Abstract
Pheochromocytomas have been described in association with vascular abnormalities like renal artery stenosis. A 48-year-old man was admitted to our hospital with the complaints of headache, sweating, anxiety, dizziness, nausea, vomiting and hypertension. For last several days, he was having a dull aching abdominal pain. Abdominal computed tomography (CT) revealed the presence of a left adrenal pheochromocytoma. An inferior vena cava (IVC) venogram via the right jugular vein demonstrated occlusion of the IVC inferior to the right atrium. Surgical removal of pheochromocytoma was done, followed by anticoagulant treatment for IVC thrombosis, initially with subcutaneous low molecular weight heparin, and then with oral warfarin, resulting in restoration of patency. To the best of our knowledge, the occurrence of pheochromocytoma in IVC thrombosis has not been reported so far from India. Possible mechanisms of such an involvement are discussed.
Keywords: Anticoagulant, hypertension, inferior vena cava thrombosis, pheochromocytoma
INTRODUCTION
Pheochromocytomas are rare catecholamine producing tumors arising from chromaffine cells in the sympatho adrenal system. Its prevalence is estimated at 0.1% to 0.6%.[1] They secrete various catecholamines, predominantly norepinephrine, and epinephrine to small extent.[2] These catecholamines are responsible for the manifestations with sustained or paroxysmal symptoms.[2] Diagnosis is established by measuring metanephrines in the urine or blood.[3] Localization of the tumor is done using computed tomography (CT) or magnetic resonance imaging (MRI) scans.[4] Surgical resection of the tumor mass is the definitive treatment.[5]
Thrombosis of the inferior vena cava (IVC) has comparable etiological factors to lower limb deep venous thrombosis.[6] Hypercoagulability related to hematological or neoplastic abnormalities, venous stasis secondary to extraluminal pressure from tumors or inflammatory processes, and vessel injury due to trauma have all been implicated as primary mechanism in the pathophysiology of IVC thrombosis.[6] However, its association with pheochromocytoma in Indian subjects has not been reported till date. We report the case of a patient who presented with pheochromocytoma and IVC thrombosis.
CASE REPORT
A 48-year-old man was admitted to our hospital with complaints of headache, sweating, anxiety, dizziness, nausea and vomiting. He also had dull aching abdominal pain. He was a nonsmoker with no significant family history. There was no history of risk factors for hypercoagulable state. The patient was 164 cm tall and weighed 57 kg. On physical examination, there were no café au lait spots or neurofibromas. His resting pulse rate was 100 beats/min. The patient's blood pressure was 240/150 mmHg. Bilateral lower limb arterial and venous examination was normal. There was no redness over feet, pedal edema or collateral formation over abdomen. Abdominal examination revealed mild lower abdominal tenderness without any hepatomegaly and ascitis. Rest of the systemic examination was unremarkable.
Hematological analysis confirmed normocytic anemia with hemoglobin 11.3 gm/dl, a raised erythrocyte sedimentation rate (ESR) (130 mm fall in the first hour), while the total and differential leukocyte counts were normal. C-reactive protein was elevated at 130 mg/L. Urine examination was normal. Biochemical parameters such as liver and kidney functions, and serum electrolytes, calcium, phosphorous, alkaline phosphatase and D-dimer were within normal limits. Electrocardiogram revealed left ventricular hypertrophy. The chest radiograph and echocardiogram were normal. The Endocrinological evaluation revealed increased urine catecholamines and urinary vanillyl mandelic acid (VMA) [Table 1]. Plasma cortisol and Adrenocorticotropic hormone (ACTH) were within normal limits.
Table 1.
