Abstract
Rhinosporidiosis is a granulomatous disease that usually affects the nasal mucosa and nasopharynx. The disease is widely prevalent in the tropics, especially in southern India and Sri Lanka. In central India, rhinosporidiosis is endemic in Raipur, Chhattisgarh. Involvement of the tracheobronchial tree is extremely rare. Only two cases of bronchial rhinosporidiosis had been reported in world literature. We report a third case of bronchial rhinosporidiosis which occurred in a patient with coexisting nasal and nasopharyngeal rhinosporidiosis. Bronchoscopic guidance excision of mass and electric cauterization of base was done under local anesthesia.
KEY WORDS: Bronchus, rhinosporidiosis, bronchoscopic excision, cauterization
INTRODUCTION
Rhinosporidiosis is a chronic granulomatous infectious disease caused by Rhinosporidum seeberi with high prevalence in parts of Indian subcontinent and Sri Lanka.[1] Rhinosporidiosis is endemic in Raipur, Chhattisgarh of Central India.[2]
It produces polypoidal lesions that are friable, bleed profusely during resection and have a high tendency to recur. The common site of occurrence is nose and nasopharynx but can present at unusual sites. The involvement of tracheobronchial tree is rare, but can pose challenging problems for diagnosis, surgical excision as well as anesthetic management. We are reporting a case of 35-year-old man who had recurrent nasal rhinosporidiosis and was found to have left bronchial and urethral involvement. The bronchial rhinosporidiosis lesion was excised successfully under local anesthesia without any complications and no recurrence on 1-year follow up noted.
CASE REPORT
A 35-year-old male presented to the Department of ENT with mass in right nasal cavity and nasal blockage of 1-year duration. No history of nasal bleeding and respiratory difficulty was found. History of pond bath was present. Patient was operated once for the nasal rhinosporidiosis 3 year back. On anterior rhinoscopic examination, pinkish mulberry like mass was present in the floor of right nasal cavity. Left nasal cavity was clear. On oral cavity examination mass was hanging in oropharynx which bleeds on touch. On nasal endoscopy examination, rhinosporodiosis mass was found attached to floor of right nasal cavity and choana hanging toward nasopharynx.
After all routine examination and X-ray chest, patient was sent for the premedical and preanaesthetic checkup regarding the fitness for the surgery. During examination, it was found that patient has decreased air entry on left lung. X-ray chest showed hyperlucent lung on left side. Chest consultation was done and advised for the bronchoscopic evaluation and HRCT of thorax. HRCT thorax showed hyperlucent left lung field suggestive of air trapping with irregular soft tissue density mass (size 1.4 × 1.2 cm) seen in distal part of left main bronchus suggestive of papillomatous lesion.
Flexible fiberoptic bronchoscopic evaluation was done under local anesthesia. Pinkish mulberry like mass was found attached to left main bronchus [Figure 1]. It was decided to do the bronchoscopic excision of mass and cauterization of base under local anesthesia. Anesthetists were kept in standby with all the facility available for general anesthesia, tracheostomy and any other emergency. Bronchoscopic excision and cauterization of mass was done by the chest physician. The mass was first cauterized and then completely excised with bronchoscopic snare [Figure 2]. Excised mass was removed with the help of basket. The area was rechecked to find that no mass should left behind and whether cauterization has been done properly. Thereafter oral intubation was done and nasal mass was cauterized and excised completely under general anesthesia. Postoperative period was uneventful. Histopathological examination from the bronchial mass confirmed as rhinosporodiosis showing squamous metaplasia of bronchial epithelium and spores within the sporangia [Figure 3]. The patient was advised for follow up at monthly interval.
Figure 1.
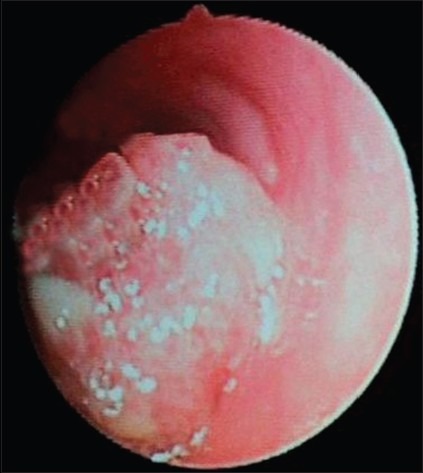
Bronchoscopy revealed pinkish mulberry like rhinosporidiosis mass in left main bronchus
Figure 2.
