Summary
A young patient with a facial trauma after a road accident was admitted to our department with incoercible epistaxis. A CT scan showed a right pterional acute epidural hematoma (EDH). Angiography demonstrated multiple sources of bleeding of the right sphenopalatine arteries, cause of the epistaxis, and an intracranial leakage of the right middle meningeal artery, responsible for the EDH. The patient immediately underwent embolization of the right internal maxillary artery and right middle meningeal artery. The procedure stopped the epistaxis and no further enlargement of the EDH was observed, avoiding its surgical treatment. Endovascular surgery may be an effective procedure to stop the arterial meningeal bleeding sustaining acute EDH and may be a useful tool in the management of special cases of post traumatic EDH.
Key words: endovascular treatment, epistaxis, epidural cerebral hematoma
Introduction
In the post-CT era the dogma of prompt surgical treatment of the epidural hematoma (EDH) has been challenged. The non surgical management of particular EDH is now widely accepted and nonsurgically managed cases are rapidly increasing reaching even 60% of all EDH in recent years 1,2,4,5,6,8,9,11,13,19. The enlargement of acute EDH is a common event but it occurs during about five hours after CT diagnosis 12,16. So this frame time may be useful for an endovascular procedure stopping the arterial epidural bleeding.
Advances in endovascular surgery have led to an increasing number of pathological conditions treated with this procedure. Recently Suzuki et Al. (2004) reported nine cases of traumatic epidural hematoma successfully managed with endovascular surgery. We report a case of an incoercible epistaxis after a craniofacial trauma, with associated bleeding of the right meningeal artery causing a temporal EDH. Both bleedings were consecutively treated during the same endovascular procedure. The patient avoided any other open surgical procedures with an excellent outcome.
Case Report
A 22-year-old woman, the victim of a road accident, was admitted to our department for a facial trauma. On admission she presented an 11 Glasgow Coma Scale (GCS) score and an incoercible epistaxis. The patient was immediately intubated due to rapid worsening of the general conditions related to the severe hemorrhage.
Brain CT showed a right acute pterional epidural hematoma of 1 cm maximum size with a cerebral temporal contusion (figure 1). The angiography demonstrated multiple sources of bleeding of the right sphenopalatine arteries and an intracranial leakage of contrast medium from the right middle meningeal artery, about 0.7 cm distal to the origin of the posterior branch, related to the epidural hematoma (figure 2).
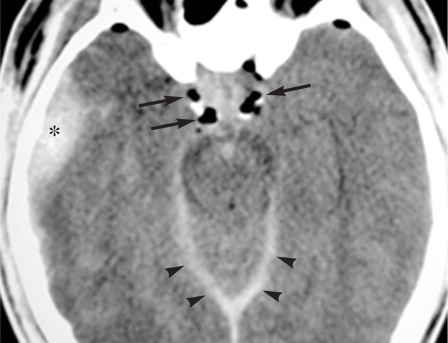
Figure 1.
Non contrast axial brain CT scan shows the right pterional epidural hematoma (asterisk), 1 cm maximum size, associated with mild right temporal contusion. Air bubbles (arrows), interpeduncular hemorrhage and subdural tentorial blood suffusion (arrowheads) are shown.
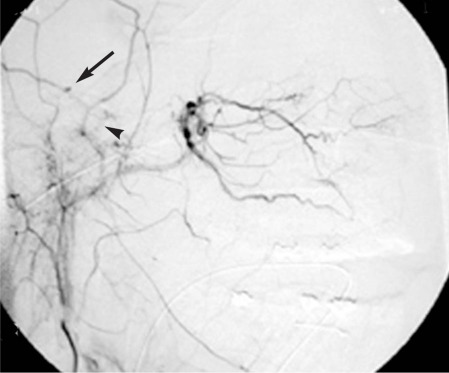
Figure 2.
Digital subtraction angiography of the right external carotid artery, lateral projection. The microcatheter is positioned in the external carotid artery. Active bleeding or a small pseudoaneurysm is seen around the middle meningeal artery, parietal branch, 7 mm after its origin, supplying the epidural hematoma (arrow). Common trunk of the middle meningeal artery (arrowhead).
