Summary
We aimed to show the anatomical relationship between a dissecting aneurysm of the posterior cerebral artery (PCA) and tentorial free edge to understand the pathophysiologic mechanism. A 52-year-old woman with a history of head trauma presented with dizziness and numbness in her left fingers. 3D DSA showed a dissecting aneurysm of the right P2-P3 segment of PCA.
The fusion of 3D DSA and 3T MRI was performed at the dedicated workstation using three pairs of landmarks including the ICA termination, MCA bifurcation and A1-A2 junction of the right ACA. Fusion of 3D DSA and 3T MRI clearly demonstrated the dissected segment of PCA crossed the tentorial free edge twice.
The fusion images support the direct trauma hypothesis of dissecting aneurysm of the P2-P3 segment of PCA. This novel imaging technique shows future potential to be used to understand the anatomical relationships between various vascular lesions and surrounding structures.
Key words: 3T MRI, 3D DSA, dissecting aneurysms, image fusion, posterior cerebral artery
Introduction
Direct trauma such as basal skull fracture may result in arterial dissection, but the mechanism of intracranial arterial dissections from minor, indirect or remote trauma is still unclear22. We report a case of a dissecting aneurysm of the PCA with a remote history of minor head trauma demonstrating 3D relationships between the dissecting aneurysm and tentorial free edge using 3 Tesla MRI (3T MRI) and 3D Digital Subtraction Angiography (DSA) fusion technology. By correlating the elegant anatomy demonstrated with the fusion technique and the microsurgical anatomy of the PCA, we propose that direct trauma of the PCA against the free edge of the tentorium is one of etiological factors of PCA dissection. This study also highlights the clinical usefulness of a fusion technique using 3-Tesla magnet and 3D DSA. To our knowledge, this is the first report of clinical application of a 3-Tesla MRI image and 3D DSA fusion technique.
Case Report
A 52-year-old woman presented with dizziness and several episodes of numbness in the fingers of her left hand. About ten years ago, she had fallen from a second story building, hitting her back and buttocks when she landed on the concrete floor. There was no recorded head injury. The patient had been well until she presented ten years later with above symptoms.
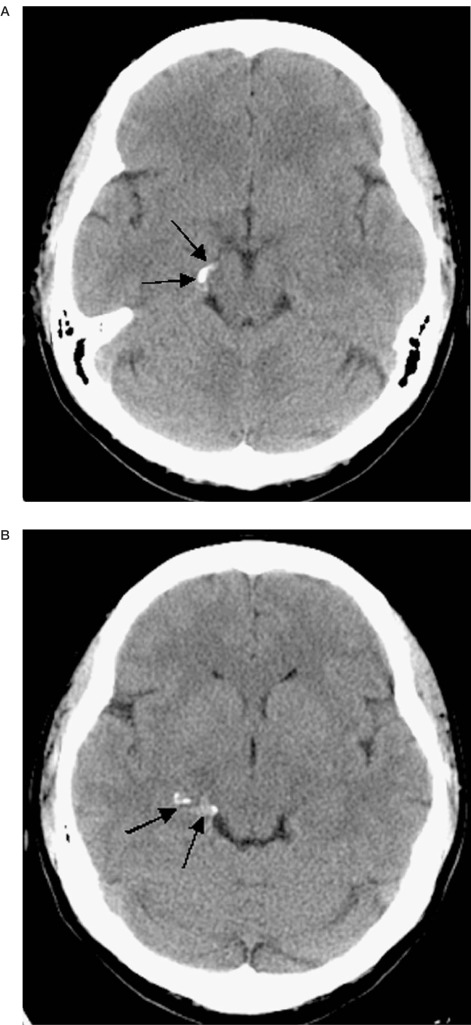
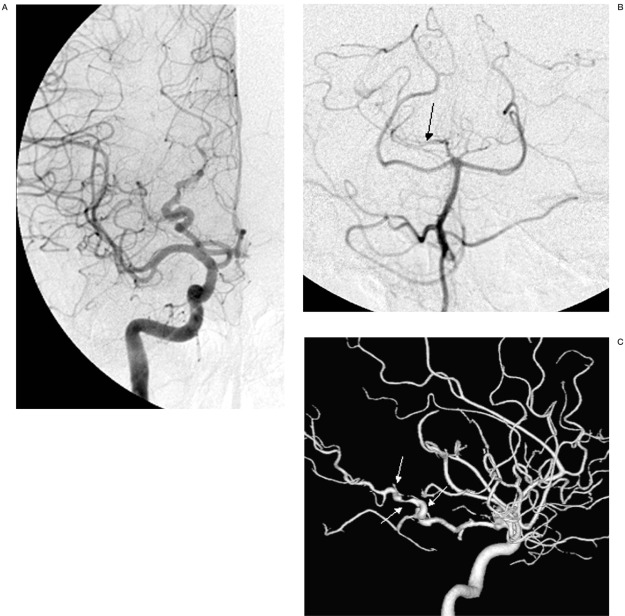
Brain CT, 3 Tesla MRI and 3D DSA were performed. The imaging studies revealed a dissecting aneurysm of the right posterior cerebral artery (PCA). CT scan demonstrated calcification in the tentorial hiatus in the region of the free edge of the tentorium cerebelli on the right side (figure 1A,B). Standard and 3D DSA demonstrated typical findings of dissection of the P3 segment of the right PCA extending from the inferior temporal branch to the origins of the parieto-occipital and calcarine arteries (figures 2A-C).
