Sir,
Glandular enlargement of the breast in males is known as gynecomastia. Normally, gynecomastia occurs in the neonates, during puberty, and with aging, as a physiological phenomenon.[1] Imbalance in androgen or estrogen action in the breast tissue through a variety of mechanisms leads to pathological gynecomastia. Drug-induced gynecomastia is one such mechanism seen commonly in day-to-day practice. Common drugs incriminated in the causation of gynecomastia are cimetidine, digitalis, phenytoin, spironolactone, protease inhibitors, and antiandrogens.[1–3] Although the incidence of drug-induced gynecomastia varies from 20 to 25%, only occasionally the antituberculous therapy (ATT) is incriminated in the causation of gynecomastia[4] Among them, only isoniazid, and rarely thiacetazone, is reported to be associated with gynecomastia.[5–16] Herein, we describe a case of isoniazid-induced gynecomastia during the treatment of tuberculous lymphadenitis.
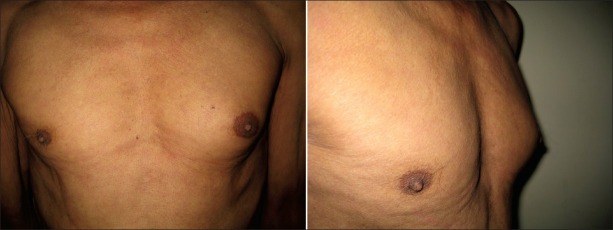
A 45-year old male presented to the chest clinic with bilateral cervical lymphadenopathy associated with evening rise of temperature and constitutional symptoms in the form of malaise, loss of appetite, and weight loss. General physical examination was unremarkable. Fine-needle aspiration from the lymph node revealed granulomatous inflammation and stain for acid-fast bacilli was positive. Chest X-ray was normal and he denied treatment with antituberculous therapy (ATT) or other drugs causing gynecomastia in the past. Tuberculin skin test was strongly positive while human immunodeficiency virus (HIV) serology was non-reactive. He was started on category III ATT (Rifampicin 600 mg, isoniazid 600 mg, pyrazinamide 1.5 g thrice a week) under directly observed treatment short course (DOTS) as per the Revised National Tuberculosis Control Programme (RNTCP) protocol. At the end of intensive phase of ATT, he showed symptomatic improvement with decrease in lymph node size. After 3 months of ATT, he visited the chest clinic with painful progressively enlarging swelling of breast on the left side [Figure 1]. On examination, a firm mound of tissue measuring approximately 4 cm around the nipple-areola complex was present, suggesting gynecomastia. No other obvious cause on detailed evaluation (normal genital and thyroid examination with normal secondary sexual characteristics) led to a presumptive diagnosis of isoniazid-induced gynecomastia due to the temporal association with ATT. This diagnosis was based on probable (WHO-UMC causality categories) role of isoniazid in causing gynecomastia. The patient was reassured and was shifted to rifampicin and ethambutol-based therapy for the next 3 months. After 1 month of stopping isoniazid, the pain subsided, and at the end of 3 months, the swelling had decreased by 25%. The patient was eventually lost to follow-up.
Figure 1.
Unilateral enlargement of left breast in frontal and lateral view
This case highlights the importance of recognizing the temporal association of this benign side effect of isoniazid therapy during the course of ATT that can be managed simply with reassurance and withdrawing the culprit drug, thus avoiding the need for unnecessary diagnostic evaluation. Withdrawing the offending drug, isoniazid as in this case, is both diagnostic and therapeutic, and should be done irrespective of presence or absence of painful reaction. True gynecomastia is defined as glandular enlargement of breast tissue of more than 4 cm in men. It should always be differentiated from the pseudogynecomastia by demonstrating firm, fibrous cord-like tissue that is concentric with nipple–areola complex in true gynecomastia by approximating thumb and forefinger together from either side of the breast during clinical examination.[1]
Pathological gynecomastia most commonly occurs due to imbalance between androgen and estrogen or because of increased aromatase activity in the adipose tissue leading to excess estrogen. Prolactin at times may be involved in the genesis of gynecomastia through negative feedback on gonadotropin hormone release.[17] When the cause for gynecomastia cannot be determined, it is termed as idiopathic. By decreasing androgen or increasing estrogen, drugs are an important and common cause of gynecomastia and should always be included in the evaluation as the possible causal agent.
