Abstract
Generalized arterial calcification of infancy (GACI) is a life-threatening disorder in young infants. Cardiovascular symptoms are usually apparent within the first month of life. The symptoms are caused by calcification of large and medium-sized arteries, including the aorta, coronary arteries, and renal arteries. Most of the patients die by 6 months of age because of heart failure. Recently, homozygous or compound heterozygous mutations for the ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) gene were reported as causative for the disorder. ENPP1 regulates extracellular inorganic pyrophosphate (PPi), a major inhibitor of extracellular matrix calcification. A newborn was diagnosed with GACI. The infant died at the age of 7 weeks of cardiac failure and the parents were referred to Molecular Biology and Cytogenetic lab for further workup. Cytogenetics analysis was performed on the parents, which showed normal karyotypes and mutational analysis for the ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) gene was also performed. The mutational analysis showed that both father and mother of the deceased infant were heterozygous carriers of the mutation c.749C>T (p.P250L) in exon 7 of ENPP1 and it was likely, that the deceased child carried the same mutation homozygous on both alleles and died of GACI resulting from this ENPP1 mutation. The couple was counseled and monitored for the second pregnancy. Amniocentesis was performed at 15 weeks of gestation for mutational analysis of the same gene in the second pregnancy. The analysis was negative for the parental mutations. One month after the birth of a healthy infant, peripheral blood was collected from the baby and sent for reconfirmation. The results again were negative for the mutation and the baby was on 6 months follow up and no major symptoms were seen. The parents of the child benefited enormously by learning about the disease much in advance and also its risk of recurrence. The main aim of this study is to emphasize on two aspects: (i) the importance of modern molecular techniques in diagnosis such a syndrome and (2) the difficulties faced by the physician to provide appropriate diagnosis and the adequate genetic counseling to the family without molecular facilities.
Keywords: Cytogenetics, ecto-nucleotide pyrophosphatase/phosphodiesterase 1 encoding gene, prenatal diagnostic testing, pyrophosphate
INTRODUCTION
Generalized arterial calcification of infancy (GACI) is a rare autosomal recessive disorder, reported till date in about 180 individuals.[1,2] Calcification of large and medium-sized arteries and marked myointimal proliferation leading to arterial stenoses, are characteristic vascular features of the GACI phenotype. An extravascular feature of foci of periarticular calcification, occurs in many of the affected subjects.[3] Initial signs of the disease may occur prenatally and most affected children die in early infancy from sequelae of vascular occlusion, typically myocardial infarction or congestive heart failure due to hypertension.[3,4] The diagnosis of GACI is often not considered until arterial calcification is incidentally detected in the affected patients. Also, arterial calcification might be too subtle to be detected by conventional radiography. In such cases, ultrasonography or computed tomography scan might demonstrate evidence of arterial calcification and establish the diagnosis.[5,6] Other potential causes of arterial calcium deposition, such as, hypervitaminosis D, advanced renal disease and hyperparathyroidism, can be excluded after basic laboratory investigations.[6] GACI is most frequently caused by mutations in ENPP1, a gene encoding ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1).[7] Prenatal diagnostic testing may not yield accurate results as the manifestations are usually seen only in the last trimester of pregnancy. However, early diagnosis by mutation analysis from amniocentesis is possible, if disease causing mutations in ENPP1 have been identified previously in an index case in the same family.
CASE REPORT
The parents of the index case were non-consanguineous. The antenatal ultra sonography during the pregnancy at 13 and 30 weeks of gestation did not reveal any abnormalities. The 36 weeks ultrasonography revealed polyhydramnios and accumulation of mild pericardial effusion in the fetus [Figure 1a and 1b]. Amniotic fluid was drawn for chromosomal analysis during the 15 weeks of gestation which was normal, and unremarkable for numerical and structural anomalies. The female infant was born at term via lower-segment cesarean section. She developed respiratory distress immediately after birth and required supplemental oxygen. The baby was pale looking, hyperglycemic and euthermic. Vesicular breath sounds were audible. Abdomen was soft. A pansystolic murmur was heard at the 3rd and 4th left intercostal space. Central nervous system examination was unremarkable, and there were no external congenital anomalies. Her femoral pulses were present; however, brachial, radial, posterior tibial and dorsalis pedalis pulses were absent. Due to severe respiratory distress, she was initially started on continuous positive airway pressure (CPAP) ventilation; however, was later shifted to mechanical ventilation in view of increasing dyspnea and severe metabolic acidosis. In view of severe anemia [Table 1], she was given packed red blood cell transfusion.