Figure 1.
A,B) Serpiginous calcification (arrows) is shown in the right tentorial hiatus corresponding to the course of the PCA.
Figure 2.
Selective injections of the right internal carotid artery (A) and right vertebral artery (B) and 3D DSA shaded surface display (SSD) reconstruction (C) demonstrate a series of alternating dilatation and segmental narrowing of the P3 segment of the right PCA (pearl and string sign, white arrows). The right vertebral artery injection (B) shows washout of the contrast media (arrow) in the right PCA due to un-opacified bloods from right posterior communicating artery.
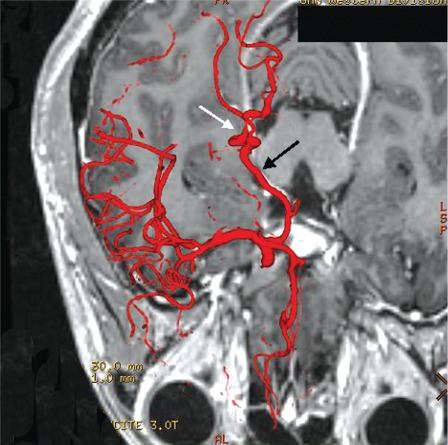
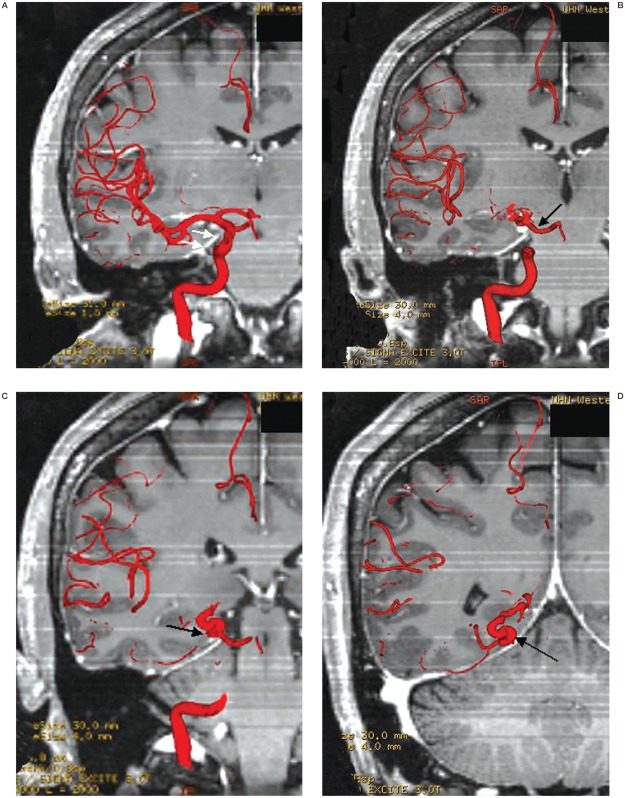
Image fusion techniques were then applied to clarify the anatomical relationship between the dissected PCA and tentorial edge. 3T MRI and 3D DSA fusion images clearly showed the tortuous P3 segment of the right PCA crossing the free edge of the tentorium twice. It also clearly defined the anatomy where the segment is impinged against the free edge (figures 3, 4A-D).
