Abstract
Objective
Continuous glucose monitoring (CGM) systems collect and store glucose data in an ongoing fashion for several days at a time. The main advantage of continuous glucose monitoring is that it can help identify fluctuations and trends that would otherwise go unnoticed with other glucose measures. Here we provide a review of CGM for behavioral researchers.
Methods
We begin with a brief review of diabetes and glucose measurement and then describe what CGM is and reference the commercial CGM systems currently available. We discuss the challenges involved in using CGM in behavioral research. We then present a broad overview of CGM in behavioral research, including data from ours and others' research programs. Finally, we cover some practical issues to be considered when using CGM, suggest reporting guidelines for the behavioral researcher, and offer suggestions for future research.
Results
Only a handful of behavioral researchers are using CGM, although its use is increasing. The main ways that CGM is being used in behavioral research is to investigate basic biobehavioral processes, to assess the effects of behavioral interventions on diabetes control, and to use CGM itself as a behavior modification and teaching tool in diabetes self-management interventions.
Conclusions
CGM holds promise to help behavioral researchers unravel the complex relationships among glucose and intrapersonal, interpersonal, and contextual factors. However, the uptake of CGM for this purpose is limited, and the possibilities for its use are largely unmet. We encourage behavioral researchers to implement CGM in their protocols, and to do so in a way that maximizes its explanatory power.
Keywords: continuous glucose monitoring, ambulatory monitoring, diabetes, glycemic, behavioral, glucose
Introduction
Diabetes is a complex chronic disease that provides a useful model for studying biobehavioral processes. Yet, far from being an academic curiosity, it is also a growing public health problem. Continuous glucose monitoring (CGM), designed to improve clinical care for patients with diabetes, has increased the sophistication of research questions that can be asked regarding diabetes. Most CGM research is aimed at demonstrating its clinical benefits for diabetes control, and at describing patient satisfaction in clinical settings. Only a handful of behavioral researchers are currently using CGM, yet it holds promise for answering key questions regarding the relationship of glucose to mood, stress, health behaviors, and behavioral interventions. Here we provide a primer and review of CGM for behavioral researchers. We begin with a brief review of diabetes and glucose measurement, and then describe what CGM is and compare the commercial CGM systems currently available. We discuss the challenges involved in using CGM in behavioral research. We then present a broad overview of CGM in behavioral research, including data from ours and others' research programs. Finally, we offer practical tips for the behavioral researcher and suggestions for future research.
Diabetes and Behavioral Diabetes Research
Most morbidity and mortality in diabetes is associated with long term complications, rather than the metabolic condition per se. Several well controlled trials with decades of follow up show that the risk of some long-term complications can be reduced with glycemic control. (1,2). Unfortunately, adequate glycemic control continues to be elusive for many people with diabetes. This is due largely to the numerous cognitive, psychological, social, cultural, and environmental barriers to diabetes self-care. And while avoidance of hyperglycemia is the overarching goal of diabetes treatment, avoidance of iatrogenic hypoglycemia is also paramount. Hypoglycemia is acutely disruptive and dangerous, and recent evidence suggests that hypoglycemia may also be associated with adverse long-term outcomes (3).
Common problems encountered by individuals with diabetes include episodes of hyper- and hypoglycemia, demands of the daily regimen, frustration with failure to meet treatment goals, and worries about and adjustment to long-term complications. Rates of self-management behaviors are problematically low, and mood, anxiety, and eating disorders are higher than in non-diabetic populations. Behavioral diabetes research elucidates the interplay of psychological, behavioral, and contextual factors in diabetes management and control, and tests non-pharmacological interventions to improve medical and psychosocial outcomes for patients with diabetes.
Traditional Measures of Glycemia and Glucose
Historically, outside the research laboratory there have been two main methods for assessing diabetes control, i.e., A1c and self-monitoring of blood glucose. A1c (hemoglobin A1c, HbA1c, A1C, or Hb1c) serves as a marker for average blood glucose levels over the previous 4-weeks to 3-months prior to the measurement. A1c assessment is ideally performed with a venous blood draw. The major limitation of A1c is that it is solely a measure of central tendency, not variability, and reflects average blood glucose over a relatively long period of time. As such, it is not sensitive to small or recent changes in glucose and does not reflect glucose variability.
Measurement of short-term glucose changes has largely relied on patient self-monitoring of blood glucose (SMBG) with fingerpricks. SMBG provides a snapshot of current glucose levels, and the main limitation of SMBG data is that blood glucose levels that occur at times when SMBG is not performed are not captured. Participant burden, compliance, and reactivity to glucose data also limit the applications of SMBG to behavioral research.
Continuous Glucose Monitoring
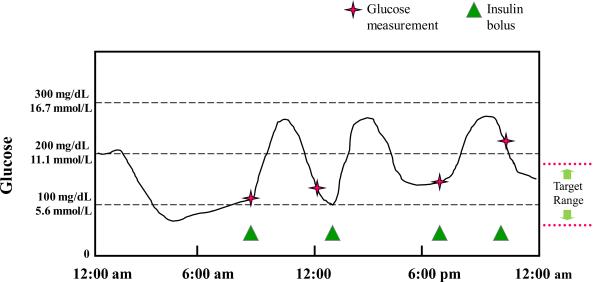
Continuous glucose monitoring systems collect and store glucose data in an ongoing fashion for several days at a time. The systems collect glucose data continuously and allow access to updated averages every few (3–5) minutes. The main advantage of continuous glucose monitoring is that it can help identify fluctuations and trends that would otherwise go unnoticed with A1c or SMBG (see figure 1). Clinically, CGM can be used diagnostically to identify hypoglycemia unawareness, and when paired with a self-management diary, to identify specific self-management problems such as patient reasoning, impulsivity, and misunderstandings. It can also be used therapeutically to reveal diurnal glucose patterns and to provide patients with near-to-real-time glucose values they can use to make minute to minute regimen modifications. Beyond diabetes, CGM shows some promise for management of medical issues including post-stroke hyperglycemia (4), and intensive care of non-diabetic pediatric patients (5).
Figure 1.
Continuous glucose monitoring provides a more comprehensive picture of the patterns.