Baseline biochemical parameters of the patient
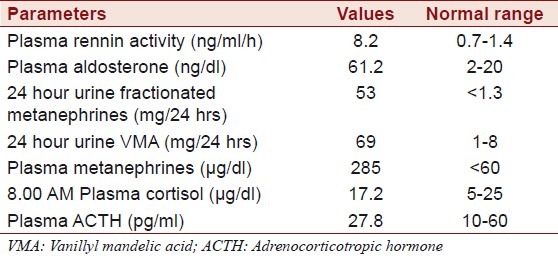
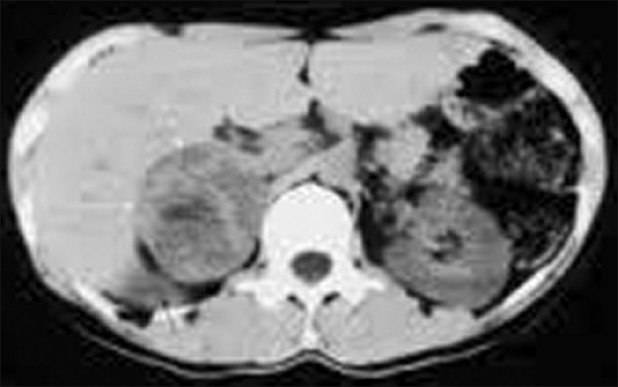
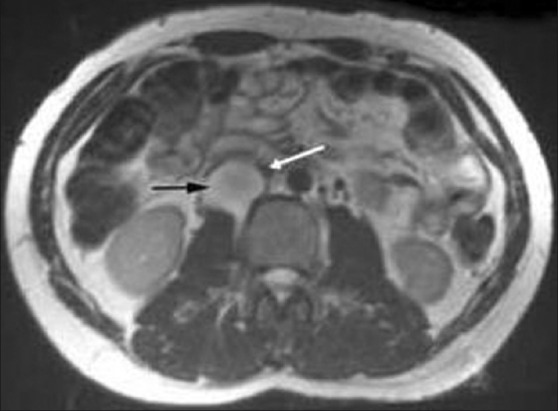
Abdominal CT revealed a well defined, heterogenous mass lesion of size 7.6 × 5.3 × 4.8 cms with attenuation score of 35 HU at the upper pole of right kidney without any calcifications [Figure 1]. Ultrasound (USG) abdomen revealed normal renal and hepatic parenchyma. There was no involvement of renal vein, hepatic veins and veins of lower limbs demonstrated by doppler ultrasound. Magnetic resonance imaging (MRI) revealed intraluminal thrombus extending proximally up to the confluence of hepatic veins immediately inferior to the right atrium without distal extension to femoral veins bilaterally [Figure 2]. MRI also revealed compression of IVC. An IVC venogram via the right jugular vein demonstrated multiple filling defects indicating occlusion of the IVC inferior to the right atrium [Figure 3]. There was simultaneous enlargement of distal part of IVC.
Figure 1.
Computed tomography of the abdomen- showing a well defined, heterogeneously enhancing mass lesion of size 7.6 × 5.3 × 4.8 cm at the upper pole of right kidney without any calcifications. The left adrenal gland appeared to be normal
Figure 2.
T2-weighted axial magnetic resonance imaging demonstrating the mass (predominantly high signal) between the inferior vena cava and right kidney (black arrow) compressing the overlying inferior vena cava (white arrow)
Figure 3.
IVC venogram showing multiple filling defects indicating occlusion of the inferior vena cava inferior to the right atrium. There is distal enlargement of inferior vena cava
A diagnosis of IVC thrombosis with pheochromocytoma was established, and surgical treatment was planned. Alpha receptor blocking therapy with prazosin was instituted, followed by β blocker, after testing for adequacy of α blockade. The patient was treated conservatively with subcutaneous low molecular weight heparin followed by oral warfarin. After 2 weeks, hypertension was well controlled and the remaining symptoms disappeared. With adequate blood pressure control, patient was subjected to laparoscopic adrenalectomy. There was no evidence of perilesional or distant invasions. Biopsy of the specimen revealed a typical organoid or zellballen pattern with no cytoplasmatic inclusion, pleomorphism, cytological alterations or necrosis; and, the mitotic index was low [Figure 4]. During the postoperative period, the blood pressure was normal, and the patient's convalescence was uncomplicated. He was discharged on the 11th postoperative day. During the next 16 months, the patient's blood pressure remained normal. A 24-hour urine specimen collected for metanephrine and VMA, revealed levels within normal limits. At present, the patient is asymptomatic, requires no medications, and is employed as an engineer. MRI imaging demonstrated resolution of the thrombosis and return of patency of the IVC at 4 months [Figure 5].
Figure 4.