Figure 1a.

Single live intrauterine fetus at 36 weeks of gestation without polyhydramnios and pericardial effusion
Figure 1b.

Single live intrauterine fetus at 36 weeks of gestation showing polyhydramnios (↔) and pericardial effusion (→)
Table 1.
Clinical manifestations of the first child in the case report
She developed extreme tachycardia. Electrocardiography (ECG) on monitor was suggestive of ventricular fibrillation. Due to severe hypertensionand oxygen saturation of 95% in room air, she was started on intravenous (IV) Furosemide. Chest radiograph revealed a massive cardiomegaly with a calcified aorta.A 2D Echo revealed severe left ventricular dysfunction [Figure 2]. She was started on Milrinone infusion and the dose was gradually increased to 0.9 micrograms per/kg per minute with minimal improvement.A repeat echocardiogram on the 49th day of life revealed severe biventricular dysfunction. The ascending aortic arch and the coronary arteries were calcified [Figure 2]. She was started on inotropic support (dobutamine and milrinone) and diuretic therapy. An ultrasound scan of the abdomen revealed a diffuse calcification of abdominal wall with right artery stenosis. A CT scan of abdomen revealed a diffuse rim calcification of the entire aorta and its branches in the chest and abdomen, and this pointed towards a rare genetic form of diffuse arterial calcification. The clinical diagnosis of generalized arterial calcification of infancy (GACI) was proposed, and therapy with IV Pamidronate (three doses given at 0.9 micrograms per/kg per minute) was started at 18 days of life intravenously. Despite therapy, a rapid evolution to multiorgan failure and death occurred on day 51 of life. Autopsy was not performed as the parents did not give consent, and also mutational analysis of ENPP1 was not performed as no tissue was available.
Figure 2.
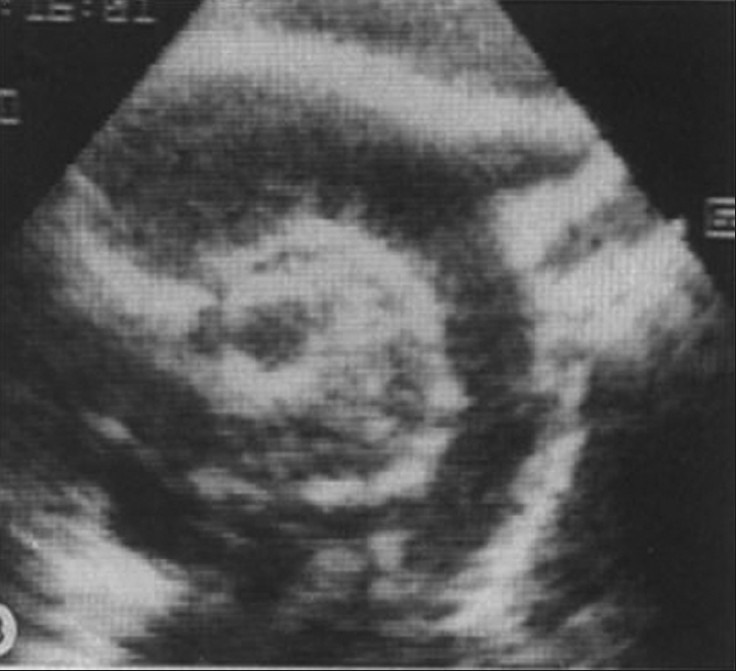
Echocardiogram at 49 day of the baby's age showing that there was a prominent calcification of the aortic arch, right pulmonary artery (RPA), innominate artery (IA), left carotid artery (LCA), and left subclavian artery (LSA)
The family was planning for the second conception; however, wanted to have an objective and measurable understanding about the risk of recurrence in a subsequent pregnancy. For this purpose the family was referred for a genetic counseling to the Molecular Biology and Cytogenetics lab, Apollo Hospitals, Hyderabad, India. After one year of the first baby's demise, the mother conceived for the second time, and the pregnancy was closely monitored.A detailed genetic analysis was carried after taking the informed consent from the couple. About 3 ml of peripheral blood sample was collected from the couple in a heparinized vacutainers and processed for cytogenetic analysis. Lymphocyte stimulated cultures were set up as described by Moorehead et al.[8] Giemsa banding (GTG banding) was performed according to Seabright et al.