Angiography was performed with selective bilateral catheterization of the internal carotid arteries and compression of the contralateral carotid artery. In this way the possible sources of bleeding from the internal carotid arteries are displayed and the correct orthodromic opacification of the ophthalmic arteries is proved. Once the absence of anastomosis between the external and internal carotid and vertebral artery systems was confirmed, the patient underwent selective embolization of the internal maxillary artery.
The endovascular embolization of the internal maxillary artery was performed with a coaxial catheter technique, inserting a 5 French guiding catheter into the common trunk of the external carotid artery. Inside the guiding catheter we used an "Excel 14" microcatheter (Target-Boston) with a "Transend" 0.014 in. microwire (Target-Boston). To embolize the multiple sources of bleeding of the right sphenopalatine arteries 10, the distal tip of the micro catheter was placed in the distal portion of the internal maxillary artery, after the origins of the middle meningeal artery, deep temporal artery and accessory meningeal artery.
By the microcatheter we released on both sides "Gelita®" (Aesculap, Tuttlingen) fragments with non ionic iodate medium contrast (Visipaque®, Shering). This embolic material is composed of hardened gelatine of porcine origin, completely biologically degradable. Once the epistaxis had been stopped, as a complementary treatment, the right middle meningeal artery was superselectively catheterized and embolized with disappearance of the leakage of the epidural hematoma (figure 3). So the endovascular treatment achieved two goals: the resolution of the epistaxis and the prevention of the enlargement of the epidural hematoma.
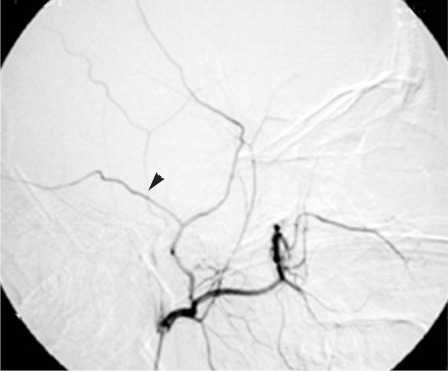
Figure 3.
Digital subtraction angiography of the right external carotid artery, lateral projection, after selective endovascular embolization. The microcatheter is before the origin of the internal maxillary artery, to perform panoramic control angiography. Compared to fig. 2 no leakage from the middle meningeal artery is observed.
After embolization the patient's general conditions and blood parameters returned to normal status with blood transfusion. Brain CT scan after twelve hours (figure 4) showed that the size of the epidural hematoma was unchanged. No neurosurgical treatment was necessary. The patient was discharged after six days. A brain CT after five weeks showed the spontaneous disappearance of the EDH.
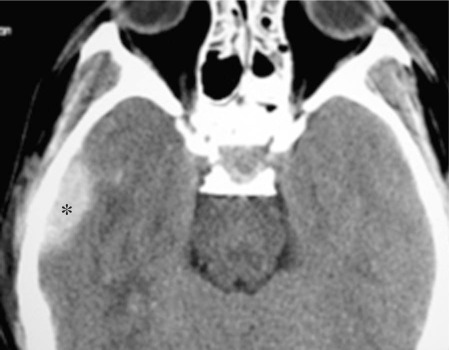
Figure 4.
Non contrast axial brain CT shows the right pterional epidural hematoma (asterisk) slightly decreased in size and in density (compare with figure 1). Right temporal contusion. Readsorption of pneumoencephalon and interpeduncular hemorrhage and subdural tentorial blood suffusion.
Discussion
Endovascular treatment in the internal maxillary artery territory is not without risk. Pain, cranial nerve lesions and stroke of different degrees of severity have been reported 10. To avoid complications and to obtain a successful result, special attention to some details is recommended. A preventive bilateral internal carotid artery angiographic study is mandatory, with alternate common carotid artery compression. In this way possible leakage from the carotid arteries is demonstrated and the orthodromic opacification of the ophthalmic artery is proved, excluding supply from the external carotid artery. To rule out the possibility of dangerous anastomosis between the external carotid artery and the vertebral artery system, a bilateral preventive study of the external carotid artery is necessary. In the majority of cases the side of active bleeding from the posterior nasal or sphenopalatine arteries cannot be directly detected. In these instances embolization to stop the epistaxis is performed on both sides. The same bilateral procedure is done if the epistaxis does not cease after embolization of only one side.