Although there has been considerable research into the development of CGM technology, only a limited number of devices have been commercialized. To-date non-invasive approaches to measurement of glucose levels have not been successful. The GlucoWatch® Biographer (developed by Cygnus Corporation, California, USA) and Pendra® (developed by Pendragon Medical Ltd., Zurich, Switzerland,) which received regulatory approval in 2001 and 2004 respectively, had limited accuracy and are no longer available commercially.
All of the currently available CGM devices are minimally invasive, and consist of a sensor probe that passes through the skin into the subcutaneous tissue (6). CGM systems can be configured in a “real-time” mode (also known as real-time CGM; RT-CGM) with the device receiver showing continuously updated glucose measurements with direction and rate-of-change information directly to the patient, or a “blind” mode that records the continuous glucose data but does not display the glucose measurements to the patient. The Abbott Freestyle Navigator® (Abbott Diabetes Care, Alameda, California) and DexCom Seven™ (DexCom Inc., San Diego, California) are RT-CGM devices, however professionals who wish to use these systems for blinded data collection can obtain software from the companies to re-configure the device modes. The Medtronic MiniMed (Medtronic Diabetes, Northridge, California) CGM devices are available in blinded or real-time mode: the original MiniMed CGMS and more recent iPro system are blinded devices, whereas the Guardian RT and Paradigm pumps are configured for real-time use. The GlucoDay®S and the newer GlucoMen®Day (A. Menarini Diagnostics, Florence, Italy), which are only available in Europe, can be configured for blinded or real-time use (7).
The currently available CGM devices use two different measurement technologies: the Abbott, DexCom and Medtronic sensors are electrochemical, whereas the Menarini system is based on microdialysis (6). The electrochemical devices use the same technological approach to fingerstick blood glucose monitors, and have a glucose measuring enzyme (glucose oxidase) directly incorporated in the sensor probe. Microdialysis involves the pumping of a buffer solution through the subcutaneous catheter, with measurement of the glucose in the effluent from the catheter by a monitor outside the body. The microdialysis approach may have some potential accuracy advantages (8); however, since microdialysis systems need to incorporate a pump and solution bag the size of these devices are considerably larger than the electrochemical CGM devices, and this could negatively affect patient satisfaction (9).
Unlike the fingerstick glucose monitoring devices in routine use, which measure the glucose concentration in the capillary blood, CGM devices measure the glucose concentration in the interstitial fluid. When the glucose level in the body is stable, the glucose concentration in the interstitial fluid will be similar to that in the blood. However, when the glucose level is changing, there is a delay (lag time) for glucose to diffuse from the blood into the interstitial fluid.
This lag time has important practical implications with regard to the calibration of glucose sensors and the evaluation of CGM device accuracy. Current CGM devices require external calibration, and the user needs to perform periodic fingerstick blood glucose measurements to calibrate the device. If the fingerstick blood glucose measurement used for calibration are taken when the glucose is changing this will shift the calibration curve of the device leading to inaccuracy. The importance of calibrating CGM devices when the glucose level is stable (i.e. the blood and interstitial glucose concentration are in steady state) needs special emphasis in the training of study subjects and patients (10).
This lag time also has research implications. Although it would be desirable from the standpoint of study design and data analysis to factor in a specific lag time between blood and interstitial glucose measurements, unfortunately there are marked inter- and intra-individual differences in lag. This is in part related to the rate of change of the glucose levels, as well as local site differences. Modeling the error in CGM systems has proven to be extremely difficult (11). Thus, the exact timing (in minutes) of the relationship between glucose and a stimulus may be difficult to determine. Yet, with careful reporting, the temporal sequence of glucose and a stimulus, and the approximate timing of their unfolding, can be determined.
Several statistical approaches have been used to evaluate CGM device accuracy (12). All of these approaches are based on comparison of paired sensor interstitial glucose and reference blood glucose measurements which can be problematic if the paired measurements are taken when the glucose is changing (13). Since there are no standardized timing protocols for the evaluation of CGM device accuracy, comparison of reports of different devices can be misleading (14). The accuracy of current glucose sensors is considerably better than first generation devices (15), and the literature suggests that the different devices currently available have comparable accuracy. However, most published reports do not provide data about other performance characteristics (signal stability, frequency of sensor failure, signal attenuation due to pressure on the sensor site leading to false low glucose measurements [more common with DexCom and Medtronic systems], or skipped measurements [which in practice is especially problematic with the DexCom device]) that can affect device utility.
Several numerical measures are commonly used to summarize CGM data. Measures that provide a picture of the deviation in glucose levels from the target range include percent time within 70–180 mg/dL, percent time below 70 mg/dL, and percent time above 180 mg/dL, and area below/above the curve (16). Some CGM devices may give spurious low and high glucose readings, and numerical measures of extreme hypoglycemia (below 50 mg/dL) and hyperglycemia (above 300 mg/dl) calculated from CGM data will sometimes need to be interpreted with caution. CGM data can also be used to obtain a more comprehensive and accurate measure of glycemic variability (17) than can be determined with intermittent SMBG. Since the distribution of glucose values in most individuals with diabetes is not normally distributed, standard deviation (a metric that is computed by most SMBG software) can be an inaccurate measure of glycemic variability. Other measures of glucose variability include pre-prandial to post-prandial change in glucose, coefficient of variation (CV) of glucose, average daily blood glucose range, the M value (18), the lability index (19), and the mean amplitude of glucose excursions (MAGE; 20).
CGM and Behavior
The Potential of Combining CGM and Daily Diary Methods
Daily diary methods, including Ecological Momentary Assessment (EMA; 21) Experience Sampling Methods (ESM; 22) and less intensive daily self-report methods, hold considerable potential when combined with CGM. Although several studies have combined these measurement approaches, it is our impression that much more can be learned from such combined applications, and that much more attention needs to be paid to creating daily diary protocols that can help answer questions of interest.
At the most basic level, obtaining repeated measures of behavior, emotional experience, and exposure to daily stressful encounters provides an opportunity to aggregate reports over the course of a day to create highly reliable indicators (23), and thus allows the investigator to examine whether measured behaviors, experiences and stressful exposures co-vary with glucose levels, and whether such co-variation is more likely among some individuals than others. In the language of multi-level modeling, evaluating individual differences in co-variation involves testing cross-level interactions. Although the ability to examine such daily co-variation is itself a virtue of daily diary methods, by retaining the disaggregated structure of diary reports, diabetes investigators can examine lead-lagged relations between changes in glucose levels or glucose variability, and everyday experience, behavior and stressful encounters, and whether such temporal patterning is more prevalent among some individuals than others. These lead-lagged associations can help investigators draw causal inferences that can be examined with greater control in the laboratory.