[9] Fifty metaphases were scored under light transmission microscope and karyotyping software (Leica CW 4000 Total solutions for cytogenetics imaging V1.3) was used for cytogenetic analysis. Metaphases were karyotyped according to International System for Human Cytogenetic Nomenclature (ISCN) criteria.[10] The cytogenetic analysis showed normal karyotype for both husband and wife without any numerical and structural anomalies. Peripheral blood sampling was repeated in the Ethylenediaminetetraacetic acid (EDTA) vacutainers and Deoxyribonucleic acid (DNA) was extracted using DNA extraction kit (Qiagen) and sent in the elution buffer for molecular analysis of ENPP1 in generalized arterial calcification. Mutation analysis of ENPP1 was performed with a set of 25 primer pairs, and all 25 exons and their flanking splice sites of the ENPP1gene were amplified from genomic DNA by polymerase chain reaction (PCR). The PCR products were directly sequenced bidirectionally using a DNA analyzer (ABI 3730) and a sequencing Kit (BigDye Terminator v1.1 Cycle) according to the manufacturer's protocol (Applied Biosystems). All primer sequences are available on request. Mutations were compared with the ENSEMBL polymorphism database.
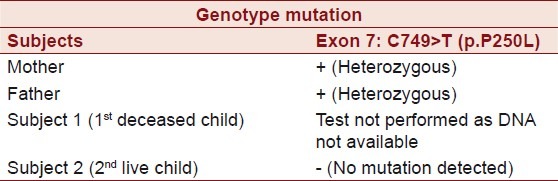
Molecular analysis of the ENPP1gene in the DNA sample of both husband and wife showed that they were both heterozygous carriers for the mutation c.749C > T (p.P250L) in exon 7 of ENPP1. The mutation led to an amino acid change of proline to leucine at position 250 of the protein [Figure 3].
Figure 3.
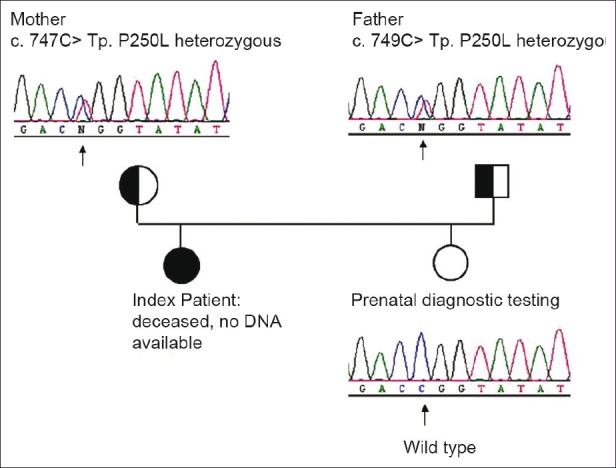
Chromatograms of the DNA sequence of exon 7 of the ENPP1 gene in the affected family. Note heterozygosity for the c.749C>T mutation in ENPP1 in both of the parents
Wife: Exon 4: C517A>Cp.K173Q
Exon 7: C749>T (p.P250L)
Exon 13: C.1273-17delT
Husband: Exon 7: C.749>T (p.P250L)
Exon 13: C.1273-17delT
All mutations were detected on one allele. The mutation c.517A> p.K173Q in exon 4 is known to be a non pathogenic polymorphism. The mutation c.1273-17delT is an intronic deletion in intron 12 and 17 base upstream of exon 13 which probably will not cause any transcriptional error (not been described previously) and is most likely not disease causing [Table 2 and Figure 3].
Table 2.
Genotype mutation showing proband and the parents in the case report
During the second pregnancy at 15 gestational weeks, the amniocentesis was performed. The amniotic fluid was collected in two vials, and the first vial was cultured for cytogenetic analysis and the results showed a normal karyotype. From the second vial, the DNA was extracted and mutational analysis of exons 4 and 7 of ENPP1 was performed from the fetal DNA, after exclusion of maternal contamination of the sample. Mutational analysis in the amniotic sample was negative for parental mutation (paternal mutation). After the baby was born, mutational analysis was repeated from a blood sample and again was confirmed to be negative for the parental mutation (paternal mutation) [Figure 3].