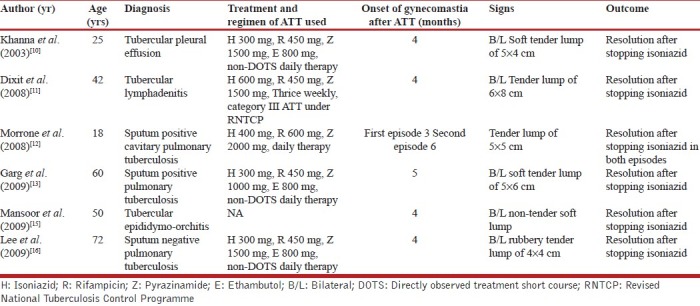
Our patients received 600 mg of isoniazid as part of thrice weekly regimen of RNTCP, similar to reported by Dixit et al., but other reported instances of gynecomastia occurred with lesser doses of isoniazid.[10–13,15,16] Gynecomastia related to the use of isoniazid was first recognized in 1953 with subsequent four more reports in French and Italian literature,[5–9] However, a systematic review of the database PubMed using text terms like: Drug-induced gynecomastia, isoniazid-induced gynecomastia, ATT-induced gynecomastia, and search of our personal files revealed only five cases of gynecomastia related to isoniazid [Table 1].[10–13,15] In all the reported cases, gynecomastia was noted after at least 3 months of isoniazid therapy, and in all cases, withdrawal of isoniazid led to its resolution, which is consistent with findings in our patient. The demonstration of temporal association of gynecomastia twice with isoniazid therapy in the same patient by Morrone et al. and resolution following its withdrawal strongly supports its causal relationship rather.[12] The exact mechanism of isoniazid causing gynecomastia remains unknown; plausible proposed mechanisms are altered androgen–estrogen due to disturbance in pyridoxine metabolism as well as refeeding syndrome during recovery from chronic illness, which needs further clarification.[11]
Table 1.
Details of all the cases of isoniazid-related gynecomastia reported in the English literature
In conclusion, the importance of this entity lies in the fact that simple recognition and withdrawal of the offending drug can lead to resolution of this problem.
REFERENCES
- 1.Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med. 2007;357:1229–37. doi: 10.1056/NEJMcp070677. [DOI] [PubMed] [Google Scholar]
- 2.Brody SA, Loriaux DL. Epidemic of gynecomastia among haitian refugees: Exposure to an environmental antiandrogen. Endocr Pract. 2003;9:370–5. doi: 10.4158/EP.9.5.370. [DOI] [PubMed] [Google Scholar]
- 3.Rahim S, Ortiz O, Maslow M, Holzman R. A case-control study of gynecomastia in HIV-1-infected patients receiving HAART. (29-32, 35-40).AIDS Read. 2004;14:23–4. [PubMed] [Google Scholar]
- 4.Braunstein GD. Gynecomastia. N Engl J Med. 1993;328:490–5. doi: 10.1056/NEJM199302183280708. [DOI] [PubMed] [Google Scholar]
- 5.Guinet P, Garin JP, Morneix A. Gynecomastia in a grave case of pulmonary tuberculosis during isonicotinic hydrazide therapy. Lyon Med. 1953;188:281–4. [PubMed] [Google Scholar]
- 6.Bassoli G, Bottero A, Romeo G. Several cases of gynecomastia in pulmonary tuberculosis patients treated with isoniazid. G Ital Della Tuberc. 1956;10:280–4. [PubMed] [Google Scholar]
- 7.Borsella C, Merelli B. Appearance of gynecomastia in pulmonary tuberculosis patients during isoniazid therapy. G Clin Med. 1957;38:1744–58. [PubMed] [Google Scholar]
- 8.Vidal J, Fourcade J, Chapel A. Gynecomastia during treatment with isoniazid. Montp Med. 1960;57:9–10. [PubMed] [Google Scholar]
- 9.Bergogne-Berezin E, Nouhouayi A, Letonturier P, Thibault B, Tourneur R. Letter: Gynecomastia caused by isoniazid.value of determination of the inactivation phenotype. Nouv Presse Med. 1976;5:213–4. [PubMed] [Google Scholar]
- 10.Khanna P, Panjabi C, Maurya V, Shah A. Isoniazid associated, painful, bilateral gynaecomastia. Indian J Chest Dis Allied Sci. 2003;45:277–9. [PubMed] [Google Scholar]
- 11.Dixit R, Sharma S, Nawal CL. Gynaecomastia during antituberculosis chemotherapy with isoniazid. J Assoc Physicians India. 2008;56:390–1. [PubMed] [Google Scholar]
- 12.Morrone N, Morrone N, Junior, Braz AG, Maia JA. Gynecomastia: A rare adverse effect of isoniazid. J Bras Pneumol. 2008;34:978–81. doi: 10.1590/s1806-37132008001100014. [DOI] [PubMed] [Google Scholar]
- 13.Garg R, Vaibhav, Mehra S, Prasad R. Isoniazid induced gynaecomastia: A case report. Indian J Tuberc. 2009;56:51–4. [PubMed] [Google Scholar]
- 14.Chunhaswasdikul B. Gynecomastia in association with administration of thiacetazone in the treatment of tuberculosis. J Med Assoc Thai. 1974;57:323–7. [PubMed] [Google Scholar]
- 15.Mansoor T, Rizvi SA, Khurram F, Ali W. Isoniazid-Induced Gynaecomastia. Internet J Surg. 2009. p. 18. [Available from: http://www.ispub.com/journal/the-internet-journal-of-surgery/volume-18-number-1/isoniazid-induced-gynaecomastia.html. ]
- 16.Lee MK, Jib Na D, Jeon H, Lee YD, Cho YS, Han MS, et al. A Case of Isoniazid Induced Gynecomastia. Tuberc Respir Dis. 2009;66:33–6. [Google Scholar]
- 17.Eckman A, Dobs A. Drug-induced gynecomastia. Expert Opin Drug Saf. 2008;7:691–702. doi: 10.1517/14740330802442382. [DOI] [PubMed] [Google Scholar]