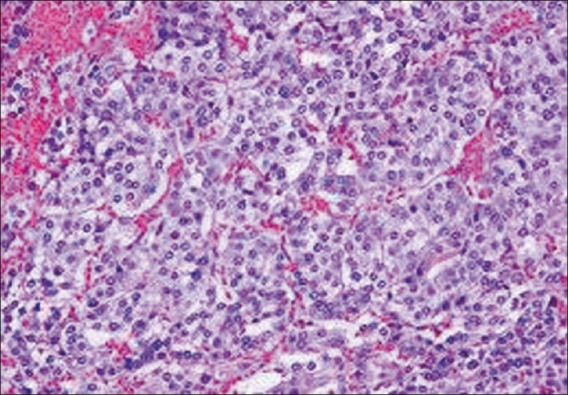
The typical growth pattern of nests of tumor cells (zellballen) surrounded by a discontinuous layer of sustentacular cells and fibrovascular stroma in the biopsy specimen of the patient in the study. Blood vessels surrounding tumor nests are composed of round to oval cells
Figure 5.
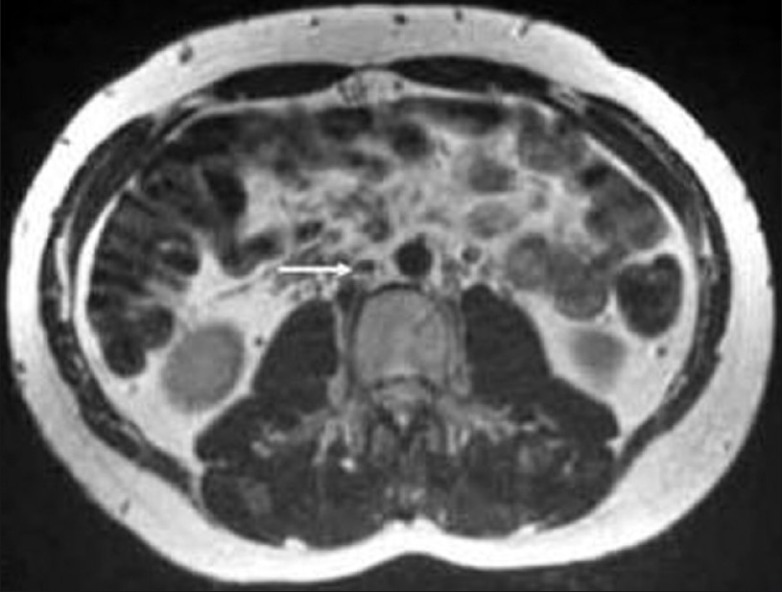
T2-weighted axial magnetic resonance imaging comparable in position and image acquisition to Figure 2 demonstrating complete resolution of inferior vena cava thrombosis (white arrow) after 4-months of oral anticoagulation therapy
DISCUSSION
Two aspects render our case unusual: 1) the coexistence of pheochromocytoma with IVC thrombosis 2) Though there are case reports citing the association between malignant pheochromocytoma and IVC thrombus,[7,8] to our sincere belief; this is the first such report citing this uncommon association from India.
Although the lifetime incidence of venous thrombosis is 0.1%, it still remains a rare condition especially in patients below 30 years of age.[9] Predisposing factors include alterations in blood flow [stasis], injury to the vascular endothelium and abnormalities in the constitution of blood hypercoagulability (Virchow's Triad).[10] Endothelial damage is invariably an acquired phenomenon, whereas hypercoagulability may result from both congenital and acquired risk factors (especially in the peri-operative period). The classical presentation of IVC thrombus varies according to the level of the thrombosis with up to 50% of patients presenting with bilateral lower extremity swelling and dilatation of superficial abdominal vessels. Whilst some patients remain asymptomatic, lower back pain, nephrotic syndrome, hepatic engorgement, cardiac failure and pulmonary embolus have also been described.[11] Tsuji et al. reported a series of 10 patients where 40% were pyrexic at presentation, with an associated elevation in D-dimer levels and inflammatory markers (white cell count, C-reactive protein).[12] Our patient had no lower limb, liver or kidney involvement, and this might be ascribed to the partial occlusion of IVC. He only had elevated inflammatory markers. We could not explain normal D-dimer levels in the backdrop of such a large thrombus in our patient.