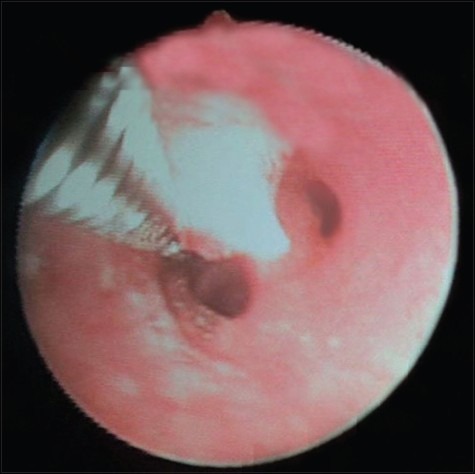
View after complete excision and cauterization of rhinosporidial mass
Figure 3.
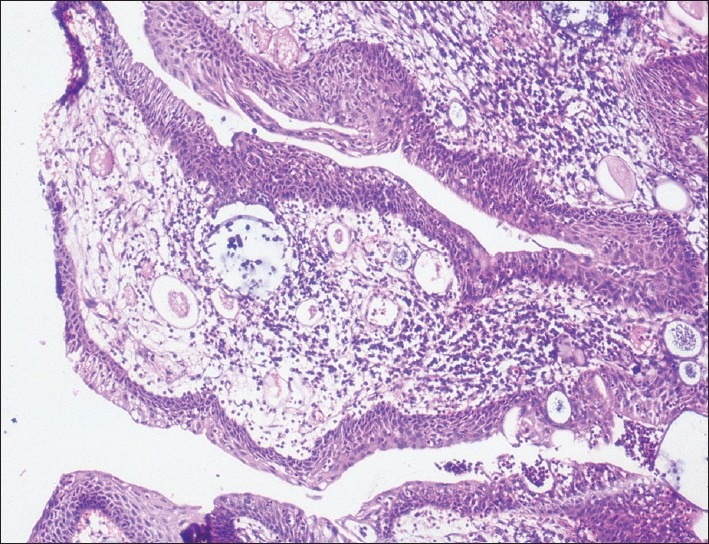
Microphotograph showing squamous metaplasia of bronchial epithelium and presence of sporangia with spores (H and E ×10)
DISCUSSION
Rhinosporidiosis is a chronic granulomatous infectious disease. The taxonomic relationship of R. seeberi with other organisms remained controversial for more than a century. The organism was first described by Malbran in 1892 as a sporozoan, as a protozoan by Seeber and as a phycomycetes by Ashworth in 1923.[3] Recently, molecular studies have shown R. seeberi to be a protistal microbe in the newly described class Mesomycetozoea at the animal-fungal boundary.[4] Though it occurs universally, rhinosporidiosis is endemic in South Asia being mainly reported from Southern India and Sri Lanka. Rhinosporidiosis is endemic in Chhattisgarh in Central India.[2] It commonly involves the nose, nasopharynx, lacrimal sac, and conjunctiva in that order of frequency.[5] It occasionally involves the lips, palate, uvula, larynx, trachea, penis, vagina, and bone.[6] Involvement of trachea and bronchial tree is very rare. Few cases have been reported of the tracheal involvement. Only two cases have been reported of the bronchial involvement.[7,8] All three cases including this are reported from India.
Thomas et al.(1956) reported a case of bronchial rhinosporidiosis in which biopsy through bronchoscopy was carried out without much bleeding, followed by a lobectomy as local excision was found to be impossible.[7]
Recently Gurudas et al. (2010) reported a case of recurrent nasopharyngeal rhinosporidiosis with right bronchial involvement which was successfully excised under general anesthesia using one lung ventilation. The surgical excision was done through tracheostoma route to avoid the external approach for the fear of implantation.[8]
Here, we presented a case of rhinosporidiosis with left bronchial involvement which was successfully excised under local anesthesia. The bronchial involvement could be more likely due to secondary implantation following the previous surgeries for nasal rhinosporidiosis. The patient has no respiratory complaints or stridor. Initially no chest pathology was suspected, it incidentally found during the preanesthetic checkup that there was a decreased air entry in the left side of lung, and then the patient was referred to the chest physician for the evaluation. The preoperative CT scan chest of the patient showed papillomatous mass in the distal part of left main bronchus. CT imaging is the preferred investigation in such situations because it provides better details about the extent of the lesion. When the confirmation was done about the extent and size of the lesion by CT, flexible fiberoptic bronchoscopy was done under local anesthesia. Mass was completely cauterized with the help of bronchoscopic snare and excised mass was removed carefully by the basket. Very minimal bleeding was observed during the excision and cauterization.