The clinical and radiological indications for surgical treatment of the EDH are well known and the outcome is generally excellent, with a low mortality 2,3,5,8,9,13,14. Nevertheless open cranial surgery is associated with some "minor" complications, never reported in the series of surgically treated EDH. Bad wound cosmetic results, atrophy of the temporal muscle, lesions of the frontotemporal branch of the facial nerve, cranial depression for the burr holes or craniotomy, painful dysfunction of the temporal mandibular joint have all been reported. These complications are of course accepted in a life-threatening condition, but are not unimportant, especially in women and in young patients, the major group in all series.
Bleeding of the EDH may stop spontaneously in many cases but this occurrence is not foreseeable. The need for surgical treatment in initial conservative EDH managed ranges from 5.5 to 32% 2,7,8,9. This significant difference is related to the different initial criteria adopted for the treatment modalities, but also demonstrates dissimilar opinions regarding the best management.
The possibility of monitoring the volume changes of the EDH by CT has favored a more conservative approach. From a large series of EDH conservatively managed it emerges that the patients admitted to hospital in good general conditions with a GCS score over 13, with stable neurological status, without neurological deficit or pupil abnormalities, with a small supratentorial epidural hematoma, under 1.5 cm or no more than 30 ml of blood volume and minimal midline shift, less than three mm, without additional intracranial pathology, are candidates for nonsurgical management 1,4,7,11,12,15,16,18. However in conservatively managed patients the maximum blood volume observed may even reach 38 ml 4, the thickness of the hematoma 40.8 mm 18 and the shift of the midline up to 5 mm 5,13. In our case the GSC was 11, related more to the compromised general conditions due to severe hemorrhage than to the brain lesions. So the criteria for a conservative management may widen.
Angiography has not been used in head trauma since the CT era. We performed diagnostic angiography for the severe epistaxis and the middle meningeal artery was embolized as a complementary treatment. Nevertheless endovascular surgery may now open new modalities of treatment in some cases of traumatic EDH. Suzuki et Al. (2004) emphasized the validity of endovascular surgery in nine cases of traumatic EDH successfully treated
A precise indication for cerebral angiography is actually not possible in head trauma apart from special cases with associated severe hemorrhagic vascular lesions, as in our case. But the important advantage of a non surgical immediate open procedure in some cases of EDH is noteworthy. In fact open cranial surgery may adversely affect a contralateral hematoma or may aggravate cardiopulmonary dysfunction in a labile trauma patient. Furthermore Suzuki et Al. (2004) stressed the benefits of a unique surgical procedure in cases of additional subdural hematomas or cerebral contusions, especially delayed or controlateral to the EDH.
So in special conditions with a high probability of delayed severe lesions, or contralateral contusion, or labile trauma patients or with cardiopulmonary dysfunction or coagulation disorders, the initial option of a conservative approach seems advisable. In these cases cerebral angiography followed by a successful endovascular procedure should be considered to avoid one or more surgical open procedures.
Conclusions
The treatment of symptomatic EDH by surgical evacuation and angiography is not recommended. Many EDH arise from vein and dural sinus lacerations or diploic bleeding demanding a direct surgical management. But in special cases the option of angiography and endovascular treatment should be considered. This technique may be an effective procedure to stop the bleeding sustaining an EDH, avoiding the risks of potential complication of open cranial surgery. Now the purpose is to identify the proper clinical criteria, imaging data and timing for the endovascular treatment of the traumatic EDH.