CT scan with contrast enhanced images and MRI scan are used to localize adrenal pheochromocytoma.[13] meta-iodobenzylguanidine (MIBG) and Positron emission tomography (PET) scanning (Gallium- DOTA-toc/noc and DOPA-PET perform better than FDG- PET) are largely reserved for extraadrenal paraganglioma, or very large tumors to rule out metastasis. Heterogeneity, high hounsfield density on CT (>HU), marked enhancement with intravenous contrast and delayed contrast washout (<60 % at 10 minutes), high signal intensity on T2 weighted MRI, cystic and hemorrhagic changes point to pheochromocytoma, adrenocortical carcinoma or metastasis. However, pheochromocytoma with lipid degeneration can result in low attenuation scores (<10 HU) and >60% washout at delayed CT scanning.[14] Benign adrenal incidentalomas are characterized by size <5 cm, sharp margins, smooth contours, lack of demonstrable growth on serial examinations, attenuation scores <10 HU, and >60% washout at delayed CT scanning.[13,15] In our patient, CT scan revealed nonhomogenous mass of HU 35 without any calcification. Histologically, pheochromocytomas are capsulated and are composed of round or polygonal epithelioid/chief cells arranged in characteristic compact cell nests (Zellballen) or trabecular patterns. The chief cells have centrally located nuclei with finely clumped chromatin, and a moderate amount of eosinophilic, granular cytoplasm. Spindle shaped sustentacular or supporting cells are located peripherally. Tumors of higher grade are characterized by a progressive loss in the relationship between chief cells and sustentacular cells, and a decrease in the number of sustentacular cells. In our patient, typical zellballen pattern was found. Presence of markers like chromogranin A (CGA), neuron specific enloase, synaptophsyin serve as additional tools to confirm the neuroendocrine nature of the chief cells.[16]
Malignant pheochromocytomas are histologically and biochemically similar to benign ones. The only reliable clue to the presence of a malignant pheochromocytoma is local invasion or distant metastases, which may occur as long as 20 years after resection.[17] Thus, even when pheochromocytomas or paragangliomas are considered “benign” on pathologic examination, long term follow-up is indicated in all patients to confirm that impression. Other markers for malignancy are absent or weak expression of inhibin/activin- beta B subunit,[18] and presence of succinate dehydrogenase B (SDH B) subunit is seen. In absence of any invasion, we considered the mass in our patient to be benign.
The simultaneous occurrence of pheochromocytoma and IVC thrombosis is reported sporadically. IVC thrombosis in this case could be because of: 1) local compression leading to alteration in blood flow and stasis 2) sustained hypertension leading to vascular endothelial injury and hypercoagulability, 3) association of pheochromocytoma with systemic lupus erythematous and Behcet's disease might explain the triggering of an autoimmune phenomenon leading to a hypercoagulable state, and 4) an underlying anatomic abnormality or coagulation disorder. It also could be a chance association between these 2 conditions. In our case, local compression due to coexisting pheochromocytoma might have been causative.
Recent advances in the utilization of ultrasound, CT and MRI imaging as well as endovascular procedures have resulted in an increase in detection rates of IVC anomalies, as well as an increase in the incidental discovery of such abnormalities during unrelated investigations, therapeutic endovascular or surgical procedures.[12] Contrast venography remains the standard for diagnosis of IVC thrombosis with a low false-positive rate, and the advantage of access for immediate treatment if required. However, it is an invasive procedure associated with a 2%-10% incidence of post-procedural Deep venous thrombosis (DVT).[6] Duplex ultrasound scanning has become an accurate non-invasive method of diagnosing IVC thrombosis and is often the first-line investigative modality.[6] However, duplex USG is operator dependant and can be limited by body habitus or the presence of bowel gas and may occasionally fail to identify any IVC anomaly.[19] CT imaging is a rapid non-invasive method which can accurately diagnose and assess the extent of thrombus as well as delineate any associated abdominal or pelvic abnormality.[6] MRI imaging is now replacing CT as the optimal investigative tool avoiding radiation and giving more accurate delineation of thrombus as well as any IVC anomaly. MRI is also used to follow-up patients to determine morphological changes in the thrombus following therapy.[20]
