Abstract
Rationale:
Factors affecting asthma course are not clearly elucidated in urban communities in developing countries. Furthermore, the interaction between factors such as atopy, environmental exposure, urbanization, and helminthic infections in modulating asthma have not been well investigated.
Objectives:
To determine factors, which affect asthma in adults being evaluated at urban tertiary health center of Southwestern part of Nigeria.
Materials and Methods:
A random sample of 24 (12 males, 12 females) consecutive asthmatics seen in the Outpatient Pulmonary Clinic of University College Hospital of Ibadan and 27 (13 males, 14 females) age and gender-matched controls underwent evaluation, which included blood tests for eosinophils, serum IgE, allergy skin tests to eight common environmental allergens, and spirometry. The modified version of the questionnaire of the International study of Asthma and Allergies in Children (ISAAC) Phase III used by the same study group of researchers in Nigeria was used to assess the asthma symptoms. Wilcoxon sign-rank tests were used to compare eosinophil counts, percentage eosinophils, and allergic skin test between the two groups, while paired t test was used to compare spirometry variables.
Results:
Asthmatics had significantly more positive skin reaction to house dust mite and mould than controls (P<0.05). Total serum IgE was also significantly higher in asthmatics than in controls (mean 210 vs 60 IU/mL; P=0.003). However, no significant differences were observed in total eosinophil counts. No significant difference in the degree of intestinal helminthes infection in the two groups, which means stool parasitism was similar. FEV1 % was significantly lower in asthmatics (P=0.02) but FEV1 was similar between the two groups (P=0.02).
Conclusion:
The elevated levels of IgE and positive skin reactions to some of the common environmental allergens suggests an important role of atopy in the expression of asthma in this developing country's urban setting. Intestinal parasites were seen in both, control and asthma subjects, but appear not to play any role in the course of asthma, so also is the blood group, genotype and G6PD status.
KEY WORDS: Adults, allergens, asthma, sensitization, urban
INTRODUCTION
Several factors have been proposed to account for the increasing prevalence of asthma. It is agreed that genetic changes in populations would be too slow to account for such a rapid change in prevalence.[1]
Environmental factors have a key role in the genesis of asthma. Among the environmental determinants are exposure to allergen such as house dust mite (HDM), grass pollens, cockroach, and animal danders.[2,3] Evidence to suggest environmental pollutants to the initial development of asthma remains very weak.[4,5]
It is generally accepted that there are both allergic (extrinsic) and non-allergic (intrinsic) forms of asthma and that the distinction would be important in relation to the management of this disease (avoidance and immunotherapy). Allergens play a dual role in many asthmatics. They incite episodes of wheezing and also cause asthma to develop in the first instance.[6]
Atopy is the single strongest factor for the development of asthma, increasing the risk to 10–20 fold compared with those who are non-atopic.[7]
Sensitization to indoor allergens like house dust, cockroach, and animal danders as well as outdoor allergens like grass pollens plays an important role in the development of atopic airways disease like allergic asthma.[6,8–10]
Numerous studies have been done in developed countries and developing countries like Nigeria based on results of skin prick tests, eosinophilia, and elevated serum IgE to distinguish extrinsic[11–18] from intrinsic type of asthma. Cockroach hypersensitivity in asthmatics is also noted in some studies.[18–20]
A number of other factors that influence the development of asthma are infection (hygiene hypothesis), low socio-economic level, overcrowding, and infection with helminthes are independently protective against atopy among school-age children living in the rural area of Latin America.[20]
Chronic infection with helminths tends to confer protection but short-lived episode of infection may exacerbate atopic disorders.[21] Much of the inverse association between infections and asthma may be attributable to atopy.
The determinants of the onset of asthma and associated factors such as atopy has not been well investigated among adults living in the urban areas of the country.
The study was conducted to identity the predisposing factors to asthma among the adults living in Ibadan, an urban part of Southwestern Nigeria, to provide insight into the disease and to look for possible solutions.
MATERIALS AND METHODS
The study was carried out among the asthmatic patients at the Chest Clinic of the University College Hospital, Ibadan, Nigeria, between 1st of February 2006 and 30th December 2006.
Male and female adult patients more than 16 years of age, diagnosed to have bronchial asthma who were in stable state clinically, were included in the study. Age and sex-matched control subjects were also recruited. The asthmatics were on short acting β agonist and beclomethasone inhalers for the control of the disease.
The exclusion criteria included patients who were unwilling to give consent, on prednisolone in the last 30 days, with associated chronic cardiopulmonary diseases, and those with recurrent exacerbations in the past 6 months.