Combining CGM and daily diary methods provides at least three other unique opportunities. First, the application of measurement burst strategies (24), where CGM and daily diaries are linked on multiple occasions over time, will allow investigators to evaluate the temporal stability of observed co-variation. Measurement burst methodology can also be invaluable in evaluating developmental changes in temporal patterning. Second, combined CGM-daily diary data can be used to create unique predictor variables that can be studied in relation to longer term diabetes outcomes and end points. Just as person-level characteristics such as gender, race and socioeconomic status may predict longer term outcomes, we suspect that the within-person associations between glucose levels/variability and daily experience hold promise in predicting longer term diabetes outcomes. Finally, despite the considerable success of psychology in developing empirically validated treatments, the assumed mechanisms of action of even its most successful treatments have been called into question (25). We believe that daily diary methods hold considerable promise to increase our understanding of the mechanisms of action of behavioral diabetes interventions. For example, diabetes interventions designed to increase the use of stress management strategies during times of high stress should produce changes in treated patients' ability to employ these strategies on high stress days. This inherently within-person temporal dynamic unfolds over the course of a day or even over several minutes. Yet diabetes researchers almost invariably measure mean levels of coping efficacy, while hypothesizing within-person daily dynamics as the mechanism of change. We encourage diabetes behavioral interventionists to match their elegant within-person temporally unfolding hypotheses with equally elegant daily diary study methods which we believe are uniquely well-suited to test such hypotheses (26).
Challenges of Combining CGM and Daily Diary Reports
Despite the promises of combining CGM and self-reports captured electronic diaries, diabetes investigators face significant challenges and decision points in designing diary protocols that synchronize optimally to CGM. In addition to decisions regarding the appropriate behaviors, experiences and exposures to measure each day, the investigator must decide how often to measure, at what times during the day, and for how many days, balancing data quality and quantity against participant burden. Decisions regarding once daily versus multiple daily reports, and whether to rely on interval-contingent, signal-contingent, or event-contingent recording (27) ultimately turns on the questions being asked, and whether the temporal dynamics of the behaviors, experiences and exposures being studied are thought to fluctuate rapidly (28). Although the nuances of such decisions are beyond the scope of this article, there is one aspect of creating a daily diary protocol that deserves attention here—participant burden. We urge researchers who plan to combine CGM with daily diary reports to limit the length and difficulty of diary assessments, use simple language, limit the number of clauses within questions and the number of response options per question, and minimize the period of retrospection to recent and specific instances (29).
Another consideration is the ability of study participants to faithfully and accurately record the behavioral variables of interest in an ambulatory setting. This is not an issue unique to CGM research, but it is particularly noteworthy here because most CGM systems have an integrated `event' recording system which could be used for this purpose. Data suggest that patient's use of CGM event markers is not sufficient to capture important glucose influencing events (30), so other data capture methods may be preferable. Hand-held electronic devices, telephonic reporting, web-based data entry portals, and paper diaries are all options. Preference for one system over another may depend in part on the frequency and immediacy of reporting, the nature of the data to be reported, the characteristics of the study population, and cost.
A final critical issue in this regard is the curve of glucose change relative to the behavioral variable of interest. Carbohydrate intake will have perhaps the fastest and most pronounced effect on glucose, followed by exercise and medication, which can have variably acute and/or prolonged effects, up to 24 hours. Emotions and stress may have variable, and small, if any, detectable effects on glucose depending upon their intensity, frequency and duration as well as the context in which they occur. Thus, the strategy for summarizing the CGM data vis-à-vis the diary data is dependent upon the phenomenon under investigation.
Measurement Reactivity
Researchers interested in combining real-time physiological measurement such as CGM with self-reports gathered in vivo are often and appropriately concerned that by repeatedly assessing study participants' self-reported thoughts, experiences, emotions and behaviors, they may inadvertently induce change in the self-reported variables. Although this concern warrants ongoing investigation, the potentially biasing effects of instrumentation and procedures on the validity of obtained data, referred to as measurement reactivity, should generate concern for any measurement method (31). Diary methods actually overcome several common sources of measurement reactivity associated with self-report methods, most notably by limiting the recall error generated by the schema-based processing typically created when research participants are required to recall experiences and behaviors that occurred days, weeks or months ago.
The self-monitoring and daily diary literatures have identified several factors that can create measurement reactivity, including awareness of and reflection upon the targeted behaviors or experiences, an individual's motivation to change the measured behavior, the desirability of the targeted behavior, explicit or implicit demands for change, the number of behaviors monitored (monitoring one behavior is more reactive than monitoring several behaviors), the sequence of monitoring (recording a behavior before it occurs creates a higher likelihood of reactivity than recording the behavior after it occurs), and feedback or opportunities to review previous diary entries. Fortunately, these reactivity inducing features can be minimized through the use of carefully crafted daily diary protocols and electronic data capture.
Overall, whereas the reactive nature of diary methods has been assumed, reviews of the literature have consistently painted a more nuanced picture. Although we caution investigators planning to integrate CGM and diary methods to pay close attention to potential measurement reactivity, there is rather clear evidence that when previous records are not accessible, multiple behaviors, cognitions and emotional states are monitored, and demand for change is minimized, reactivity to even very intensive monitoring, such as EMA, is reduced (32).
Reactivity to glucose (as opposed to diary) data may be an issue with CGM, given that even with blinded systems, participants must calibrate the CGM system using observable fingerstick glucose levels. To the degree that the frequency of calibrations exceeds the frequency of a participant's typical SMBG, there may be increased awareness of and attention to glucose levels. Notwithstanding, research that uses SMBG for collection of glucose data has exactly the same problem, and the benefit of continuous glucose data is generally thought to outweigh any potential reactivity to calibrations.