Management of patients with coexisting pheochromocytoma and IVC thrombosis needs operative resection of the adrenal mass and medical/interventional management of IVC thrombosis. The goals of operation include 1) removal of the tumor with postoperative normotension, and 2) IVC luminal restoration and anticoagulation. Minimally invasive techniques are being increasingly used for resection of adrenal tumors and to treat renal artery lesions. Laparoscopic adrenalectomy is performed by either the transperitoneal or retroperitoneal approach.[21] Our patient was subjected to laparoscopic adrenalectomy after adequate preoperative blood pressure control by α blockers, followed by β blockers. Treatment options in the case of IVC thrombus without anatomical variance include anticoagulation, mechanical thrombectomy, systemic thrombolytic therapy, transcatheter regional thrombolysis, pulse-spray pharmacomechanical thrombolysis and angioplasty.[22] There is no specific literature describing the ideal duration of anticoagulation in these instances; however, case evidence identifies a trend toward treatment for a minimum of one year with the interplay of hypercoagulability disorders needing to be factored into any decision. Surgical reconstruction of the IVC and bypass of an aberrant section are both recognized modalities reserved for the most severe cases and are associated with morbidity and mortality risk.[23] Endovascular stent placement in combination with angioplasty is recommended in the cases of residual stenosis and chronic IVC occlusion.[23]
In the case of IVC thrombus associated with an aberrant IVC, with no other predisposing factors, treatment involves anti-coagulation. The duration of this treatment is widely debated with no extensive literature to provide an evidence based approach. Dean et al. took a view, which is quite similar to that of ours, that a caval anomaly is a permanent risk factor for venous stasis and thrombosis and that anticoagulant treatment should be lifelong.[24] Since our patient had no anatomic abnormality or any other predisposing factors, we decided to give the treatment for 4 months only and stopped it then after documenting radiologic luminal restoration.
CONCLUSION
Pheochromocytoma is known to be associated with vascular abnormalities. Though cases of renal artery stenosis, renal artery aneurysm and inferior vena cava thrombosis have been described, we found the uncommon association with IVC thrombosis in an Indian patient. IVC thrombosis is associated with a significant acute and chronic morbidity. A high index of suspicion is warranted for IVC thrombus. CT or preferably MRI imaging are required to delineate IVC anatomy and ascertain proximal extent of the thrombus. Although invasive therapeutic modalities exist, long-term and commonly life-long anticoagulation is often required. Pheochromocytoma does not seem to have any effect on the outcome of the coexisting IVC thrombosis. Our article calls for more research to confirm or refute the proposed hypothetical association.
ACKNOWLEDGMENT
All the authors would like to express their heartfelt thanks to Dr. Jagadeesh Tangudu, M Tech, MS, PhD, and Sowmya Jammula, M Tech for their immense and selfless contribution towards manuscript preparation, language editing and final approval of text.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Anderson GH, Jr, Blakeman N, Streeten, David HP. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–15. doi: 10.1097/00004872-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A, Yumita S, Kimura S, Miura Y, Yoshinaga K. Immunoreactive corticotropin-releasing hormone, growth hormone-releasing hormone, somatostatin, and peptide histidine methionine are present in adrenal pheochromocytomas, but not in extra-adrenal pheochromocytoma. J Clin Endocrinol Metab. 1990;70:996–9. doi: 10.1210/jcem-70-4-996. [DOI] [PubMed] [Google Scholar]
- 3.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of Pheochromocytoma which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 4.Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H, et al. Adrenocortical carcinomas and adrenal pheochromocytomas: Mass and enhancement loss evaluation at delayed contrast enhanced CT. Radiology. 2005;234:479–85. doi: 10.1148/radiol.2342031876. [DOI] [PubMed] [Google Scholar]
