Summary
Endovascular coil occlusion of cerebral aneurysms is increasing as a viable treatment for both ruptured and unruptured aneurysms. The purpose of this study was to evaluate the safety and performance of a newer generation of complex-shaped, geometrically conformable, platinum coils, the TRUFILL DCS Detachable Coil System. From September 2000 to December 2002, 112 patients with 116 aneurysms, either ruptured or unruptured, deemed by an attending neuro-interventionalist to be acceptable candidates for endovascular coil embolization, were recruited into an open-label, prospective, multi-center, international registry study from 23 centers in Europe. Information on relevant clinical characteristics, device and procedure performance, and angiographic occlusion data were collected for all patients. An Independent Medical Monitor collected and reviewed information on all device-and procedure-related complications resulting in serious adverse events. Angiographic evaluation immediately following treatment of 116 aneurysms showed a mean ± SD percent of aneurysm occlusion of 93.5% ± 14.2, with 90.2% of aneurysms occluded at least 90%. The desired occlusion was achieved in 94.9% of aneurysms. Success was defined as the ability to obtain ≥ 90% aneurysm occlusion. The proportion achieving greater than 90% occlusion was statistically equivalent (at least as good) to the 80% registry standard. Complication rates were 6.9% device-related and 2.6% procedure-related. Only two complications were categorized as serious adverse events. The TRUFILL DCS coil system provided good to excellent complete occlusion of the aneurysm at initial treatment, as compared to other published studies, and proved effective and safe to use in treating both ruptured and unruptured cerebral aneurysms.
Key words: endovascular treatment, cerebral aneurysms, coil occlusion, aneurysm therapy, interventional neuroradiology
Introduction
The endovascular treatment of cerebral aneurysms has increased significantly with the publication of the International Subarachnoid Aneurysm Trial results, which demonstrated a 24% increased benefit for patients treated by coil embolization vs. surgical clipping 1, and of multiple retrospective reviews comparing coiling with surgical clipping for the treatment of unruptured cerebral aneurysms 2-4. The number of patients with intracranial aneurysms who have been treated using detachable platinum coil technology has grown steadily to more than 230,000 worldwide 5. Yet aneurysmal recanalization as a result of incomplete occlusion at initial treatment remains a limitation of the current technology 6-9. Recently, a new detachable platinum coil system that specifically addresses this limitation was introduced for endovascular aneurysm therapy (TRUFILL DCS Detachable Coil System; Cordis Neurovascular, Inc., Miami Lakes, FL). We report the results of the initial, multi-center, prospective, patient registry study that used TRUFILL DCS coils to treat intracranial aneurysms, either ruptured or unruptured. The purpose of this study was to evaluate the safety and performance of this new coil device in terms of percent aneurysm occlusion at initial treatment as well as all device-and procedure-related adverse events in the peri-procedural period up to the time of discharge.
Methods
Patient Selection
From September 2000 to December 2002,112 patients with 116 angiographically documented aneurysms, either ruptured or unruptured, were recruited into this open-label, registry study from 23 centers in Europe. The patients were deemed by the attending neuro-interventionalist to be acceptable candidates for endovascular coil embolization therapy. Inclusion criteria included patients with an intracranial aneurysm who were referred for endovascular treatment. Patients were excluded from the registry when superselective placement of the microcatheter was not possible; when there was severe atheromatous disease; or when angiograms showed that the aneurysm was not appropriate for endovascular treatment (i.e., severe intracranial vessel tortuosity or stenosis, intracranial vasospasm not responsive to medical therapy, etc.). Informed patient consent was obtained in all cases. Information on device-and procedure-related complications resulting in all serious adverse events was collected and reviewed by an Independent Medical Monitor.
Description of Aneurysms
Sixty-three percent of the aneurysms that were treated were ruptured. Of the aneurysms treated, 89.6% had not been previously treated; 7% were previously treated with other types of coils, 0.9% with clipping, and 2.6% with other methods. Table 1 shows the locations and sizes of the treated aneurysms.
Table 1.