The Current Range of Applications of CGM to Behavioral Research
Because CGM is relatively new and many studies using it have not yet published findings, in addition to searching PubMed for relevant manuscripts using the search term `continuous glucose monitor', we also searched the National Institutes of Health RePORTER database at http://projectreporter.nih.gov/reporter.cfm, and contacted the listserves of North American and European professional societies of behavioral diabetes researchers requesting information on studies currently underway. The review below is a representative, but not exhaustive, description of the ways in which CGM is being used in behavioral research. We found that CGM is being used 1) to study basic bio-behavioral processes among experiences, exposures, behaviors, and glucose, 2) to measure the effect of behavioral interventions on glucose, and 3) as a behavior modification and teaching tool in diabetes self-management interventions.
CGM for Studying Basic Bio-behavioral Processes
The most basic use of CGM in studying bio-behavioral processes is to use CGM as a short-term measure of glycemic control, and to link glycemic control to time-invariant reports of behavior. For example, McDonnell et al. (33) studied externalizing behaviors and glucose in pediatric type 1 diabetes patients. Subjects wore CGM for 72 hours, and parents completed a standard assessment of their child's behavior at two time points. Externalizing scores were positively associated with percent of time spent in the hyperglycemic range, negatively associated with the percent of time spent in the euglycemic range, and unrelated to percent of time spent in the hypoglycemic range. However, externalizing behaviors were assessed retrospectively, and the investigators could not test for directionality.
The continuous aspect of continuous glucose monitoring can also be exploited to study real-time or near-to-real-time influences on, and consequences of, glucose. In daily studies, CGM glucose data can be collected in tandem with reports of daily experiences. This allows researchers to characterize the temporal unfolding of basic bio-behavioral processes. For example, the relationship between mental stress and glucose has been studied for several decades. Controlled trials show a benefit of stress management on A1c, suggesting that stress does in fact have a deleterious effect on glucose (34). Yet, laboratory studies investigating the effects of experimental stressors on glucose have been inconsistent, perhaps because of the brief, contrived, and decontextualized nature of laboratory stressors. CGM offers the opportunity to investigate the relationship between stress and glucose in an ecologically valid manner.
Exposure to racism has recently been identified as a source of mental stress that is associated with poor health outcomes such as hypertension and blood pressure reactivity to stress. We investigated the relationship between self-reported racism and glucose among 80 women with T2DM. Participants completed the Schedule of Racist Events (SRE; 35) which asks respondents to rate the frequency and stressfulness of racist events over their lifetime. Participants wore blinded CGM for 72 hours. We found that higher lifetime racism predicted higher average glucose over 72 hours, even after controlling for same-day medication compliance.
Our working model suggested that self-reported lifetime racism is not only associated with higher average glucose levels, but also with glucose reactivity to mental stress of any type – racial or non-racial. We are currently extending the finding reported above by investigating whether higher lifetime racism is associated with greater glucose reactivity to mental stress. Here we report preliminary findings from nine African American women with T2DM. After completing the SRE these women were fitted with blinded CGM and they were instructed in the use of an interactive voice response (IVR) system, which they used to report twice-daily experiences of stressors and diabetes self-care behaviors for 7 days. The twice daily IVR reports were date and time stamped, and participants were locked out of the system if they did not report during the reporting windows, noon-4pm and 8–11pm.
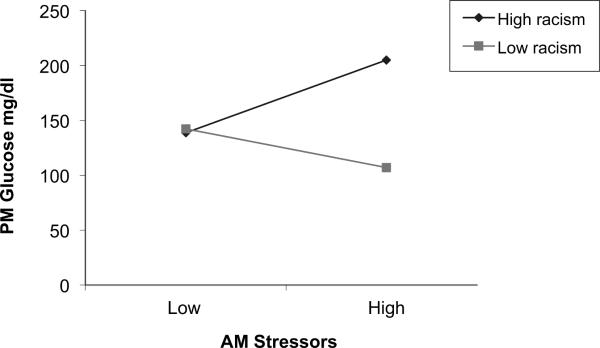
In this preliminary analysis, we examined how lifetime racism and IVR-reported morning daily stressors interacted in predicting glucose levels later in the day after controlling for morning glucose levels. For parsimony, we calculated a single racism summary z-score comprised of lifetime frequency and stressfulness which we refer to as `zracism' in figure 2. We also standardized the remaining predictor to obtain standardized coefficients (betas) in our multilevel model. Results for the interaction indicated a trend (p = .12) consistent with the prediction that racism will exacerbate the effect of stress on glucose levels. The form of this effect is shown in figure 2; simple effects tests revealed that women who reported greater lifetime racism (1 SD above the mean),, showed a significant positive association (beta = .30, p = .02, 95% CI: .04 to .54) between morning stress and glucose later in the day. For women reporting low lifetime racism (1 SD below the mean), morning stressors were unrelated to later day glucose (beta = −.15, p = .49, 95% CI: = −.57 to .28).
Figure 2.
CGM glucose reactivity to stressors varies by self-reported lifetime racism.
Several studies have demonstrated that stress management interventions improve A1c (e.g., 34). However, it is not known whether the intervention decreases only average glucose levels, or whether the intervention actually decreases glucose reactivity to mental stress. A new randomized controlled trial (36) is evaluating the effects of a stress management intervention on glucose and glucose reactivity to stress among Latinos with T2DM. To determine the intervention's effects on glucose reactivity to stress, participants will wear blinded CGM for 72 hours at baseline, and again post-treatment while reporting daily experiences of stressors. We hypothesize that stress reactivity will decrease from baseline to post-stress for the intervention group, and that decreased stress reactivity at post-test will predict improved A1c at 6 month follow-up.
The association between mood and glucose has also been the topic of ongoing investigation. Hermanns et al. (37) fitted patients with T1DM with blinded CGM for 49 hours. During the CGM recording time, participants rated their current mood states 15 times using hand held computers, and they described their mood using an adjective checklist. Results show that high glucose values had a negative impact on mood, yet glucose variability was not associated with subsequent mood ratings. Also using EMA, Fritschi and colleagues (38) are examining the associations among baseline depressive symptoms, physical activity collected via accelerometers, and glucose gathered from CGM among patients with T2DM.
In addition to being used to examine the relationship of glucose to stress and mood, CGM is particularly useful for examining the relationship of glucose to eating behaviors, hypoglycemia, and sleep.