Figure 3.
Axial fusion image clearly showing the normal P2 segment of the right PCA located between the cerebral peduncle and the tentorium. The abnormal P3 segment (white arrow) is seen to cross the free edge twice from medial to lateral proximally and lateral to medial more distally. Note that there is nearly perfect registration of the fused DSA image to the corresponding enhanced right PCA in the MRI (black arrow).
Figure 4.
A-D) Coronal fusion images showing the P3 segment of the right PCA as it crosses the tent (white arrows) with the artery (black arrows) clearly impinged by the free edge.
Materials and Methods
MRI-3D DSA Fusion Technique
3D DSA
The procedure was performed under local anesthesia. A 5 French catheter was guided into the both internal carotid and vertebral arteries. 3D DSA was performed using a 3D DSA angiographic unit (LCLP BPL Angio; GE Medical Systems, Milwaukee, WI). Twenty milliliters (ml) of contrast agent (Omnipaque 300mgI/ml; Amersham Health, Oakville, ON, Canada) was injected at 4 mls/s into the right internal carotid artery. The images were acquired in a 512x512 matrix using a 22 cm field of view and reconstructed using a 512x512x 512 matrix (0.3 mm voxel size). Reconstructed images were displayed using maximum intensity projections, surface shaded display, and volume rendering techniques by a dedicated workstation (GE Advantage AW 4.2; GE Medical Systems, Milwaukee,WI).
MRI
The MRI was performed using a 3-Tesla superconducting unit (Signa Excite; GE Medical Systems, Milwaukee, WI) with an 8-channel head coil. A 3D FSPGR sequence was used with the following parameters: TR/TE-7.7/3.1, ET-1.O,TI-450, F/A-12, Matrix-256 x 256, FOV-22 x 22 cm, slice thickness-0.8 mm/0 skip, scan time-12 min. The examination is performed after an intravenous injection of a bolus of 10 ml gadodiamide (Omniscan 0.5 mmol/ml; Amersham Health, Oakville, Ontario, Canada).
3T MRI-3D DSA Fusion Technique
The fusion was performed at the same GE workstation used for 3D DSA reconstruction using the 3D Xray/MR Fusion software (version 1.0.56; GE Medical Systems, Milwaukee, WI). The registration of the 3D DSA images onto the MRI requires three pairs of landmark points. A pair consists of a manually placed point on an anatomical feature on the 3D DSA image followed by placing the corresponding point on the same anatomical feature on the MRI. Arterial bifurcations such as internal carotid artery termination, middle cerebral artery bifurcation and A1-A2 segment junction of anterior cerebral artery were chosen as landmarks. The software then registered (fused) the 3D DSA and MRI.
Discussion
Intracranial Dissection
Intracranial arterial dissections (dissecting aneurysms) can involve both the anterior and posterior circulation. The distal vertebral and basilar arteries are most often involved in the posterior circulation 3,5,7,9,20,26. The other arterial branches, posterior inferior cerebellar (PI-CA)4,27, posterior cerebral (PCA)3,9,12, superior cerebellar (SCA) 6 and the anterior inferior cerebellar arteries (AICA) 23 are rarely involved.
The supraclinoid internal carotid (ICA)13,18, anterior cerebral (ACA) 13,14 and middle cerebral arteries (MCA) 15 are involved most commonly in the anterior circulation. The anterior choroidal artery is rarely involved11. Arterial dissections in both the anterior and posterior circulation often have no associated causative factors (spontaneous) or may be associated with minor and major head trauma and a variety of other factors such as connective tissue disease. Adults and children may be affected 8,17,19,21,22,24,25. The angiographic criteria for diagnosis of dissecting aneurysm have been described9,13. These are: segmental narrowing (string sign), segmental narrowing with dilatation (pearl and string sign), fusiform dilatation and double lumen sign (presence of false lumen or intimal flap).