Size and location of aneurysms.
| Location | N=116 | |
|---|---|---|
| ICA | 20 | 17.2% |
| ACA | 9 | 7.8% |
| ACom | 28 | 24.1% |
| PCom | 19 | 16.4% |
| MCA | 14 | 12.1% |
| BA | 11 | 9.5% |
| Other | 15 | 12.9% |
| Size (mm) | Mean ± SD | Range |
| Sac length | 8.1 ± 4.5 | 2.0 – 7.0 - 24.0 |
| Sac width | 7.3 ± 5.3 | 2.0 – 6.0 - 40.0 |
| Neck | 4.1 ± 2.2 | 0.5 – 3.7 - 15.0 |
|
ICA, Internal Carotid Artery; ACA, Anterior Cerebral Artery; ACom, Anterior Communicating Artery; PCom, Posterior Communicating Artery; MCA, Middle Cerebral Artery; BA, Basilar Artery | ||
Aneurysm Location: Ninety aneurysms (77.6%) were located in the anterior circulation. The most common locations were the anterior communicating artery (24.1%), the internal carotid artery (17.2%), and the posterior communicating artery (16.4%).Other locations included the basilar artery (9.5%).
Size of the Aneurysms: Of 114 aneurysms with measurement data, 92 (80.7%) were small (<10 mm), 20 (17.5%) were large (10-25 mm), and two (1.8%) were giant (>25 mm).
Size of the Neck: Of 115 aneurysms with neck measurement data, 59 (51.3%) had small necks (<4 mm), and 56 (48.7%) had wide necks (≥ 4 mm).
The TRUFILL DCS Detachable Coil System
The TRUFILL DCS Detachable Coil System is a newer generation embolic device system that consists of a platinum detachable coil, a delivery tube, and a detachable coil syringe.
The DCS Coil: The platinum coil has a three-dimensional (3D), random, complex shape that enables the coil to conform to a variety of aneurysm shapes. The coil loops conform to the periphery of the available space, facilitating concentric filling and leading to higher packing densities 9. TRUFILL DCS coils are offered in a variety of sizes, softness, and shapes. They are available in two grades of softness and are either 3D complex-shaped (figure 1) or 2D helical-shaped (figure 2). The Complex Fill coils are softer than other currently available 3D coils, which, when combined with their random shape, allows them to conform better to the shape of the aneurysm, resulting in better aneurysm packing and better neck coverage than standard 2D and 3D coils currently available.
Figure 1.
Image shows 3D configuration of the Complex Trufill DCS detachable coil. Note the random loops.
Figure 2.
Image shows 2D configuration of the Helical Trufill DCS detachable coil.
The Delivery Tube: The delivery tube is used to deliver and detach the embolic coil within the aneurysm. It is a pushable "mini" microcatheter with an elastomeric gripper at the distal end that holds the coil during delivery and positioning.
The Detachable Coil Syringe: To detach the coil from the delivery tube, hydraulic pressure is transferred from the manually operated detachable coil syringe to the distal tip of the delivery tube. The syringe delivers the controlled hydraulic pressure used to purge the system before entry into the body and for subsequent actuation of the elastomeric gripper during coil detachment.
The pressure causes the elastomeric gripper to manually release, gently separating the coil from the delivery tube. The syringe includes a pressure gauge to provide visual feedback. If, upon delivery and prior to detachment, it is felt that the coil is inappropriate for the aneurysm, the coil can be completely removed so that another, more appropriate coil can be delivered.
The TRUFILL DCS Detachable Coil System is compatible with braided microcatheters with an inner diameter of 0.021 inches, a length of 150 cm, and 3-cm dual tip markers. Compatibility tests were carried out specifically with the Rapid Transit and PROWLER Plus microcatheters (Cordis Neurovascular, Inc., Miami Lakes, FL).
The Embolization Procedure
All participating centers adhered to standard and routine interventional neuroradiology patient care as outlined according to their individual hospital protocols. A 6F Envoy (Cordis Neurovascular, Inc., Miami Lakes, FL) guiding catheter was inserted into the right or left femoral artery using the standard technique for percutaneous interventional neurovascular procedures. Angiographic assessment was performed using the appropriate orthogonal views that best showed the aneurysm height, width, and neck. Each neuro-interventionalist decided on the number and type of TRUFILL DCS coils to be deployed into the aneurysm depending on the configuration and size of the aneurysm and on his/her individual experience for achieving satisfactory aneurysm occlusion. The number and size of each coil placed, as well as whether each coil detachment was rated to be acceptable or unacceptable, was documented on the Device Evaluation Form. Following detachment and removal of the final coil, post-embolization procedure angiograms were performed using the same orthogonal views as used in the pre-embolization angiograms.