Foods can have a profound and quite variable effect on glucose. Nansel and colleagues have conducted a series of small studies comparing the effects of low vs. high glycemic meals on glucose using blinded CGM. Laboratory (39) and ambulatory feeding studies in youth with diabetes (40) suggest that a low glycemic index diet may improve post-prandial glucose control. Patton (41) is conducting a series of studies examining children's mealtime behaviors and post-prandial glucose excursions and hypoglycemia as measured by CGM. Barbor and colleagues (42) are using CGM to compare nutrition protocols in women with gestational diabetes, pairing post-prandial glycemic profiles with lipemia profiles, and relating these parameters to neonatal outcomes.
CGM is particularly useful for studying hypoglycemia, because it can detect hypoglycemic episodes that occur outside the patient's awareness. Munshi and colleagues (43) used blinded CGM in an elderly sample and observed high frequency and long duration of hypoglycemic episodes unrecognized by patients (not captured by SMBG or by subjective symptoms). Fifty percent of the patients with hypoglycemia had an A1c >9% suggesting that simply relaxing the A1c goal will not be sufficient to reduce the incidence of hypoglycemia in the elderly. Rizzo et al. (44) found that greater glucose variability, as measured by mean amplitude of glucose excursions (MAGE) over 48 hours of CGM, predicted worse scores on neurocognitive tests independent of multiple controls including fasting plasma glucose, post-prandial glucose, and A1c. Another study currently underway by Tsalikian and colleagues (45) is using CGM to relate hypoglycemia to neurocognitive and neuroimaging parameters in youth with T1DM.
CGM is also an excellent tool for sleep researchers because CGM unobtrusively measures glucose during sleep. Bialasiewicz et al. (46) used CGM to examine the effects of sleep disordered breathing on the normal decline in glucose observed during rapid eye movement (REM) sleep. They found that the occurrence of disordered breathing during REM reversed the glucose decline, whereas in non-REM sleep disordered breathing had no effect on glucose. Using CGM, Berndt-Zipfel et al. (47) reported that waking up in response to an alarm-clock leads to an arousal reaction that causes significant elevations in glucose concentrations compared to undisturbed sleep or being awakened by a person. Two other studies (48,49) are currently using CGM to investigate associations between sleep disturbance and glucose, and to evaluate whether continuous positive airway pressure normalizes glucose.
Perhaps the most exciting research use of CGM is its potential role in `closing the loop' between glucose sensing and insulin delivery, i.e., the development an artificial pancreas. Kovatchev and colleagues (50) are developing incremental modules for a closed loop system, including a behavioral observer that will track over time key recurrent elements of a patient's glucose-relevant behaviors. This module will ultimately be linked to a physiological observer and advisory and/or controlling systems. This line of research not only holds potential for a major clinical breakthrough, but may also yield novel information about patient behavior and decision making.
Measuring the Effect of Behavioral Interventions on Glucose
Another use of CGM in behavioral research is as an alternative to A1c or SMBG for assessing the glycemic effect of behavioral interventions. Several such studies are underway. For example, Streisand and colleagues are currently evaluating the efficacy of a parenting support program for parents of young children with T1DM (51) on glucose levels collected with CGM at baseline and again at post-treatment. CGM is also being used to investigate the glycemic effects of pre-meal insulin bolus timing protocols in T1DM (52) and physical activity protocols in older patients with impaired glucose tolerance (53).
In some intervention studies, clinical use of CGM itself is the focus of a behavioral intervention. For example, Shaw and colleagues are using CGM in a trial to determine if prevention of recurrent hypoglycemia, through use of CGM, restores hypoglycemia awareness (54). RT-CGM is the intervention, and glucose levels collected via retrospective CGM serve as the outcome. Laffel and colleagues (55), as well as Wysocki and colleagues (56), are testing family focused behavioral interventions designed to optimize CGM use in pediatric patients. Because these designs emulate clinical practices, each patient selects the commercially available CGM device that meets their needs and uses RT and retrospective analyses of the CGM data as appropriate. CGM as a Behavior Modification and Teaching Tool in Diabetes Self-management Interventions
Several researchers have exploited the potential of CGM as a motivational tool to improve self-management behaviors. For example, Allen et al. (57) fitted adults with T2DM with and blinded CGM and accelerometers for 72 hours and were instructed to enter meal, exercise, and medication events. Data were subsequently reviewed with each participant to demonstrate the effects of physical activity and nutrition on their own glucose levels. They found that the CGM data produced numerous potential teaching opportunities, and also increased physical activity, and decreased BMI and A1c, even after controlling for medication changes.
Practical Issues
While CGM allows researchers to ask novel and nuanced questions, there are several practical issues about which any researcher interested in using CGM should be aware.
Shelf Life
Depending on the commercial system, sensors have variable shelf-life (usually in the range of several months), requiring that the researcher anticipate recruitment flow and order sensors in batches. Similarly, some systems have an integrated battery that has time-limited shelf-life and recharging capability. Depending upon the commercial system, a single CGM recorder may not last the duration of, say, a 5-year study, so replacement costs should be factored into budgets.
Advances in Technology
As CGM technology continues to evolve, new systems are coming onto the market, and older systems are being replaced by newer generations. CGM software is typically more rapidly updated than CGM hardware. Furthermore, systems are occasionally recalled. Converting to a new system during the course of a study can be problematic if the algorithm for converting interstitial fluid to glucose readings differs between systems. Our team experienced this problem several years ago, and the situation temporarily interrupted data collection.
Loss of Data
Data may be lost for various reasons (e.g., including sensor failure, download failure, lack of calibration, user error). While any one of these may be a relatively infrequent event, their combination could potentially result in substantial amounts of missing data. Reasonable precautions can prevent many including checking the integrity of the system and replacing batteries before each use, proper training of participants, frequent participant prompts for calibrations, take home handouts with simple instructions and a phone number to call with any problems, and encouragement and incentives for wearing the sensor for the full recording period.
Data Analysis
Each CGM system generates its own summary report which is clinically useful, but the data it provides may be too crude to answer most research questions. CGM systems also generate raw data files in spreadsheet or comma separated value (CSV) formats which can be imported to most commercially available statistical software. Yet, as with other frequently measured physiological data, the researcher is faced with handling a large amount of raw data, on the order of several hundred glucose readings per 24 hours per participant. The best approach for data reduction is highly idiosyncratic to the research question at hand.