The course of the PCA in relation to the free edge of the tentorium is shown to have four variations 2. In the medial variant, the main trunk is located medial to the free edge of the tentorium. It sends its cortical branches over and above the free edge of the tentorium and thus all the main branches are predisposed to be compressed during transtentorial herniation but the brain stem branches are spared. In the lateral variant, the proximal main trunk crosses the free edge before it gives off its cortical branches, and therefore the main trunk may be compressed but the cortical branches are spared. However, the brain stem branches may be compressed as they cross the tentorium back to the brain stem.
In the mediolateral variant, the PCA divides into two secondary trunks in the middle incisura space. The medial branch gives rise to both the parieto-occipital and the calcarine branches which cross the free edge and thus either may be compressed. The lateral branch crosses the free edge before it gives rise to the temporal branches. The most common variant is the tortuous PCA, where the main trunk crosses the free edge several times and thus it could be compressed at various points along its course. Correlation of the involved segments of the PCA and the surgical anatomy 2 could explain why the P1-P2 segments are involved most frequently. In the lateral and tortuous variants (the most common variants), the main trunk crosses the free edge, and in the case of the tortuous variant, it does that several times. Although the P3 segment may be involved in the tortuous variant, in the lateral variant, it is the proximal main trunk, (P1/P2) that crosses the free edge and thus the distal P3 portion is not related to the free edge. Hence, the P1-P2 segments are involved more frequently. In the less common mediolateral and tortuous variants, the P3 segment near or at the junction of the calcarine and parieto-occipital origins crosses the free edge and is thus liable to trauma. This explains the angiographic findings that the P3 lesion either extends up to the calcarine parieto/occipital junction (current patient) or extends from P3 through to involve P4. The parieto-occipital and calcarine branches in the medial variant (least common) and in the mediolateral variant divide in the ambient cistern before crossing the free edge as individual branches and thus may be individually involved. However, these are the least common of the variants and may explain why there has been no reported case of individual P4 segments lesion.
The PCA in this case report is a tortuous variant. The fusion image shows that the P3 segment of the PCA weaves across the free edge twice. It also indicates the points of contact of the P3 segment of the PCA with the free edge of the tentorium. This gives strong support to the theory that the direct trauma of the PCA against the free edge of the tentorium can be a cause of the P3 segment dissection. The calcification of the PCA wall on CT implies that this is a chronic lesion associated with her fall ten years ago. It is unclear how indirect or remote trauma can cause dissection22. It has been suggested that shearing and rotational injury with significant intensity can disrupt the vessel wall, but not severe enough to cause complete disruption 1.
Various contributory etiological factors such as polyarteritis nodosa, cystic medial necrosis, atherosclerosis, fibromuscular disease, Marfan's syndrome, mixed connective tissue disease, migraine and electrocution have been reported9,22.
The close proximity of the posterior cerebral artery to the free edge of the tentorium may not require much force to directly traumatize the artery against the tentorium. Hence, it is possible that minor trauma or severe physical exertion such as continuous shaking of the head may be enough force to traumatize the artery against the free edge.
The precipitating event may be minor or in cases where there is no recorded history of such, the trauma may be so trivial as to be considered insignificant by the patient. Underlying vascular disease such as polyarteritis nodosa, cystic medial necrosis, atherosclerosis, fibromuscular dysplasia, Marfan's syndrome, and mixed connective tissue disease, appear to be uncommon but could weaken the wall predisposing the artery to dissection.
3D DSA and 3 Tesla MRI fusion
Image fusion of three dimensional digital subtraction angiography images (3D DSA) with 1.5 Tesla unit magnetic resonance images (MRI) to visualize the perforating arteries of the brain has been described using of the "common cursor" technique of displaying the fused images 16. However, the clinical usefulness of the final product of fused images sets was not discussed. In this case report, the demonstrated relationship between the free edge of the tentorium and adjacent dissected PCA segment may give a clue to the pathophysiology of PCA dissection. We have also shown the clinical usefulness and technical feasibility of the MRI and 3D DSA fusion technique in brain arteriovenous malformation (AVM) cases10. Thus, the MRI and 3D DSA fusion technique can be regarded as a very helpful tool to understand the anatomical relationship between non-vascular structures and intracranial arteries. In addition, it can also provide very useful information for surgical planning of potentially challenging cases such as deep seated or postoperative AVMs.