Study methods and procedures
Pulmonary function test
Spirometry was performed with the PC-based full function KoKo spirometer. After withholding short-acting inhaled bronchodilator therapy in asthmatics for at least 6 h before the study, the best of three efforts of forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and FEV1 % were recorded. Similar determinations were made in control subjects.
Blood sample
A blood sample (10 mL) was obtained from all patients and controls and processed at the IMRAT Research Laboratory. Full blood count including hemoglobin phenotype, blood group, and G6PD status were determined. The white blood cell count was performed with the Swelab 910EO+ auto counter, (Swelab, Stockholm, Sweden) while the Swelab autoheater was used for the absolute eosinophil count.
Serum IgE level
Total serum IgE was determined by ELISA method. 10 μL of calibrators (0.0, 100, 250, 500 IU/mL IgE calibrators), controls, or test samples were pipetted into IgE antibody-coated wells of microtiter plates. After this, 250 μL of enzyme antibody conjugate (goat anti-IgE labeled with horseradish peroxidase +0.02% thiomersal +0.002% gentamycin sulphate) was added. This was incubated at room temperature (22°C) for 45 min. This was discarded and the well was washed four times with diluted wash buffer (buffered detergent solution +0.02% thiomersal +0.002% gentamycin sulphate). 100 μL of substrate chromogen (buffered H2O2 and 3,3’,5,5’ tetramethylbenzidine solution) and 100 μL of 1 μN H2SO4 were added for 15 min at room temperature. The absorbance was read at 450 nm wavelength. The concentration of IgE was extrapolated from the calibration curve.
Allergy skin testing
Allergy skin test was performed using the Skintestor OMNI™ (Greer Laboratories Inc. Lenoir, North Carolina, USA) on the volar aspect of the forearm with standardized extracts of eight allergens: Dermatophagoides farinae – Mite, Dermatophagoides pteronyssinus- Mite, Pty Felis catus domesticus – Cat, Mangifera indica- Mango, Mus musculus – Mouse, Blattella germanica- German cockroach, Periplaneta Americana- American cockroach, Canis familiaris – Dog, Alternaria alternata, Aspergillus niger, Cladosporium spherospermum, Penicillium notatum – Mold, Mix 3, Bermuda, Johnson, June, Meadow Fescue, Orchard, Red Top, Timothy, Sweet Vernal, Perennial, and Rye- 9 Southern Grass Mix. Histamine was used as positive control while 50% gly +50% cocas was used as negative control. All subjects were told not to scratch or scrub the points of application of allergens. Within 15 to 20 min, assessment of both the wheal (swelling and edema) and erythema (flame/redness) reactions were measured with transparent meter rule and recorded.
Stool and urine evaluation for parasites
All subjects produced fresh stool and urine samples, which were sent to the parasitology laboratory for evaluation to exclude the presence of parasites and ova in stool as a contributing factor to eosinophilia or asthma flare-up.
Data analysis
Categorical variables such as gender, blood group, G6PD status, and parasite infections were compared between the asthmatics and the control subjects using Fisher's exact tests. Continuous variables were compared between the two groups using t tests (for body mass index, WBC, IgE, and lung function measures) or Wilcoxon-sign-rank tests (for eosinophil counts and percentage eosinophil) based on their distributions. Allergy skin reactions were compared between the two groups using Wilcoxon sign-rank. A P value equal to or less than 0.05 was considered statistical significance.
Ethical approval
This study was approved by the Ethical Committee of University College Hospital/University of Ibadan, Nigeria.
RESULTS
Study subjects
A total of 24 asthmatic patients (12 male, 12 female) seen at the chest clinic and 27 control subjects (13 male, 14 female) underwent the evaluation.
The mean age and sex distribution is as shown in Table 1 (32.5±9.2). The mean of age asthmatic was comparable to that of the control subjects (32.9±8.9).
Table 1.