Insertion and Removal
Medical personnel are not required for sensor insertion or removal. However, patients naïve to CGM may not be comfortable doing these tasks themselves. Our experience is that a research assistant can be trained to insert the device using universal precautions and care with the sharps, and then participants can be trained to calibrate it at home and remove the subcutaneous sensor. This allows flexibility with follow-up visits to collect the recorder and download data. While infection at sensor sites is rare, it is a risk, so confirmation of the fact that a participant has removed the sensor at the end of the study is desirable.
Calibration
Interstitial glucose levels and blood glucose levels are less concordant when glucose levels are changing. Thus participants should be instructed to calibrate when blood glucose levels from SMBG are stable, i.e., not during or after exercise, and not within 2 hours of a meal. Sensor lots also vary with regard to accuracy. However, unfortunately there is no published reference data that can be used by investigators to determine the performance of specific sensor lots.
Participants
Another consideration is whether the population of interest may have unique challenges with CGM. Our work with African American adult women with T2D showed that on a scale from 1–10, with 1= `extremely easy' and 10= `extremely difficult,' participants rated the difficulty of using the CGM as mean=1.2. Other studies similarly show high acceptability from pediatric research participants and their parents (41,58). However, participants with low numeracy, low vision, memory deficits, or impaired manual dexterity may have difficulty performing the required tasks. Increasing age and discomfort with technology may also be an impediment (30).
Future Directions
Reporting Guidelines
Stone and Shiffman (59) have offered thoughtful reporting guidelines for daily diary studies. Behavioral research using CGM will benefit from uniformity in reporting in order to increase interpretability of reports and to allow comparison across studies. Below we suggest information that should be included in reports of behavioral CGM studies. Our suggestions are not exhaustive, and we encourage their revision as the technology changes and the field progresses.
Details of CGM system used: Brand, model, and whether blinded or RT-CGM is used.
CGM data collection procedure: Density and scheduling of CGM, participant training, details of the CGM data acquisition (e.g., from raw data or from system generated reports); changes in CGM system over the course of data collection.
Quality of data: Ratio of number of glucose readings to number of expected glucose readings; number of calibrations; number of paired sensor-meter readings; correlation between sensor and meter readings.
Glucose metric employed: Specific information about the metric of glucose control (e.g., percent of time within range) and glucose variability (e.g., mean amplitude of glucose excursions) employed for analyses.
Potential Applications
Below we suggest potential research applications that would further our understanding of the interplay of psychosocial phenomena and glucose. First, examine the relationships among different types of experiences and exposures and glucose. Determine the rate and direction of change, the duration of the response, and the characteristics of patients that render them vulnerable to such effects on glucose. Second, employ CGM to elucidate patient problem solving and decision making processes. CGM may be helpful in characterizing the ways in which patients use data to make day to day treatment decisions. Third, use CGM to demonstrate active ingredients of behavioral interventions. In order to demonstrate the mechanisms of action of behavioral interventions, the target behavior must be measured during the timeframe of behavioral contingencies and linked to subsequent glucose. Fourth, within parameters of acceptable participant burden, combine CGM with other ambulatory monitoring such as ambulatory blood pressure monitors, holter monitors, and accelerometers. Fifth, consider data from more than one respondent simultaneously. CGM data from the participant with diabetes, combined with paired self-reports from the participant with diabetes and his/her parent, spouse, or caretaker, may yield rich information regarding social processes (e.g., 60).
Moving beyond CGM's application to the study of diabetes, increasing evidence points to the possibility that low levels of glucose and poor glucose tolerance are associated with compromised self-control. Gailliot and Baumeister (61) reviewed published evidence that self-control, whether in the form of dietary restraint, coping with stress, resisting impulsive behaviors, abstaining from alcohol or other effortful controlled processes, impairs subsequent self-regulation efforts, and they summarize findings documenting that these various manifestations of self-control consume large amounts of glucose. Yet the mechanisms through which glucose affects self-control, and whether certain individuals are uniquely vulnerable to glucose related self-control impairments are not known. Although the most convincing evidence of linking self-control to blood glucose currently comes from laboratory studies (e.g., 62), CGM linked to repeated within-day reports of various self-control efforts in daily life could provide a unique opportunity to examine the dynamic temporal interplay of self-control and glucose in real time, documenting `when-then' sequences (26) within individuals, while simultaneously addressing whether some individuals show a particularly strong association between self-control and poor glucose tolerance, and whether others are resilient. A clear clinical application of CGM in clarifying the association between self-control and glucose is in the study of bulimia, where dietary restraint is thought to play a central role (63). Although the association between restrained eating and subsequent overeating has not received consistent support (e.g.,64) the theoretical prominence of chronic dietary restraint in eating disorders, and the promising support for Gailliot and Baumeister's (61) hypothesis that poor glucose tolerance is associated with compromised self-control, suggests that CGM may find an important place in the eating disorders literature.
Conclusions
CGM holds promise to unravel the complex relationships among glucose and intrapersonal, interpersonal, and contextual factors. However, the uptake of CGM for this purpose is limited, and the possibilities for its use are largely unmet, in part because its use requires the management of numerous practical issues (65). We encourage behavioral researchers to implement CGM in their protocols, and to do so in a way that maximizes its explanatory power.