DISCUSSION
GACI is a rare, fatal, autosomal-recessive disorder that results in arterial stenosis and decreased elasticity of the blood-vessel walls, secondary to unregulated hydroxyapatite deposition. GACI is associated with inactivating mutations in the ENPP1 gene, which lead to decreased levels of inorganic pyrophosphate, a potent physiological inhibitor of hydroxyapatite-crystal deposition in the blood-vessel walls.[7] Till date, only 180 cases have been published on this disease, and, many cases go undiagnosed or unreported. GACI is associated with a high mortality rate owing to the development of severe hypertension and cardiovascular complications in early infancy. Several case studies have described patients who survived into adulthood with persistent hypertension and cardiovascular squealae; however, approximately 85% of affected infants do not survive beyond 6 months of age.[6] Infants often present with nonspecific signs, including poor feeding, cyanosis and respiratory distress. The diagnosis of GACI is often not considered until arterial calcification is incidentally detected as in the index case presented here. Arterial calcification might be too subtle to be detected by conventional radiography. In such cases, ultrasonography or CT might demonstrate evidence of arterial calcification and establish the diagnosis.[5,6,11]
GACI should also be considered when hydrops fetalis is detected antenatally by ultrasonography as calcification is very rarely detected in the early stages of pregnancy. During pregnancy there might be polyhydramnios and mild pericardial effusion seen in the fetus is compared with normal, which was also present in our index case [Figure 1a and 1b]. The clinical presentation is variable, and most cases are diagnosed postmortem. Affected patients usually present with respiratory distress, feeding difficulties, hypertension and progressive heart failure. Electrocardiography can reveal evidence of myocardial ischemia, which suggests coronary-artery involvement, and might also show ventricular hypertrophy or impaired ventricular function consistent with infarction.[11] Coronary artery involvement can be lethal within the first 6 months.[12] The syndrome is histologically characterized by intima proliferation and diffuse deposition of hydroxyapatite at the internal elastic lamina of medium-sized arteries and correlates to the loss of function to mutations in the ENPP1 gene (chromosome 6q22–q23, OMIM 173335), encoding for ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP1). This cell surface enzyme is a type II membrane glycoprotein generating inorganic pyrophosphate PPi in vascular smooth muscle cells, chondrocytes and osteoblasts. PPi is a strong inhibitor of hydroxyapatite deposition. The pathogenesis of GACI is related to reduced NPP1 enzymatic activity leading to low extracellular PPi levels.[13–15]
Rutsch and colleagues demonstrated that homozygous or compound heterozygous loss-of-function mutations in ENPP1 result in GACI in about 80% of the cases.[16] Accordingly, in the family presented here, ENPP1 mutational analysis was performed in the parents of an index case with clinically proven GACI. The parents were found to be heterozygous carriers of two different ENPP1 mutations. This makes is very likely that the deceased child must have been compound heterozygous for the same mutations. However, we were not able to prove this hypothesis, because there was no DNA available from the deceased child. The couple was counseled accordingly since they wanted another child.
It was reported earlier in 2009 that GACI is a rare autosomal recessive disorder and is characterized by vascular calcification and myointimal proliferation in infancy and thus results in stenosis and reduced vascular elasticity. There is an urgent need to use molecular tests to confirm the diagnosis and understand the risk-benefit ratio and also the potential risks involved. Genetic counseling for GACI is very important as the disease is transmitted in an autosomal recessive manner. Considering the objective evidence of risk of recurrence of ENPP1 mutations in a subsequent conceptus, the parents made a conscious decision to try to have another child. Although the clinical diagnosis of GACI is based on clinical features and typical radiographic signs, molecular analysis of ENPP1 is mandatory for genetic counseling in the affected family. Hence, it is recommended that along with investigations like CT, more advanced investigations such as fetal echo scan and molecular analysis of ENPP1 should be included as this would help the clinicians and the prospective anxious parents to plan their families in a better way. In cases such as the present one, where a family has significant emotional and social issues to grapple with, the conflict as to whether to go for another pregnancy or not, can be answered with molecular diagnosis methods and the anxiety of the parents can be reduced. Given these concerns, a thorough explanation of the risk-benefit ratio should be considered whenever diagnostic tests are considered in patients with GACI, along with a detailed discussion with the parents about the potential risks involved.