Age and sex distribution of asthmatics and normal adults

Blood tests for asthmatics and controls
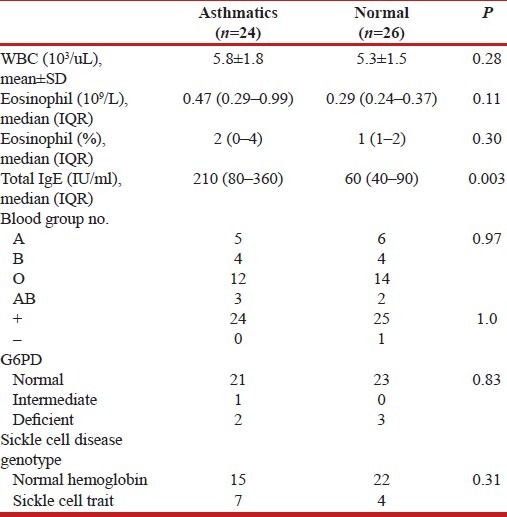
Table 2 shows the results of blood tests of asthmatics and normal adults. The total eosinophil counts, although much higher in the asthmatics than in the control subjects, is not statistically significant. The total IgE is significantly higher in asthmatics than controls (P=003). The blood groups, genotype, and G6PD status shows no difference in both groups.
Table 2.
Blood tests of asthmatics and normal adults
Allergic skin test and stool parasitism
Table 3 shows that asthmatics had significantly more positive skin reaction to HDM and mould than the control subjects but no significant difference was noted with cat hair, dog epithelium, grass, mango blossom, mouse epithelium, and cockroach.
Table 3.
Allergic tests of asthmatics and normal adults
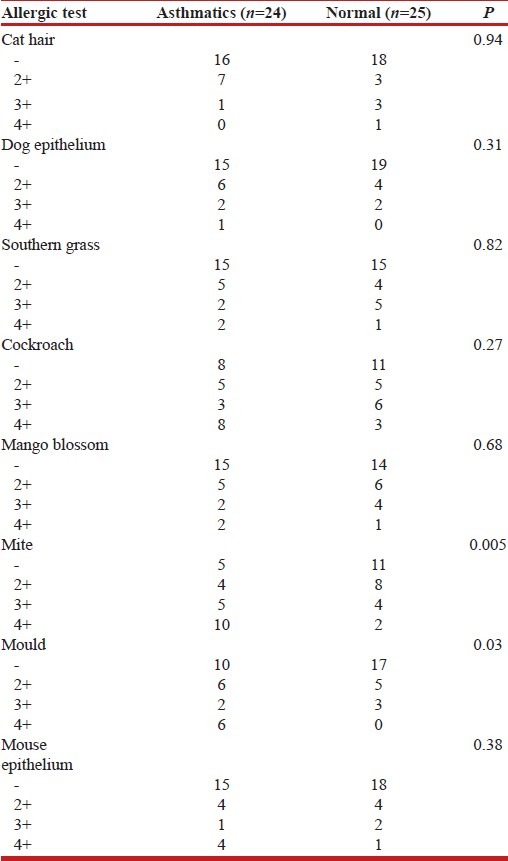
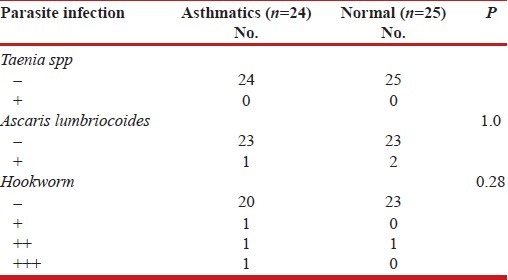
Table 4 shows that there is no significant difference between intestinal parasite infestation between the asthmatics and the control subjects.
Table 4.
Parasite infection of asthmatics and normal adults
Pulmonary function test
Table 5 shows that asthmatics have lower mean values of the lung function tests but of no statistical significance (FEV1, P value=0.21).
Table 5.
Spirometry tests of asthmatics and normal adults
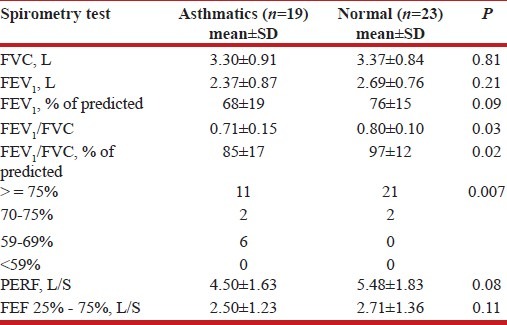
The FEV1/FVC was significantly lower in the asthmatics (P=0.02) compared with the controls.
DISCUSSION
The worldwide variation in the factors affecting the course of asthma indicates that environmental factors may be critical. Exposure to indoor air pollutants is at least as important as that of the outdoor air pollutants with regard to asthma development because many allergens and irritants can be found indoors.[22] There is growing evidence that infections and helminthes parasites, including geohelminth, may be protective against atopy and symptoms of asthma.[21,23–25]
There were significant differences in the pattern of skin reactions among asthmatics in this study. Asthmatics had significantly more positive skin reactions to HDM and mould than the control subjects, which was similar to the findings reported by Aderele et al.[26] This group of asthmatics may be classified as extrinsic asthmatics.