Abbreviations
- CGM
continuous glucose monitoring
- SMBG
self-monitoring of blood glucose
Footnotes
Conflicts of interest: Dr. Wolpert is a paid consultant for Abbott Diabetes Care
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 3.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S, ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 4.Allport LE, Baird TA, Davis SM. Hyperglycaemia and the ischaemic brain: Continuous glucose monitoring and implications for therapy. Curr Diabetes Rev. 2008;4:245–57. doi: 10.2174/157339908785294433. [DOI] [PubMed] [Google Scholar]
- 5.Allen HF, Rake A, Roy M, Brenner D, McKiernan CA. Prospective detection of hyperglycemia in critically ill children using continuous glucose monitoring. Pediatr Crit Care Med. 2008;9:153–8. doi: 10.1097/PCC.0b013e3181668b33. [DOI] [PubMed] [Google Scholar]
- 6.McGarraugh G. The chemistry of commercial continuous glucose monitors. Diabetes Technol Ther. 2009;11(Suppl 1):S17–24. doi: 10.1089/dia.2008.0133. [DOI] [PubMed] [Google Scholar]
- 7.Valgimigli F, Lucarelli F, Scuffi C, Morandi S, Sposato I. Evaluating the clinical accuracy of GlucoMen®Day: A novel microdialysis-based continuous glucose monitor. J Diabetes Sci Technol. 2010;4:1182–92. doi: 10.1177/193229681000400517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wentholt IM, Vollebregt MA, Hart AA, Hoekstra JB, DeVries JH. Comparison of a needle-type and a microdialysis continuous glucose monitor in type 1 diabetic patients. Diabetes Care. 2005;28:2871–2876. doi: 10.2337/diacare.28.12.2871. [DOI] [PubMed] [Google Scholar]
- 9.Kubiak T, Worle B, Kuhr B, Nied I, Glasner G, Hermanns N, Kulzer B, Haak T. Microdialysis-based 48-hour continuous glucose monitoring with GlucoDay: Clinical performance and patients' acceptance. Diabetes Technol Ther. 2006;8:570–575. doi: 10.1089/dia.2006.8.570. [DOI] [PubMed] [Google Scholar]
- 10.Wolpert HA. The nuts and bolts of achieving end points with real-time continuous glucose monitoring. Diabetes Care. 2008;31(Suppl 2):S146–149. doi: 10.2337/dc08-s238. [DOI] [PubMed] [Google Scholar]
- 11.Facchinetti A, Sparacino G, Cobelli C. Modeling the error of continuous glucose monitoring sensor data: critical aspects discussed through simulation studies. J Diabetes Sci Technol. 2010;4:4–14. doi: 10.1177/193229681000400102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke WL, Kovatchev B. Continuous glucose sensors: Continuing questions about clinical accuracy. J Diabetes Sci Technol. 2007;1:669–675. doi: 10.1177/193229680700100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taub MB, Peyser TA, Erik Rosenquist J. Numerical simulation of the effect of rate of change of glucose on measurement error of continuous glucose monitors. J Diabetes Sci Technol. 2007;1:685–694. doi: 10.1177/193229680700100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King C, Anderson SM, Breton M, Clarke WL, Kovatchev BP. Modeling of calibration effectiveness and blood-to-interstitial glucose dynamics as potential confounders of the accuracy of continuous glucose sensors during hyperinsulinemic clamp. J Diabetes Sci Technol. 2007;1:317–322. doi: 10.1901/jaba.2007.1-317. idiographic approach to personality. Soc Personal Psychol Compass 20091;3:292–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31:1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther. 2009;11:S-45–S-54. doi: 10.1089/dia.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11:551–65. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 18.Schlichtkrull J, Munck O, Jersild M. The M-value, an index of blood glucose control in diabetics. Acta Med Scand. 1965;177:95–102. doi: 10.1111/j.0954-6820.1965.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 19.Ryan EA, Shandro T, Green K, Paty BW, Senior PA, Bigam D, Shapiro AMJ, Vantyghem MC. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 20.Service FJ, Molner GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 21.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 22.Conner TS, Barrett LF, Tugade MM, Tennen H. Idiographic personality: The theory and practice of experience sampling. In: Robins RW, Fraley RC, Kreuger R, editors. Handbook of Research Methods in Personality Psychology. Guilford Press; New York: 2007. pp. 79–96. [Google Scholar]
- 23.Epstein S. The stability of behavior: I. On predicting most of the people much of the time. J Pers Soc Psychol. 1979;37:1097–1126. [Google Scholar]
- 24.Sliwinski MJ. Measurement-burst designs for social health research. Soc Personal Psychol Compass. 2008;2:245–261. [Google Scholar]
- 25.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 26.Conner TS, Tennen H, Fleeson W, Barrett LF. Experience sampling methods: A modern idiographic approach to personality research. Soc Personal Psychol Compass. 2009;3:292–313. doi: 10.1111/j.1751-9004.2009.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler L, Reis HT. Self-recording of everyday life events: Origins, types, and uses. J Pers. 1991;59:339–354. [Google Scholar]
- 28.Tennen H, Affleck G, Coyne JC, Larsen RJ, DeLongis A. Paper and plastic in daily diary research: Comment on Green, Rafaeli, Bolger, Shrout, and Reis. Psychol Methods. 2006;11:112–118. doi: 10.1037/1082-989X.11.1.112. [DOI] [PubMed] [Google Scholar]
- 29.Reis HT, Gable SL. Event sampling and other methods for studying everyday experience. In: Reis HT, Judd CM, editors. Handbook of research methods in social and personality psychology. Cambridge University Press; New York: 2000. pp. 190–222. [Google Scholar]
- 30.Allen N, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring in non-insulin-using individuals with type 2 diabetes: acceptability, feasibility, and teaching opportunities. Diabetes Technol Ther. 2009;11(3):151–8. doi: 10.1089/dia.2008.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb EJ, Campbell DT, Schwartz RD, Sechrest L. Unobtrusive measures: Nonreactive research in the social sciences. Rand McNally; NY: 1966. [Google Scholar]
- 32.Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain. 2003;104:343. doi: 10.1016/s0304-3959(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 33.McDonnell CM, Northam EA, Donath SM, Werther GA, Cameron FJ. Hyperglycemia and externalizing behavior in children with type 1 diabetes. Diabetes Care. 2007;30:2211–5. doi: 10.2337/dc07-0328. [DOI] [PubMed] [Google Scholar]
- 34.Surwit RS, van Tilburg MA, Zucker N, McCaskill CC, Parekh P, Feingolos MN, Edwards CL, Williams P, Lane JD. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34. doi: 10.2337/diacare.25.1.30. [DOI] [PubMed] [Google Scholar]
- 35.Landrine H, Klonoff EA. The Schedule of Racist Events: A measure of racial discrimination and a study of its negative physical and mental health consequences. J Black Psychol. 1996;22:144–168. [Google Scholar]
- 36.Perez-Escamilla R, Wagner J. Stress management among Latinos with type 2 diabetes. R01 MD005879-01. Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=8147012&icde=11927609.