References
- 1.Bejjani GK, Donahue DJ, Rusin J. Radiological and clinical criteria for the management of epidural hematomas in children. Pediatr Neurosurg. 1996;25:302–308. doi: 10.1159/000121144. [DOI] [PubMed] [Google Scholar]
- 2.Bezircioglu H, Ersahin Y, et al. Nonoperative treatment of acute extradural hematomas: analysis of 80 cases. J Trauma. 1996;41:696–698. doi: 10.1097/00005373-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bricolo AP, Pasut LM. Extradural hematoma: toward zero mortality. Neurosurgery. 1984;14:8–12. doi: 10.1227/00006123-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bullock R, Smith RM, Dellen JR. Non-operative management of extradural hematoma. Neurosurgery. 1985;16:602–605. doi: 10.1227/00006123-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chen TY, Wong CW, et al. The expectant treatment of "asymptomatic" supratentorial epidural hematomas. Neurosurgery. 1993;32:176–179. doi: 10.1227/00006123-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Cooper PR. Non surgical extradural hematomas (comments) Neurosurgery. 1986;18:700. [Google Scholar]
- 7.Hamilton M, Wallace C. Non operative management of acute epidural hematoma diagnosed by CT: the neuroradiologist's role. Am J Neuroradiol. 1992;13:853–859. [PMC free article] [PubMed] [Google Scholar]
- 8.Knukey NW, Gelbard S, Epstein MH. The management of "asymptomatic" epidural hematomas. J Neurosurg. 1989;70:392–396. doi: 10.3171/jns.1989.70.3.0392. [DOI] [PubMed] [Google Scholar]
- 9.Korinth M, Weinhzierl M, Gilsbach JM. Treatment options in traumatic epidural hematomas (in German) Unfallchirurg. 2002;105:224–230. doi: 10.1007/s001130100316. [DOI] [PubMed] [Google Scholar]
- 10.Kurata A, Kitahara T, et al. Superselective embolization for severe traumatic epistaxis caused by fracture of the skull base. Am J Neuroradiol. 1993;14:343–345. [PMC free article] [PubMed] [Google Scholar]
- 11.Pozzati E, Tognetti F. Spontaneous healing of acute extradural hematomas: study of twenty-two cases. Neurosurgery. 1986;18:696–700. doi: 10.1227/00006123-198606000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Sakai H, Takagi H, et al. Serial changes in acute extradural hematoma size and associated changes in level of consciousness and intracranial pressure. J Neurosurg. 1988;8:566–570. doi: 10.3171/jns.1988.68.4.0566. [DOI] [PubMed] [Google Scholar]
- 13.Servadei F, Faccani G, et al. Asymptomatic extradural hematomas: results of multicentric study of 158 cases in minor head injury. Acta Neurochir (Wien) 1989;96:39–45. doi: 10.1007/BF01403493. [DOI] [PubMed] [Google Scholar]
- 14.Servadei F, Vergoni G. Extradural hematomas: surgical and non surgical treatment. Am J Neuroradiol. 1993;14:506–507. [PMC free article] [PubMed] [Google Scholar]
- 15.Servadei F, Vergoni G, et al. Extradural hematomas: how many deaths can avoided? Protocol for early detection of hematoma in minor head injuries. Acta Neurochir (Wien) 1995;133:50–55. doi: 10.1007/BF01404947. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan TP, Jarvik JG, Cohen WA. Follow-up of conservatively managed epidural hematomas: implications for timing of repeat CT. Am J Neuroradiol. 1999;20:107–113. [PubMed] [Google Scholar]
- 17.Suzuky Sachio, Masakata Endo, et al. Efficacy of endovascular Surgery for the treatment of acute epidural hematomas. Am J Neuroradiol. 2004;25:1177–1180. [PMC free article] [PubMed] [Google Scholar]
- 18.Tuncer R, Kazan S, et al. Conservative management of epidural haematomas. Prospective study of 15 cases. Acta Neurochir (Wien) 1993;121:48–52. doi: 10.1007/BF01405182. [DOI] [PubMed] [Google Scholar]
- 19.Tuncer R, Acikbas C, et al. Conservative management of extradural haematomas: effects of skull fractures on resorption rate. Acta Neurochir (Wien) 1997;139:203–207. doi: 10.1007/BF01844752. [DOI] [PubMed] [Google Scholar]