Sensitivity to aeroallergens had also been observed in Europe and United States of America (USA). Kang et al.[20] reported cockroach and ragweed sensitive subgroups among patients in an inner city asthma study in Chicago, while Hendricks et al.[27] reported skin test reactions among asthmatics in London.
More asthmatics showed strong positive skin reaction (4+) to HDM. This finding is similar to the findings reported by Onadeko et al.[28] and Awotedu et al.[29] HDM was also the most common allergens with positive reactions among the asthmatics in studies in Europe and USA.[27,30–32]
Cockroach allergen was not recognized in this study. Franser[33] in South Africa reported a prevalence of 16% for cockroach allergen among the asthmatics while Kang et al.[20] had a prevalence of 21% with skin prick test among the asthmatics in the low socio-economic class of the inner city.
Adenijo et al.[34] documented a case of cockroach allergen sensitivity in Lagos, which he ascribed to the increased density population and cockroach infestation.
In this study, the absence of cockroach allergen sensitivity may be a reflection of the level of cockroach infestation in this urban community compared with the finding in Lagos, which is more densely populated.[34]
The findings in this study agree with that of Cooper et al.[35] that no significant positive reactions were noted with cat hair and dog epithelium. The absence of an association with pets (specifically cats and dogs) may be explained by essentially a different relationship that families in the urban tropics have with such animals. They have dogs to warn off intruders and cats to control worm-like structures (vermis), and these animals generally live outside the house and do not have close contact with their owners. Exposure to pet allergens may, therefore, be low. Significant reaction to dog dander were the findings of Onadeko et al.[28] Awotedu et al.,[29] and Aderele et al.,[26] which contradicts the finding in this study.
An important observation from this study was the high prevalence of atopy observed.
This agrees with the observation by Godfrey[35] that urban asthmatics had significantly high serum IgE level than their controls. The finding of significantly higher level of IgE among the asthmatic compared with the controls also stresses the importance of atopy in the expression of asthma in this community.
In this study, no statistically significant differences were observed in the total and percentage eosinophil counts between the asthmatic patients and the controls although asthmatic patients have higher eosinophil counts.
Eosinophilia has been reported in several studies done in the tropics. Kartasamita et al.[36] reported statistically significant increased levels of eosinophils among asthmatic children. Neequaye gave similar report of significant eosinophilia among the asthmatic patients in Ghana.[37] Eosinophils have been shown to play a significant role in the pathogenesis of bronchial asthma. The presence and extent of eosinophilia in the peripheral blood may reflect the severity of the asthma. In this study, it is possible that the presence of no significant eosinophilia among the asthmatics may suggest that the asthma control is optimal, as the level of peripheral eosinophils tends to fall with better control in stable persistent asthma.
In this study, there was no significant difference between intestinal parasite infestation among the asthmatic and the control subjects, so the protective effect on atopy and development of asthma could not be ascertained as in other studies.[38–42]
In a large cross-sectional study conducted in the urban areas of Butajira in Ethiopia by Davey and co-workers,[38] no evidence of a protective effect of intestinal helminthic infections against wheeze or asthma was found – a finding that seems to contradict their previous data obtained in Jimma, another urban area in Ethiopia.[39] It was noted that, in Butarija, the prevalence and intensity of helminth infections were lower (33.8%) than the prevalence found in Jimma (77.3%). So, again in Butarija, lower prevalence of Ascaris infections, which would predict light infections, did not protect against allergies and asthma, emphasizing the possible importance of intensity of helminthes infection in modulating allergic responses.[40] Indeed it has been argued that high intensity of helminthes infection might exacerbate allergic disorders.[41] The mechanisms behind this is purely speculative with light infections, helminthes-associated molecules that drive Th2 responses might potentiate IL-4, IL-13 and IgE, whereas only with heavy infections, molecules that lead to regulatory immune responses, might reach a sufficient level to modulate the immune system and down-regulate Th2 responses.[42]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sears MR. Epidemiology of childhood asthma. Lancet. 1997;350:1015–20. doi: 10.1016/S0140-6736(97)01468-2. [DOI] [PubMed] [Google Scholar]