- 37.Hermanns N, Scheff C, Kulzer B, Weyers P, Pauli P, Kubiak T, Haak T. Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia. 2007;50:930–3. doi: 10.1007/s00125-007-0643-y. [DOI] [PubMed] [Google Scholar]
- 38.Fritschi CJ. Momentary biobehavioral effects on physical activity in adults with T2 diabetes. 1K99NR012219-01. Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=7957872&icde=0.
- 39.Nansel TR, Gellar L, McGill A. Effect of varying glycemic index meals on blood glucose control assessed with continuous glucose monitoring in youth with type 1 diabetes on basal-bolus insulin regimens. Diabetes Care. 2008;31:695–7. doi: 10.2337/dc07-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rovner AJ, Nansel TR, Gellar L. The effect of a low-glycemic diet vs a standard diet on blood glucose levels and macronutrient intake in children with type 1 diabetes. J Am Diet Assoc. 2009;109:303–7. doi: 10.1016/j.jada.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patton SR, Williams LB, Eder SJ, Crawford MJ, Dolan L, Powers SW. Use of continuous glucose monitoring in young children with type 1 diabetes: implications for behavioral research. Pediatr Diabetes. 2011;12:18–24. doi: 10.1111/j.1399-5448.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbour L. Role of macronutrient diet composition on maternal and infant metabolic outcomes. 1R21DK088324-01. http://projectreporter.nih.gov/project_info_details.cfm?aid=8063883&icde=11927695.
- 43.Munshi MN, Suhl E, Sternthal A, Giusti J, Staum E, Lee Y, McCartney R, Desrochers L, Bonsignore P, Segal A. Frequent hypoglycemia among older adults with A1c>8% detected by continuous glucose monitoring. American Diabetes Association Abstract Book; 2010. Abstract Number: 123-OR. [Google Scholar]
- 44.Rizzo MR, Marfella R, Barbieri M, Boccardi V, Vestini F, Lettieri B, Canonico S, Paolisso G. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33:2169–74. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsalikian E. Prevention of hypoglycemia and associated complications in type 1 diabetes. 5U10HD041915-09. Available at: http://projectreporter.nih.gov/project_info_details.cfm?aid=8120958&icde=11927854.
- 46.Bialasiewicz P, Czupryniak L, Pawlowski M, Nowak D. Sleep disordered breathing in REM sleep reverses the downward trend in glucose concentration. Sleep Med. 2011;12:76–82. doi: 10.1016/j.sleep.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Berndt-Zipfel C, Köthe L, Nawrodt B, Mraz B, Patzelt-Bath A, Nauck MA. Glycaemic rises after waking up in response to an alarm clock in type 1-diabetic patients analysed with continuous glucose monitoring (GlucoDay® S) Exp Clin Endocrinol Diabetes. 2011;119:56–8. doi: 10.1055/s-0030-1265162. [DOI] [PubMed] [Google Scholar]
- 48.Chasen ER. OSA, sleepiness, and activity in diabetes management. 5R21HL089522-02. Availabile at: http://projectreporter.nih.gov/project_info_description.cfm?aid=7789449&icde=11927916.
- 49.Tasali E. Slow wave sleep and type 2 diabetes risk in aging. 5P01AG011412-14; subproject 8400. Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=8245077&icde=11928007.
- 50.Kovatchev BP. Modular bio-behavioral closed-loop control of type 1 diabetes. 5R01DK085623-02. Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=8137258&icde=11927986.
- 51.Streisand R. Parenting and control among young children with T1 diabetes. 5R01DK080102-03 Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=8054224&icde=11928059.
- 52.Schade DS. The importance of insulin timing in type 1 diabetes. 5M01RR000997-35; subproject 6492. [Google Scholar]
- 53.DiPietro L. Post-meal exercise and glycemic control imaging. 5R21AG031550-02. http://projectreporter.nih.gov/project_info_description.cfm?aid=7686781&icde=11928132.
- 54.Shaw J. Prevention of recurrent severe hypoglycaemia: A definite RCT comparing optimized MDI and CSII with our without adjunctive real-time continuous glucose monitoring. DRN 430. http://www.ukdrn.org/eastern/hypocompass.html.
- 55.Laffel LM. Optimizing CGM use and metabolic outcomes in youth with type 1 diabetes. 1R01DK089349-01A1. Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=8147728&icde=11928230.
- 56.Wysocki T. Use of continuous glucose sensors in adolescents with inadequate diabetic control. R01-DK080831. Available at: http://projectreporter.nih.gov/project_info_description.cfm?aid=8207299&icde=11928270.
- 57.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: A randomized clinical trial. Diab Res Clin Pract. 2008;80:371–9. doi: 10.1016/j.diabres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin RR, Peyrot M. Patient-reported outcomes and diabetes technology: a systematic review of the literature. Pediatr Endocrinol Rev. 2010;7(Suppl 3):405–12. [PubMed] [Google Scholar]
- 59.Stone AA, Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. Ann Behav Med. 2002;24:236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- 60.Iida M, Seidman G, Shrout PE, Fujita K, Bolger N. Modeling support provision in intimate relationships. JPSP. 2008;94:260–78. doi: 10.1037/0022-3514.94.3.460. [DOI] [PubMed] [Google Scholar]
- 61.Gailliot MT, Baumeister RF. The physiology of willpower: Linking blood glucose to self-control. Personality Social Psychology Review. 2007;11:303–327. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- 62.Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM, Brewer LE, Schmeichel BJ. Self-control relies on glucose as a limited energy source: Willpower is more than metaphor. JPSP. 2007;92:325–6. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- 63.Stice E. A prospective test of the dual-pathway model of bulimic pathology: Mediating effects of dieting and negative affect. J Abnorm Psychol. 2001;110:124–35. doi: 10.1037//0021-843x.110.1.124. [DOI] [PubMed] [Google Scholar]
- 64.Van Strien T, Engels RCME, Van Leeuwe J, Snoek HM. The Stice model of overeating: Tests in clinical and non-clinical samples. Appetite. 2005;45:205–13. doi: 10.1016/j.appet.2005.08.004. 2005. [DOI] [PubMed] [Google Scholar]
- 65.Heinemann L. Continuous glucose monitoring and clinical trials. Diabetes Sci Technol. 2009;3:981–5. doi: 10.1177/193229680900300447. [DOI] [PMC free article] [PubMed] [Google Scholar]