Statistical Methods
A point estimate and confidence interval was calculated for the proportion of cases presenting with angiographic occlusion of at least 90% using the TRUFILL DCS coils (primary endpoint). A test of hypotheses was performed to determine if the primary hypothesis that "not less than 80% of the subjects satisfy this condition" defined procedure success. In addition, a point estimate and confidence interval was calculated for the proportion of cases suffering any adverse event as defined in the protocol.
Illustrative Cases Using the TRUFILL DCS Detachable Coil System
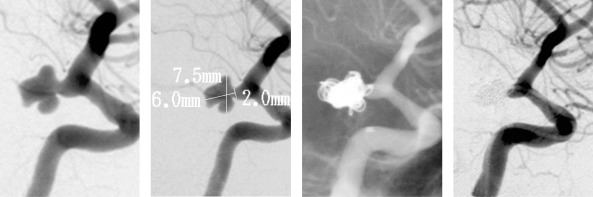
Case 1: A 47-year-old female presented with an acute subarachnoid hemorrhage, Hunt and Hess Grade II, due to an irregularly shaped aneurysm arising from the internal carotid artery at the region of the posterior communicating artery segment (figure 3A). The aneurysm measured 6.0 mm in height, 7.5 mm in width, with a 2.0 mm neck (figure 3B). A preshaped PROWLER Plus microcatheter was placed into the aneurysm, and 6 TRUFILL DCS coils were placed into the aneurysm without complications (figure 3C). The follow-up angiogram revealed excellent conformability of the coils within the irregular shape and excellent occlusion of the aneurysm (figure 3D). One year after the procedure, the patient has made an excellent recovery, with continued occlusion of the aneurysm documented by follow-up angiography.
Figure 3.
This 47-year-old female presented with an acute subarachnoid hemorrhage due to an irregularly shaped aneurysm arising from the internal carotid artery at the region of the posterior communicating artery segment. Pretreatment, treatment, and follow-up images are presented. Pretreatment angiogram shows an irregularly shaped aneurysm arising from the internal carotid artery at the region of the posterior communicating artery segment. Lateral view angiogram shows the aneurysm to measure 6.0 mm in height, 7.5 mm in width, with a 2.0 mm neck. A pre-shaped Prowler microcatheter is placed in the aneurysm, and 6 TRUFILL DCS coils are placed into the aneurysm without complications. Follow-up angiogram shows excellent conform ability of the coils within the irregular shape, and occlusion of the aneurysm. At one year follow-up the patient has made an excellent recovery with continued occlusion of the aneurysm.
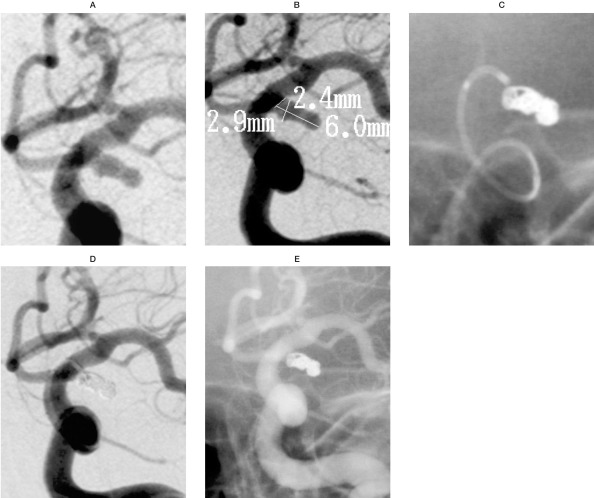
Case 2: A 42-year-old female presented with a Hunt & Hess Grade III, acute subarachnoid hemorrhage. An angiogram showed an elongated, irregularly shaped aneurysm arising at the junction of the posterior communicating artery (figure 4A). The aneurysm measured 6.0 mm in length, 2.9 mm in height, and had a neck width of 2.4 mm (figure 4B). A preshaped PROWLER Plus microcatheter was navigated into the aneurysm, and four TRUFILL DCS coils were placed into the aneurysm for occlusion (figure 4C, 4D). The six-month follow-up angiogram shows continued occlusion of the aneurysm and excellent flow in the carotid artery (figure 4E).
Figure 4.