- 2.Holt PG, Sly PD, Borksten B. Atopic versus infectious diseases in childhood: A question of balance? Pediatr Allergy Immunol. 1997;8:53–8. doi: 10.1111/j.1399-3038.1997.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 3.Nicolai T. Asthma prevalence lessons from the reunification of Germany. Eur Respir J. 1997;1:1–6. [Google Scholar]
- 4.Van Niekerk CH, Weinberg EG, Shore SC, Heese HV, Van Schalkwyk J. Prevalence of asthma: A comparative study of urban and rural Xhosa children. Clin Allergy. 1979;9:319–24. doi: 10.1111/j.1365-2222.1979.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 5.Von Mutius, Nicolai T, Martinez F. Relation of indoor heating with asthma, allergic sensitization and bronchial responsiveness. BMJ. 1996;312:1448–50. doi: 10.1136/bmj.312.7044.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Mutius E, Weiland SK, Fritzsch C, Duhme H, Keil U. Increasing prevalence of hay fever and atopy among children in Leipzig, Reast Germany. Lancet. 1998;351:862–6. doi: 10.1016/S0140-6736(97)10100-3. [DOI] [PubMed] [Google Scholar]
- 7.Hopkin JM. The rise of atopy and links to infection. Allergy. 2002;57(Suppl 72):5–9. doi: 10.1034/j.1398-9995.57.s72.8.x. [DOI] [PubMed] [Google Scholar]
- 8.Von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149:358–64. doi: 10.1164/ajrccm.149.2.8306030. [DOI] [PubMed] [Google Scholar]
- 9.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997;350:85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg R, Vinker S, Zakut H, Kizner F, Nakar S, Kitai E. An unusually high prevalence of asthma in Ethiopian immigrants to Israel. Fam Med. 1999;31:276–9. [PubMed] [Google Scholar]
- 11.Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402(6760 Suppl):B12–7. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 12.Yazdanbakhsh M, Kremsner PG, Van Ree R. Allergy, parasites and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 13.Matricardi PM, Bonini S. High microbial turnover rate preventing atopy: A solution to inconsistencies impinging on the Hygiene hypothesis? Clin Exp Allergy. 2000;30:1506–10. doi: 10.1046/j.1365-2222.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper PJ, Chico ME, Rodrigues LC, Strachan DP, Anderson HR, Rodriguez EA, et al. Risk factors for atopy among school children in a rural area of Latin America. Clin Exp Allergy. 2004;34:845–52. doi: 10.1111/j.1365-2222.2004.01958.x. [DOI] [PubMed] [Google Scholar]
- 15.Addo-Yobo EO, Custovic A, Taggart SCO, Thomson L. Risk Factors for asthma in urban Ghana. Allergy Clin Immunol. 2001;108:363–8. doi: 10.1067/mai.2001.117464. [DOI] [PubMed] [Google Scholar]
- 16.Falade AG, Olawuyi JF, Osinusi K, Babatunde Onadeko BO. Prevalence and severity of symptoms of asthma, allergic rhino-conjunctivitis and atopic eczema in secondary school children in Ibadan, Nigeria. East Afr J Med. 1998;(4):695–8. [PubMed] [Google Scholar]
- 17.Holgate ST. The epidemic of asthma and allergy. Nature. 1999;402(Suppl 11):2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 18.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Wright SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 19.Falade AG, Olawuyi JF, Osinusi K, Onadeko BO. Prevalence and severity of symptoms of asthma, allergic rhino-conjunctivitis and atopic eczema in 6-7 year old Nigerian Primary school children: The International Study of Asthma and allergies in Childhood. Med Princ Pract. 2004;13:20–5. doi: 10.1159/000074046. [DOI] [PubMed] [Google Scholar]
- 20.Kang BC, Johnson J, Veres-Thorner C. Atopic profile of inner-city asthma with a comparative analysis on the cockroach-sensitive and ragweed-sensitive subgroups. J Allergy Clin Immunol. 1993;92:802–11. doi: 10.1016/0091-6749(93)90057-m. [DOI] [PubMed] [Google Scholar]