3 Tesla MRI enables whole head vascular coverage with fine slices in an acceptable examination time and to achieve better spatial resolution in a limited region. The study performed with a 1.5 Tesla magnet required nearly eight minutes to cover 76 mm (covering the circle of Willis region) with 1 mm slices 16. Whereas using a 3 Tesla unit we can cover twice the distance (140.8 mm) in only half as much time (12 minutes) with finer slices (0.8 mm) and thus achieving better spatial resolution. Thus, 3T MRI could generate more anatomically correct fusion images than 1.5T MRI.
The accuracy of the fusion technique can be validated by three different methods. The first method is to check an error measurement. The fusion software automatically displays residual distance between the two points of the landmark pair in mm as an error measurement. The second method is using the common cursor technique. For example, when you put a cursor on an anatomical point on the 3D DSA, the software automatically displays the corresponding anatomical point on each of the three cross sectional MR images including axial, coronal and sagittal planes. The accuracy of the registration can therefore be visually checked and adjusted before the software generates fused images. Finally, one can visually assess the accuracy of the 3D DSA images superimposed on the corresponding enhanced vessels on the Fused MR images. In our case report, the average residual registration error was 1.5 mm or less. The other two methods also verified the minimal error of this study.
Further research using this fusion technique for the anatomical relationship of dissecting aneurysms of other arteries against fixed structures such as the anterior cerebral artery against the falx cerebri and the superior cerebellar artery against the free edge of the tentorium cerebelli may further strengthen trauma as a potential explanation of this disease. There may also be a role in this technology in determining the location of the neck of large and giant carotid cavernous and cave aneurysms to determine whether they are intradural or extradural as this knowledge will help in the decision-making process for their future management options.
Conclusions
3D DSA and 3T MRI fusion technique clearly demonstrated the anatomical relationship of the tentorial free edge and dissecting aneurysm of the PCA's P3 segment of the patient. This novel technique supports the direct trauma of the segmental PCA against the free edge of the tentorium as a valid pathophysiologic hypothesis of the dissecting aneurysm involving the P2-P3 segment of PCA.
References
- 1.Bigelow NH. Intracranial dissecting aneurysms; an analysis of their significance. AMA Arch Pathol. 1955;60(3):271–275. [PubMed] [Google Scholar]
- 2.Blinkov SM, Gabibov GA, Tanyashin SV. Variations in location of the arteries coursing between the brain stem and the free edge of the tentorium. J Neurosurg. 1992;76(6):973–978. doi: 10.3171/jns.1992.76.6.0973. [DOI] [PubMed] [Google Scholar]
- 3.Caplan LR, Estol CJ, Massaro AR. Dissection of the posterior cerebral arteries. Arch Neurol. 2005;62(7):1138–1143. doi: 10.1001/archneur.62.7.1138. [DOI] [PubMed] [Google Scholar]
- 4.Dinichert A, Rufenacht DA, Tribolet N. Dissecting aneurysms of the posterior inferior cerebellar artery: report of four cases and review of the literature. J Clin Neurosci. 2000;7(6):515–520. doi: 10.1054/jocn.2000.0757. [DOI] [PubMed] [Google Scholar]
- 5.Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg. 1984;60(2):325–334. doi: 10.3171/jns.1984.60.2.0325. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Shoin K, et al. Postpartum dissecting aneurysm of the superior cerebellar artery-case report. Neurol Med Chir (Tokyo) 1999;39(12):852–857. doi: 10.2176/nmc.39.852. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi S, Sakaki T, et al. Management of dissecting aneurysms of the posterior circulation. Acta Neurochir (Wien) 1994;1-2:26–31. doi: 10.1007/BF01401451. [DOI] [PubMed] [Google Scholar]
- 8.Kurino M, Yoshioka S, Ushio Y. Spontaneous dissecting aneurysms of anterior and middle cerebral artery associated with brain infarction: a case report and review of the literature. Surg Neurol. 2002;57(6):428–438. doi: 10.1016/s0090-3019(02)00725-5. [DOI] [PubMed] [Google Scholar]