- 5.Assalia A, Gagner M. Laparoscopic adrenalectomy. Br J Surg. 2004;91:1259–74. doi: 10.1002/bjs.4738. [DOI] [PubMed] [Google Scholar]
- 6.Giordano P, Weber K, Davis M, Carter E. Acute thrombus of the inferior vena cava. Am J Emerg Med. 2006;24:640–6. doi: 10.1016/j.ajem.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Shigemura K, Tanaka K, Arakawa S, Hara I, Kawabata G, Fujisawa M. Malignant pheochromocytoma with IVC thrombus. Int Urol Nephrol. 2007;39:103–6. doi: 10.1007/s11255-005-4969-4. [DOI] [PubMed] [Google Scholar]
- 8.Dossett LA, Rudzinski ER, Blevins LS, Chambers EP., Jr Malignant pheochromocytoma of the organ of Zuckerkandl requiring aortic and vena caval reconstruction. Endocr Pract. 2007;13:493–7. doi: 10.4158/EP.13.5.493. [DOI] [PubMed] [Google Scholar]
- 9.Rosendaal FR. Thrombosis in the young: Epidemiology and risk factors, a focus on venous thrombosis. Thromb Haemost. 1997;78:1–6. [PubMed] [Google Scholar]
- 10.Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol. 2001;114:878–80. doi: 10.1046/j.1365-2141.2001.03025.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson BT, Thomas ML. Post-thrombotic inferior vena caval obstruction. A review of 24 patients. Br Med J. 1970;1:18–22. doi: 10.1136/bmj.1.5687.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji Y, Inoue T, Murakami H, Hino Y, Matsuda H, Okita Y. Deep vein thrombosis caused by congenital interruption of the inferior vena cava – A case report. Angiology. 2001;52:721–5. doi: 10.1177/000331970105201010. [DOI] [PubMed] [Google Scholar]
- 13.Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H, et al. Adrenocortical carcinomas and adrenal pheochromocytomas: Mass and enhancement loss evaluation at delayed contrast enhanced CT. Radiology. 2005;234:479–85. doi: 10.1148/radiol.2342031876. [DOI] [PubMed] [Google Scholar]
- 14.Blake MA, Krishnamoorthy SA, Boland GW, Sweeney AT, Pitman MB, Harisinghani M, et al. Low-density Pheochromocytoma on CT. A mimicker of Adrenal Adenoma. AJR Am J Roentgenol. 2003;181:1663–8. doi: 10.2214/ajr.181.6.1811663. [DOI] [PubMed] [Google Scholar]
- 15.Boraschi P, Braccini G, Grassi L, Campatelli A, Di Vito A, Mosca F, et al. Incidentally discovered adrenal masses: Evaluation with gadolinium enhancement and fat-suppressed MR imaging at 0.5 T. Eur J Radiol. 1997;24:245–52. doi: 10.1016/s0720-048x(97)01046-2. [DOI] [PubMed] [Google Scholar]
- 16.Kliewer KE, Wen DR, Cancilla PA, Cochran AJ. Paragangliomas: Assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques. Hum Pathol. 1989;20:29–39. doi: 10.1016/0046-8177(89)90199-8. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein RE, O’Neill JA, Jr, Holcomb GW, 3rd, Morgan WM, 3rd, Neblett WW, 3rd, Oates JA, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–66. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmenkivi K, Arola J, Voutilainen R, Ilvesmäki V, Haglund C, Kahri AI, et al. Inhibin/activin betaB-subunit expression in pheochromocytomas favors benign diagnosis. J Clin Endocrinol Metab. 2001;86:2231–5. doi: 10.1210/jcem.86.5.7446. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Lee JB, Han MC, Choi BI, Im CK, Chang KH, et al. Sonographic evaluation of inferior vena caval obstruction: Correlative study with vena cavography. AJR Am J Roentgenol. 1985;145:757–62. doi: 10.2214/ajr.145.4.757. [DOI] [PubMed] [Google Scholar]
- 20.Soler R, Rodriguez E, Lopez MF, Marini M. MR imaging in inferior vena cava thrombosis. Eur J Radiol. 1995;19:101–7. doi: 10.1016/0720-048x(94)00587-3. [DOI] [PubMed] [Google Scholar]
- 21.Del Pizzo JJ, Schiff JD, Vaughan ED. Laparoscopic adrenalectomy for pheochromocytoma. Curr Urol Rep. 2005;6:78–85. doi: 10.1007/s11934-005-0071-9. [DOI] [PubMed] [Google Scholar]
- 22.Yamada N, Ishikura K, Ota S, Tsuji A, Nakamura M, Ito M, et al. Pulse-spray pharmacomechanical thrombolysis for proximal deep vein thrombosis. Eur J Vasc Endovasc Surg. 2006;31:204–11. doi: 10.1016/j.ejvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Robbins MR, Assi Z, Comerota AJ. Endovascular stenting to treat chronic long-segment inferior vena cava occlusion. J Vasc Surg. 2005;41:136–40. doi: 10.1016/j.jvs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Dean SM, Tytle TL. Acute right lower limb extremity iliofemoral deep venous thrombosis secondary to an anomalous inferior vena cava: A report of two cases. Vasc Med. 2006;11:165–9. doi: 10.1177/1358863x06074829. [DOI] [PubMed] [Google Scholar]