- 21.Martinez FD. Role of helminthes infection in the inception of asthma and allergies during childhood: Could they be protective? Thorax. 1994;49:1189–91. doi: 10.1136/thx.49.12.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt PG. Environmental factors and primary T-cell sensitization to inhalant antigens: Reappraisal of the role of infectious and air pollution. Pediatr Allergy Immunol. 1995;6:1–10. doi: 10.1111/j.1399-3038.1995.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 23.Shaheen SO, Aaby P, Hall AJ, James AL. Measles and atopy in Guinea Bissau. Lancet. 1996;347:1792–6. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 24.Lauener RP, Birchler T, Adamski J, Braun-Fahrlander C, Buff A, Herz U. Expression of CD14 and Toll-like receptor 2 in farmers’ and non-farmers’ children. Lancet. 2002;360:465–6. doi: 10.1016/S0140-6736(02)09641-1. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FD. Role of microbial burden in etiology of allergy and asthma. Lancet. 1999;354(Suppl):S1112–5. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 26.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–9. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 27.Hendricks DJ, Davies FJ, D-sonal MF, Goodwin RD, Eaton WW, Bright M, et al. An analysis of skin prick test for allergic diseases. Ann Allergy Asthma Immunol. 1995;74:23–8. [Google Scholar]
- 28.Onadeko BO, Sofowora EO. Skin sensitivity tests in Nigerians with bronchial asthma. Trans R Soc Trop Med Hyg. 1979;73:269–71. doi: 10.1016/0035-9203(79)90081-6. [DOI] [PubMed] [Google Scholar]
- 29.Awotedu AA, Oyejide C, Ogunlesi A, Onadeko BO. Skin sensitivity patterns to inhalant allergens in Nigerian asthmatic patients. Centr Afr J Med. 1992;38:187–91. [PubMed] [Google Scholar]
- 30.Burrows B, Lobowitz MD, Barbee RA. Respiratory disorders and allergy test reaction. Ann Intern Med. 1976;84:134–9. doi: 10.7326/0003-4819-84-2-134. [DOI] [PubMed] [Google Scholar]
- 31.Turner KY, Dowse GK, Stewart AG, Stewart HG. Studies on bronchial hyperreactivity, allergic responsiveness and asthma in rural and urban children of the high land of Papna New Guinea. J Allergy Clin Immunol. 1986;77:558–66. doi: 10.1016/0091-6749(86)90345-3. [DOI] [PubMed] [Google Scholar]
- 32.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99:594–9. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 33.Franser BN. Cockroaches in relation to bronchial asthma. Br J Dis Chest. 1972;66:61–6. [Google Scholar]
- 34.Adenijo OA, Bamidele EO. Cockroach hypersensitivity in Asthmatics in Lagos Nigeria. East Afr Med J. 2000;(2):622–6. doi: 10.4314/eamj.v77i11.46745. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey RC. Asthma and IgE levels in rural and urban communities of the Gambia. Clin Allergy. 1975;5:201–7. doi: 10.1111/j.1365-2222.1975.tb01853.x. [DOI] [PubMed] [Google Scholar]
- 36.Kartasamita CD, Mayudi D ROS, Demedts M. Total Serum IgE and eosinophil count in children with or without a history of asthma, wheezing or atopy in an urban community in Indonesia. The Respiratory Disease Working Group. J Allergy Clin Immunol. 1994;94:981–8. doi: 10.1016/0091-6749(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 37.Neequaye AR. Brochial asthma in Ghanians: A study of 200 patients. N Engl J Med. 1991;325:1067–71. [Google Scholar]
- 38.Davey G, Venn A, Belete H, Berhane Y, Britton J. Wheeze allergic sensitization and geohelminth infection in Butajira, Ethiopia. Clin Exp Allergy. 2005;35:301–7. doi: 10.1111/j.1365-2222.2005.02181.x. [DOI] [PubMed] [Google Scholar]
- 39.Dagoye D, Bekele Z, Woldemickad K, Boulet LP. Wheezing, allergy and parasite infective in children in urban and rural Ethiopia Am. T. Urban and rural Ethiopia Am. T. Respire. Crit Care Med. 2003;167:1369–73. doi: 10.1164/rccm.200210-1204OC. [DOI] [PubMed] [Google Scholar]
- 40.YazdanBaklish M, van den Biggelaar A, Maizela RM. Th2 responses without atopy: Immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22:372–7. doi: 10.1016/s1471-4906(01)01958-5. [DOI] [PubMed] [Google Scholar]
- 41.Cooper PJ. Can intestinal helminth infections (geohelminths) affect the development and expression of asthma and allergic disease? Clin Exp Immunol. 2002;128:398–404. doi: 10.1046/j.1365-2249.2002.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper PJ. Intestinal worms and human allergy. Parasite Immunol. 2004;26:455–67. doi: 10.1111/j.0141-9838.2004.00728.x. [DOI] [PubMed] [Google Scholar]