This 42-year-old female presented with a Hunt & Hess Grade III acute subarachnoid hemorrhage. Pretreatment,treatment,and follow-up images are presented. A) A right carotid, frontal view pretreatment angiogram shows an elongated, irregularly shaped aneurysm arising at the junction of the posterior communicating artery. B) The aneurysm measures 6.0 mm in length, 2.9 mm in height, and has a neck width of 2.4 mm. C,D) A pre-shaped PROWLER Plus microcatheter is navigated into the aneurysm, and 4 TRUFILL DCS coils are placed into the aneurysm for occlusion. E) The 6-month followup angiogram shows continued excellent follow-up, with complete occlusion of the aneurysm and excellent flow in the carotid artery.
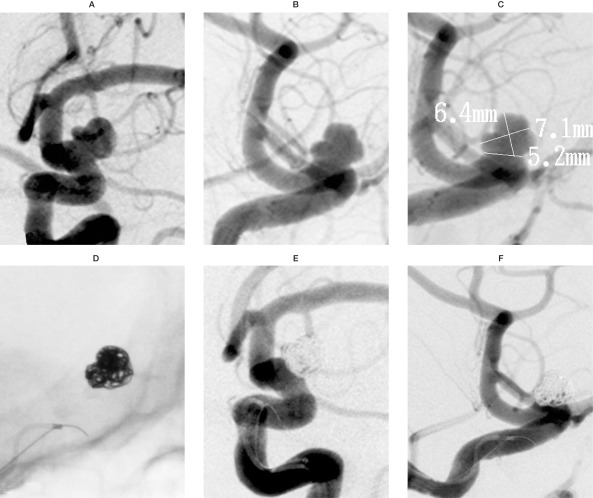
Case 3: A 56-year-old male presented with an acute subarachnoid hemorrhage from a broadbased aneurysm arising just above the ophthalmic artery segment. Angiograms in both oblique frontal (figure 5A) and lateral (figure 5B) views clearly show the irregularity of the aneurysm and the broad-based neck. The aneurysm measured 6.4 mm in height, 7.1 mm in width, and the neck was 5.2 mm (figure 5C). A 45-degree pre-shaped PROWLER Plus microcatheter was navigated into the aneurysm; with the use of the balloon-assist technique, four TRUFILL DCS coils were successfully placed within the aneurysm, with excellent conformability to the irregular shape of the aneurysm (figure 5D). The immediate posttreatment and six-month follow-up angiograms in the oblique frontal (figure 5E) and lateral (figure 5F) views show occlusion of the aneurysm and excellent conformability across the broad neck. The patient has done well clinically at six months of follow-up.
Figure 5.
This 56-year-old male presented with an acute subarachnoid hemorrhage from a broad-based aneurysm arising just above the ophthalmic artery segment. A,B) Angiogram in both the oblique frontal (A) and the lateral (B) views clearly show the irregularity of the aneurysm's shape and the broad-based neck. C) The aneurysmal height measures 6.4 mm, the width 7.1 mm, and the neck 5.2 mm. D) A 45 -degree pre-shaped PROWLER Plus microcatheter is navigated into the aneurysm, and with the use of the balloon-assist technique, 4 TRUFILL DCS coils are successfully placed within the aneurysm, with excellent conformability to the irregular shape of the aneurysm. E,F) Immediate post-treatment angiograms in the oblique frontal (E) and lateral (F) views show excellent occlusion of the aneurysm and excellent conformability across the broad neck. The patient has done well at 6 months of follow-up.
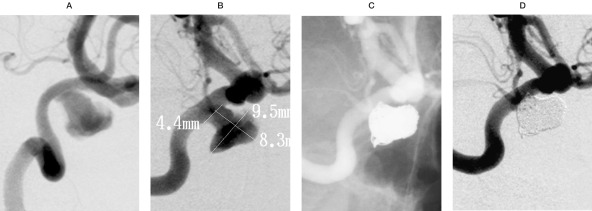
Case 4: A 61-year-old female presented with retro-orbital pain, headache, and diplopia. The cerebral angiogram showed a large, partially thrombosed aneurysm arising from the cavernous internal carotid artery (figure 6A). The residual aneurysm measured 8.3 mm in height, 9.5 mm in length, and had a 4.4 mm neck (figure 6B). A 90-degree pre-shaped PROWLER Plus microcatheter was guided into the aneurysm, and six TRUFILL DCS coils were placed into the aneurysm (figure 6C). The follow-up angiogram at six months showed continued occlusion of the large cavernous aneurysm, and clinically the patient has significantly improved from her presenting symptoms, with almost complete resolution of her symptoms (figure 6D).
Figure 6.
