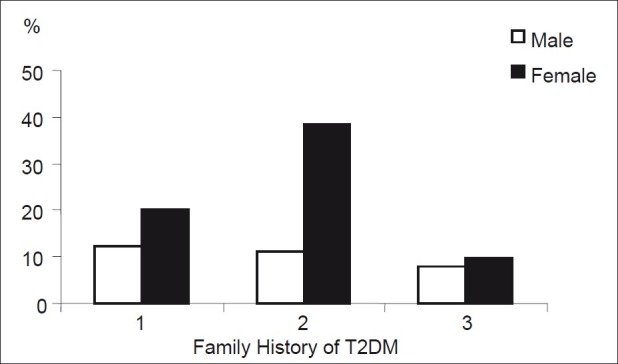
Gender differences in prevalence of metabolic syndrome
DISCUSSION
Evidence suggests that family history by itself is most useful for predicting disease when there are multiple family members affected, the relationship among relatives is close, and disease is premature, that is, it occurs at younger ages than would be expected. It has been mentioned that family history information in combination with other known risk factors could be used to provide more personalized information about our risk for common diseases.[1] Yang et al.,[14] further suggested that adding family history of diabetes could provide significant improvements in detecting undiagnosed diabetes; however, it needs further validation. In an another study,[15] it was found that not only the adults, even the youths with a positive family history showed signs of increased risk for these conditions which indicates the importance of family history approach to screening for children at risk of diabetes and cardiovascular disease.
In addition to risk assessment, family history information can be used to personalize health messages, which are potentially more effective in promoting healthy lifestyles than standardized health messages. More research is needed on the evidence for the effectiveness of such a tool.[16] The accuracy of self-reported family history of diabetes and hypertension is strongly influenced by the accuracy of self-reported personal health status of relatives. Raising awareness of personal health status is crucial to ensure the utility of family history for the assessment of risk and disease prevention.[17] Having a family history of a disease increases its salience and does not change one's perceived ability to prevent the disease.[18] To establish family history as a public health tool, it needs to be evaluated within the ACCE (analytical validity; clinical validity; clinical utility; and ethical, legal, and social issues) framework. These advances will help realize the potential of family history as a public health tool.[4]
In the present study, it was found that individuals with positive family history of diabetes had significantly higher prevalence of MS and its confounding factors as compared to their counterparts. It suggests that family history could be used as a tool for genomic studies among the Asian Indians. It is, however, important to mention that in developing countries including India, a large section of the community remains undiagnosed and therefore accuracy of self-reported family history could be challenging.
The major limitation of the study was the cross-sectional nature, and moreover, it was performed on a relatively small sample size and, therefore, is not representative of the Asian Indian population. Large-scale, nationally representative data, which is actually lacking in India, would have provided a better insight into the role of family history for the early diagnosis of chronic diseases, e.g., MS. It may also be argued that population screening would be of substantial importance for further research as family history may play a vital role in better understanding the etiological factors related to such complex traits.
CONCLUSION
The present study indicates the importance of positive family history associated with adverse CVD risk factors like MS among the adult Asian Indians. Family history, thus, could be used as a tool for genomic studies in order to understand the underlying shared gene-environment interrelation associated with complex traits. This in turn would help to develop comprehensive risk stratification protocol for better assessment and successful management of CVD risk factors in this part of the world.
ACKNOWLEDGMENTS
Arnab Ghosh received financial support (Ref. No. 5/9/48/2006-RHN vide RFC No. RHN/Adhoc/1/2009-10) from the Indian Council of Medical Research (ICMR), Government of India, New Delhi. Mithun Das received partial funding [Ref. No. F. PSW-176/09-10(ERO)] from the University Grants Commission (UGC), Government of India, New Delhi. The authors are grateful to the staff and technicians of the HGERC, Calcutta, India, for their sincere help in analyzing the metabolic profiles. The authors are also indebted to all the subjects who participated in the study.
Footnotes
Source of Support: Ref. No. 5/9/48/2006-RHN vide RFC No. RHN/Adhoc/1/2009-10) from the Indian Council of Medical Research (ICMR), Government of India, New Delhi.
Conflict of Interest: None declared.