- 9.Lazinski D, Willinsky RA, et al. Dissecting aneurysms of the posterior cerebral artery: angioarchitecture and a review of the literature. Neuroradiology. 2000;42(2):128–133. doi: 10.1007/s002340050031. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Findlay I, et al. 3D X-ray angiography and MRI fusion: preliminary report.. Abstract; Proceedings of the 17th Symposium Neuroradiologicum; 2002; Paris, France. [Google Scholar]
- 11.Matsuura H, Otawara Y, et al. Dissecting aneurysm of the anterior choroidal artery: angiographic and MR imaging findings. Surg Neurol. 2000;53(4):334–336. doi: 10.1016/s0090-3019(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 12.Moron F, Benndorf G, et al. Spontaneous thrombosis of a traumatic posterior cerebral artery aneurysm in a child. Am J Neuroradiol. 2005;26(1):58–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkuma H, Suzuki S, Ogane K. Study Group of the Association of Cerebrovascular Disease in Tohoku, Japan: Dissecting aneurysms of intracranial carotid circulation. Stroke. 2000;33(4):941–947. doi: 10.1161/01.str.0000013564.73522.05. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuma H, Suzuki S, et al. Neuroradiology and clinical features of arterial dissection of the anterior cerebral artery. Am J Neuroradiol. 2003;24(4):691–699. [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkuma H, Suzuki S, et al. Dissecting aneurysms of the middle cerebral artery: neuroradiological and clinical features. Neuroradiology. 2003;45(3):143–148. doi: 10.1007/s00234-002-0919-3. [DOI] [PubMed] [Google Scholar]
- 16.Olsen ER. Intracranial hemorrhage and amphetamine usage. Review of the effects of amphetamines on the central nervous system. Angiology. 1997;28(7):464–471. doi: 10.1177/000331977702800704. [DOI] [PubMed] [Google Scholar]
- 17.O'Sullivan RM, Robertson WD, et al. Supraclinoid carotid artery dissection following unusual trauma. Am J Neuroradiol. 1990;11(6):1150–1152. [PMC free article] [PubMed] [Google Scholar]
- 18.Pozzati E, Galassi E, et al. Regressing intracranial carotid occlusions in childhood. Pediat Neurosurg. 1994;21(4):243–247. doi: 10.1159/000120844. [DOI] [PubMed] [Google Scholar]
- 19.Pozzati E, Padovani R, et al. Benign arterial dissections of the posterior circulation. J Neurosurg. 1991;75(1):69–72. doi: 10.3171/jns.1991.75.1.0069. [DOI] [PubMed] [Google Scholar]
- 20.Pozzati E, Andreoli A, et al. Dissecting aneurysms of the vertebrobasilar system: study of 16 cases. Surg Neurol. 1994;41(2):119–124. doi: 10.1016/0090-3019(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 21.Rabinov JD, Hellinger FR, et al. Endovascular management of vertebrobasilar dissecting aneurysms. Am J Neuroradiol. 2003;24(7):1421–1428. [PMC free article] [PubMed] [Google Scholar]
- 22.Rutherford GS, Dada MA, Nel JP. Cerebral infarction and intracranial arterial dissection in closed head injury. Am J Forensic Med Pathol. 1996;17(1):53–57. doi: 10.1097/00000433-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Saito A, Ezura M, et al. An arterial dissection of the distal anterior inferior cerebellar artery treated by endovascular therapy. No Shinkei Geka. 2000;28(3):269–274. [PubMed] [Google Scholar]
- 24.Schmitt HP, Miltner E. Dissection of the anterior and middle cerebral artery with fatal ischemia following kicks to the head. Forensic Sci Int. 1991;49(1):113–120. doi: 10.1016/0379-0738(91)90178-l. [DOI] [PubMed] [Google Scholar]
- 25.Sharif AA, Remley KB, Clark HB. Middle cerebral artery dissection: a clinicopathologic study. Neurology. 1995;45(10):1929–1931. doi: 10.1212/wnl.45.10.1929. [DOI] [PubMed] [Google Scholar]
- 26.Shimoji T, Bando K, et al. Dissecting aneurysm of the vertebral artery. Report of seven cases and angiographic findings. J Neurosurg. 1984;61(6):1038–1046. doi: 10.3171/jns.1984.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 27.Yamaura A, Isobe K, et al. Dissecting aneurysms of the posterior inferior cerebellar artery. Neurosurgery. 1991;6:894–898. doi: 10.1097/00006123-199106000-00021. [DOI] [PubMed] [Google Scholar]