This 61-year-old female presented with retro-orbital pain, headache, and diplopia. A) The lateral view, internal carotid artery angiogram, shows a large, partially thrombosed aneurysm arising from the cavernous internal carotid artery. B) The residual aneurysm measures 8.3 mm in height, 9.5 mm in length, and has a 4.4 mm neck. A 90-degree pre-shaped PROWLER Plus microcatheter is guided into the aneurysm, and 6 TRUFILL DCS coils are placed into the aneurysm. Follow-up angiogram at 6 months shows continued excellent occlusion of the large cavernous aneurysm, and clinically the patient has significantly improved.
Results
One hundred and twelve patients with 116 aneurysms were treated by 29 physicians in 23 centers during the period of the study. A total of 670 (174 Complex Fill + 17 Complex Basket + 479 Helical Fill) coils were used. The duration of the procedure and number of coils used are shown in Table 2. The procedure time reflects the time from when the first TRUFILL DCS coil was used up to the detachment of the last coil.
Table 2.
Procedure characteristics (N=116).
| Procedure Time (107 Aneurysms) |
Minutes |
|---|---|
| Mean ± SD | 71.5 ± 56.2 |
| Range (min.–median–max.) | 5.0 – 60.0 – 255.0 |
| Number of Coils Deployed | Percent of Aneurysms |
| 0 | 2.6% |
| 1-5 | 56.0% |
| 6-10 | 29.3% |
| 11-15 | 8.6% |
| >15 | 3.4% |
Angiographic evaluation showed a mean ± SD percent of initial aneurysm occlusion of 93.5% ± 14.2%, with 90.2% of aneurysms occluded at least 90%. The desired occlusion, as judged by each treating physician, was achieved in 94.9% of aneurysms. Success was defined as the ability to obtain ≥ 90% aneurysm occlusion. The proportion achieving greater than 90% occlusion was statistically equivalent (at least as good) to the 80% registry standard.
Complication rates were 6.9% (8/116) device-related and 2.6% (3/116) procedure-related. The device-related complications included 1.7% (2/116) devices that were stretched or broken, 1.7% (2/116) devices not properly purged of air, 0.9% (1/116) aneurysm perforation/rupture, 1.7% (2/116) coil loop prolapse into the parent vessel, and 0.9% (1/116) microcatheter tip excess movement. The procedure-related complications included 0.9% (1/116) side branch vessel occlusion, 0.9% (1/116) transient vessel occlusion, and 0.9% (1/116) transient right-sided monoparesis and aphasia. Only two complications – (1) aneurysm rupture and hemorrhage, and (2) coil stretching with breakage and extrusion into the carotid lumenwere categorized as serious adverse events. No patients died. No emboli, neurological deterioration, transient ischemic attack, or vasospasm were observed.
Information on device performance was collected for all patients. Over 91% of the responses ranged from satisfactory to excellent in all categories. Physicians were also asked to subjectively compare the performance of the TRUFILL DCS Detachable Coil System to their experience with other endovascular coil occlusion devices. Over 91% of responses were either equivalent or superior to other endovascular coil occlusion devices in all categories.
Discussion
The goal of endovascular coil embolization of aneurysms is the long-term exclusion of the lesion from the circulation. One of the main predictors of successful, long-term aneurysmal obliteration is the degree of angiographic occlusion in the period immediately following treatment with coils. Aneurysmal recanalization and/or recurrence are more common in lesions that were incompletely occluded with coils at initial treatment 6,8-10. Incomplete initial occlusion remains a limitation of current detachable coil technology.
Recent advances in coil technology have included the introduction of such new materials as softer coils, 3D coils, and complex fill coils. Three-dimensional and complex fill coils such as the TRUFILL DCS Complex Fill and Complex Basket coils are designed to be more anatomically conformable to the aneurysm than other coils and to therefore enable true concentric filling, which leads to better packing densities and neck coverage, creating a more stable filling of the aneurysm and thus providing a more efficient exclusion of the aneurysm from the circulation.
In this current clinical evaluation, the delivery and detachment of the TRUFILL DCS coils was safe and effective. No coils failed to detach. There was no coil migration. There was no premature or involuntary coil detachment, and the hydraulic detachment was consistent and reliable (5.7 s ± 3.4). The hydraulic mechanical detachment system has been shown to be easy to use, to provide consistent detachment, and to provide visual confirmation of coil detachment from the pusher wire by a rapid decline in the manometer.