Rekha et al. reported that diagnosis of tracheobronchial rhinosporidiosis by means of bronchoscopic biopsy is dangerous because of the high risk of bleeding and the inability to visualize the full extent of the mass, especially when it obstructs the distal view. They excised the mass by using a combined approach of rigid bronchoscopy and wide tracheostomy.[5] In our case bleeding was very minimal due to prior good cauterization and the smaller size of the mass. Thomas et al. also reported the bronchoscopic biopsy without much bleeding.[7]
The mainstay of treatment for rhinosporidiosis is surgical excision by laser or electric diathermy. Several antibacterial and antifungal drugs have been tested clinically but the only drug which was found to have some antirhinosporidial effect is Dapsone which appears to arrest the maturation of the sporangia but the result are not satisfactory and the disease may recur after months or years.[9,10] We did not consider any drug therapy for the prevention of recurrence in follow up period. After 1 year of follow, no sign of recurrence was noted in bronchus.
CONCLUSION
Rhinosporidiosis in bronchus could be secondary to implantation of spores from previous surgeries for nasal and nasopharyngeal rhinosporidiosis. To the best of our knowledge and review, this is the third case of bronchial rhinosporidiosis reported in world literature; and the first case where the bronchial rhinosporidiosis mass was excised under local anesthesia without complication.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fredricks DN, Jolly JA, Lepp PW, Kosek JC, Relman DA. Rhinosporidum seeberi: A human pathogen from novel group of aquatic protistan parasites. Emerg Infect Dis. 2000;6:273–82. doi: 10.3201/eid0603.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudarshan V, Goel NK, Gahine R, Krishnani C. Rhinosporidiosis in Raipur, Chhattisgarh: A report of 462 cases. Indian J Pathol Microbiol. 2007;50:718–21. [PubMed] [Google Scholar]
- 3.Ashworth JH. On Rhinosporidium seeberi with special reference to its sporulation and affinities. Trans Royal Soc Edinb. 1923;53:301–42. [Google Scholar]
- 4.Silva V, Pereira CN, Ajello L, Mendoza L. Molecular evidence for multiple host-specific strains in the genus Rhinosporidum. J Clin Microbiol. 2005;43:1865–8. doi: 10.1128/JCM.43.4.1865-1868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rekha P, Thomas B, Pappachan JM, Venugopal KP, Jayakumar TK, Sukumaran P. Tracheal rhinosporidiosis. J Thorac Cardiovasc Surg. 2006;132:718–9. doi: 10.1016/j.jtcvs.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Makannavar JH, Chavan SS. Rhinosporidiosis - A Clinicopthological Study of 34 Cases. Indian J Pathol Microbiol. 2001;44:17–21. [PubMed] [Google Scholar]
- 7.Thomas T, Gopinath N, Betts RH. Rhinosporidiosis of the bronchus. Br J Surg. 1956;44:316–9. doi: 10.1002/bjs.18004418517. [DOI] [PubMed] [Google Scholar]
- 8.Gurudas Kini, Gopalkrishna A, Daniel Thomas. Anaesthesia for unusual presentation of rhinosporidiosis: A case report. Sri Lankan J Anaesthesiol. 2010;18:39–42. [Google Scholar]
- 9.Nair KK. Clinical trial of diaminodiphenylsulfone (DDS) in nasal and nasopharyngeal rhinsporidiosis. Laryngoscope. 1979;89:291–5. doi: 10.1288/00005537-197902000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Job A, Venkateswaran S, Mathan M, Krishnaswami H, Raman R. Medical therapy of rhinosporidiosis with dapsone. J Laryngol Otol. 1993;107:809–12. doi: 10.1017/s002221510012448x. [DOI] [PubMed] [Google Scholar]