ACKNOWLEDGEMENTS
The authors acknowledge the family presented here for accepting to share the information for publication, Y.N. and F.R. were supported by the Interdisciplinary Center for Clinical Research, Muenster, Germany.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Rutsch F, Böyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, et al. Hypophosphatemia, Hyperphosphaturia, and Bisphosphonate Treatment Are Associated With Survival Beyond Infancy in Generalized Arterial Calcification of Infancy. Circ Cardiovasc Genet. 2008;1:133–40. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dlamini N, Splitt M, Durkan A, Siddiqui A, Padayachee S, Hobbins S, et al. Generalized arterial calcification of infancy: Phenotypic spectrum among three siblings including one case without obvious arterial calcifications. Am J Med Genet Part A. 2009;149:456–60. doi: 10.1002/ajmg.a.32646. [DOI] [PubMed] [Google Scholar]
- 3.Moran JJ. Idiopathic arterial calcification of infancy: A clinicopathologic study. Pathol Annu. 1975;10:393–417. [PubMed] [Google Scholar]
- 4.Rutsch F, Schauerte P, Kalhoff H, Petrarulo M, August C, Diekmann L. Low levels of urinary inorganic pyrophosphate indicating systemic pyrophosphate deficiency in a boy with idiopathic infantile arterial calcification. Acta Paediatr. 2000;89:1265–9. doi: 10.1080/080352500750027682. [DOI] [PubMed] [Google Scholar]
- 5.Spear R, Mack LA, Benedetti TJ, Cole RE. Idiopathic infantile arterial calcification, in utero diagnosis. J Ultrasound Med. 1990;9:473–6. doi: 10.7863/jum.1990.9.8.473. [DOI] [PubMed] [Google Scholar]
- 6.Glatz AC, Pawel BR, Hsu DT, Weinberg P, Chrisant MR. Idiopathic infantile arterial calcification: Two case reports, a review of the literature and a role for cardiac transplantation. Pediatr Transplant. 2006;10:225–33. doi: 10.1111/j.1399-3046.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 7.Rutsch F, Ruf N, Vaingankar S, Toliat M, Suk A, Höhne W, et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–81. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 8.Moorehead PS, Nowell PC, Mellman WJ, Battips DM, Hungerford DA. Chromosome preparation of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960;20:613–6. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 9.Seabright MA. A rapid banding technique for human chromosomes. Lancet. 1971;2:971–97. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 10.Mitelman F. ISCN. An International System for Human Cytogenetic Nomenclature. Basel: Karger; 2005. pp. 1–115. [Google Scholar]
- 11.Ciana G, Trappan A, Bembi B, Benettoni A, Maso G, Zennaro F, et al. Generalized arterial calcification of infancy: Two siblings with prolonged survival. Eur J Pediatr. 2006;165:258–63. doi: 10.1007/s00431-005-0035-6. [DOI] [PubMed] [Google Scholar]
- 12.Champ C, Byard RW. Pulmonary thromboembolism and unexpected death in infancy. J Pediatr Child Health. 1994;30:550–1. doi: 10.1111/j.1440-1754.1994.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 13.Morton R. Idiopathic arterial calcification in infancy. Histopathology. 1978;2:423–32. doi: 10.1111/j.1365-2559.1978.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 14.Van der Sluis IM, Boot AM, Vernooij M, Meradji M, Kroon AA. Idiopathic infantile arterial calcification: Clinical presentation, therapy and longterm follow-up. Eur J Pediatr. 2006;165:590–3. doi: 10.1007/s00431-006-0146-8. [DOI] [PubMed] [Google Scholar]
- 15.Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P, Rutsch F. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI) Hum Mutat. 2005;25:98. doi: 10.1002/humu.9297. [DOI] [PubMed] [Google Scholar]
- 16.Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, et al. PC1-nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic arterialcalcification. Am J Pathol. 2001;158:543–54. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