Only two complications were considered serious adverse events: (1) aneurysm rupture and hemorrhage in one case (0.9%), and (2) coil stretching with breakage in one case out of 670 total coils deployed (0.15%), with extrusion into the carotid lumen. According to the physician, the suspected cause of the aneurysm rupture and hemorrhage was due to the microcatheter. The coil stretching with breakage occurred during withdrawal, after attempted positioning within the aneurysm sac. No further attempt was made to retrieve the coil. The postprocedure CT scan showed that the coil had migrated to the middle cerebral artery, but there was no evidence of infarction. The patient was treated with heparin therapy and was discharged on aspirin following a full recovery three weeks after the procedure. The patient had no residual symptoms at the three-month follow-up.
This study was designed to demonstrate an acceptable peri-procedural safety and performance analysis in the first clinical study using this new, second generation, detachable coil system to treat intracranial aneurysms. Additional studies on the intermediate and long-term performance of this new device are on-going.
Conclusions
The TRUFILL DCS Detachable Coil System allowed a more complete occlusion of the aneurysm at initial treatment. It proved effective and safe to use in treating both ruptured and unruptured cerebral aneurysms.
Acknowledgments
We wish to thank all the investigators and staff at the participating centers. This study was supported by a grant from Cordis Neurovascular, Inc.
Participating Centers and Physicians
U. Pasquini, Azienda Ospedaliera, Ancona, Italy; P. Courtheoux, Caen, CHU, Caen, France;J. Gabrillargues, Clermont Ferrand University Hospital, Clermont-Ferrand, France; J. Moret, Fondation Rothschild, Paris, France; A. Bonafe, Gui de Chavliac, Montpelier, France; F. Ricolfi, Henri Mondor Hôpital, Paris, France;L. Lopez Ibor, Hospital De La Ribiero, Valencia, Spain;E. Houdart, R. Chapot, Lariboisiere Hôpital, Paris, France; O. Levrier, La Timone, CHU, Marseille, France; F. Bouquingny, L. Pierot, Maison Blanche, CHU, Reims, France;F. Turjman, Neuro-Cardio Hôpital de Lyon, Lyon, France; S. Bracard, Neurologique Hôpital, Nancy, France;S. Berge, Pellegrin, CHU, Bordeaux, France;J. Vallee, N. Souror, B. Alessandra, Pitie Salpetriere, CH, Paris, France;N. McConachie, Queens Medical Center, Nottingham, UK;J. Byrne, Radcliffe Infirmary, Oxford, UK; H. Pederse, Rihshospitalet, Oslo, Norway;X. Leclerc, J.Y. Gauvrit, Roger Sallengro, Lille, France; J.F. Meder, Sainte Anne, CHU, Paris, France;C. Cognard, Toulouse University Hospital, Toulouse, France;A. Valavanis, University Hospital, Zurich, Switzerland;H. Nahser, Walton Centre for Neurology, Liverpool, UK; R. Sellar, P. White, Western General Hospital Center, Edinburgh, UK.
We also thank Dagmar Schnau for editorial assistance.
References
- 1.Molyneux A, Kerr R, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 2.Johnston SC, Wilson CB, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol. 2000;48:11–19. doi: 10.1002/1531-8249(200007)48:1<11::aid-ana4>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Zhao S, et al. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001;32:597–605. doi: 10.1161/01.str.32.3.597. [DOI] [PubMed] [Google Scholar]
- 4.Raftopoulos C, Mathurin P, et al. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg. 2000;93:175–182. doi: 10.3171/jns.2000.93.2.0175. [DOI] [PubMed] [Google Scholar]
- 5.Murayama Y, Nien YL, et al. Guglielmi Detachable Coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 6.Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. Am J Neuroradiol. 2002;23:762–767. [PMC free article] [PubMed] [Google Scholar]
- 7.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. Am J Neuroradiol. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond J, Guilbert F, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 9.Piotin M, Iijima A, et al. Increasing the packing of small aneurysms with complex-shaped coils: an in vitro study. Am J Neuroradiol. 2003;24:1446–1448. [PMC free article] [PubMed] [Google Scholar]
- 10.Vallee JN, Pierot L, et al. Endovascular treatment of intracranial wide-necked aneurysms using three-dimensional coils: predictors of immediate anatomic and clinical results. Am J Neuroradiol. 2004;25:298–230. [PMC free article] [PubMed] [Google Scholar]