REFERENCES
- 1.Yoon PW, Scheuner MT, Peterson-Oehlke KL, Gwinn M, Faucett A, Khoury MJ. Can family history be used as a tool for public health and preventive medicine. Genet Med. 2002;4:304–10. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Scheuner MT, Wang SJ, Raffel LJ, Larabell SK, Rotter JI. Family history: A comprehensive genetic risk assessment method for the chronic conditions of adulthood. Am J Med Genet. 1997;71:315–24. doi: 10.1002/(sici)1096-8628(19970822)71:3<315::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Williams RR, Hunt SX, Heiss G, Province MA, Bensen JT, Higgins M, et al. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Health Study) Am J Cardiol. 2001;87:129–35. doi: 10.1016/s0002-9149(00)01303-5. [DOI] [PubMed] [Google Scholar]
- 4.Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: A genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 5.Hariri S, Yoon PW, Qureshi N, Valdez R, Scheuner MT, Khoury MJ. Family history of type 2 diabetes: A population-based screening tool for prevention? Genet Med. 2006;8:102–8. doi: 10.1097/01.gim.0000200949.52795.df. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Wang Y, Ware RS, Tse LA, Dunstan DW, Liang Y, et al. Physical activity, family history of diabetes and risk of developing hyperglycaemia and diabetes among adults in mainland China. Diabet Med. 2011 doi: 10.1111/j.1464-5491.2011.03495.x. [In press] [DOI] [PubMed] [Google Scholar]
- 7.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U. S. population: The 6-year results from the National Health and Nutrition Examination Survey (1999-2004) Diabetes Care. 2007;30:2517–22. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh A, Liu T, Khoury MJ, Valdez R. Family history of diabetes and prevalence of the metabolic syndrome in U. S. adults without diabetes: 6-year results from the National Health and Nutrition Examination Survey (1999-2004) Public Health Genomics. 2010;13:353–9. doi: 10.1159/000262330. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM. Metabolic syndrome: A multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 10.Padaki S, Vijayakrishna K, Dambal A, Ankad R, Manjula R, Surekharani C, et al. Anthropometry and physical fitness in individuals with family history of type-2 diabetes mellitus: A comparative study. Indian J Endocrinol Metab. 2011;15:327–30. doi: 10.4103/2230-8210.85595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 12.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL. Human Kinetics. 1988 [Google Scholar]
- 13.Das M, Pal S, Ghosh A. Synergistic effects of ACE (I/D) and ApoE (HhaI) gene polymorphisms among the adult Asian Indians with and without metabolic syndrome. Diabetes Res Clin Pract. 2009;86:e58–61. doi: 10.1016/j.diabres.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Liu T, Valdez R, Moonesinghe R, Khoury MJ. Improvements in ability to detect undiagnosed diabetes by using information on family history among adults in the United States. Am J Epidemiol. 2010;171:1079–89. doi: 10.1093/aje/kwq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez R, Greenlund KJ, Khoury MJ, Yoon PW. Is family history a useful tool for detecting children at risk for diabetes and cardiovascular diseases.A public health perspective? Pediatrics. 2007;120(Suppl 2):S78–86. doi: 10.1542/peds.2007-1010G. [DOI] [PubMed] [Google Scholar]
- 16.Claassen L, Henneman L, Janssens AC, Wijdenes-Pijl M, Qureshi N, Walter FM, et al. Using family history information to promote healthy lifestyles and prevent diseases; a discussion of the evidence. BMC Public Health. 2010;10:248. doi: 10.1186/1471-2458-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssens AC, Henneman L, Detmar SB, Khoury MJ, Steyerberg EW, Eijkemans MJ, et al. Accuracy of self-reported family history is strongly influenced by the accuracy of self-reported personal health status of relatives. J Clin Epidemiol. 2012;65:82–9. doi: 10.1016/j.jclinepi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Acheson LS, Wang C, Zyzanski SJ, Lynn A, Ruffin MT, 4th, Gramling R, et al. Family history and perceptions about risk and prevention for chronic diseases in primary care: A report from the family healthcare impact trial. Genet Med. 2010;12:212–8. doi: 10.1097/GIM.0b013e3181d56ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]


