Abstract
We investigated the effect of punicalagin (PC) on benzo[a]pyrene (BP)-induced DNA adducts in vitro and in vivo. Incubation of BP (1 μM) with rat liver microsomes, appropriate co-factors and DNA in the presence of vehicle or punicalagin (1–40 μM) showed dose-dependent inhibition of the resultant DNA adducts, with essentially complete (97%) inhibition at 40 μM. However, PC failed to inhibit anti-BPDE-induced DNA adducts when tested in an in vitro non-microsomal system, suggesting that the inhibition of the microsomal BP-DNA adducts occurred due to inhibition of P450 1A1 by PC. To determine its efficacy in vivo, female S/D rats were administered punicalagin via the diet (1,500 ppm; ~19 mg/day/animal) or subcutaneous polymeric implants (two 2-cm, 200 mg with 20% drug load; 40 mg PC/implant) and then treated with continuous low-dose of BP by a subcutaneous polymeric implant (2 cm, 200 mg with 10% load; 20 mg BP/implant) and euthanized after 10 days. Analysis of the lung DNA by 32P-postlabeling showed significant (60%; p = 0.029) inhibition of DNA adducts by PC administered via the implants; the dietary route showed modest (34%) but statistically insignificant inhibition. Furthermore, total PC administered by implants was approximately 38-fold lower compared with the dietary route. Analysis of the lung microsomes showed significant inhibition of cytochrome P450 1A1 activity and induction of glutathione. Release of PC from the implants was found to be biphasic starting with a burst release, followed by a gradual decline. Ultra performance liquid chromatography analysis showed no detectable PC in the plasma but its hydrolyzed product, ellagic acid was readily detected. The plasma concentration of ellagic acid was over two orders of magnitude higher (589 ± 78 ng/mL) in the implant group compared with diet (4.36 ± 0.83 ng/mL). Together, our data show that delivery of PC by implants can reduce its effective dose substantially, and that the inhibition of DNA adducts in vivo occurred presumably due to the conversion of PC to ellagic acid.
Keywords: Benzo[a]pyrene, DNA adducts, 32P-postlabeling, Punicalagin, Polymeric implants, Bioavailability
1. Introduction
Lung cancer is the leading cause of cancer death in both men and women in the United States, as well as in the world. Due to heavily coordinated emphasis on cigarette smoking, there has been significant declined in lung cancer incidence, particularly in the males, however, no significant decline has occurred in the lung cancer mortality [1]. Chemoprevention that can reverse, suppress, prevent, or delay the carcinogenic process either by blocking the development of early lesions or by inhibiting the progression to invasive cancer offers one such alternative. Therefore, effective chemopreventive agents are needed to reduce the burden of lung cancer. Strategies should be developed not only for early detection of lung cancer but also for its prevention using simple interventions.
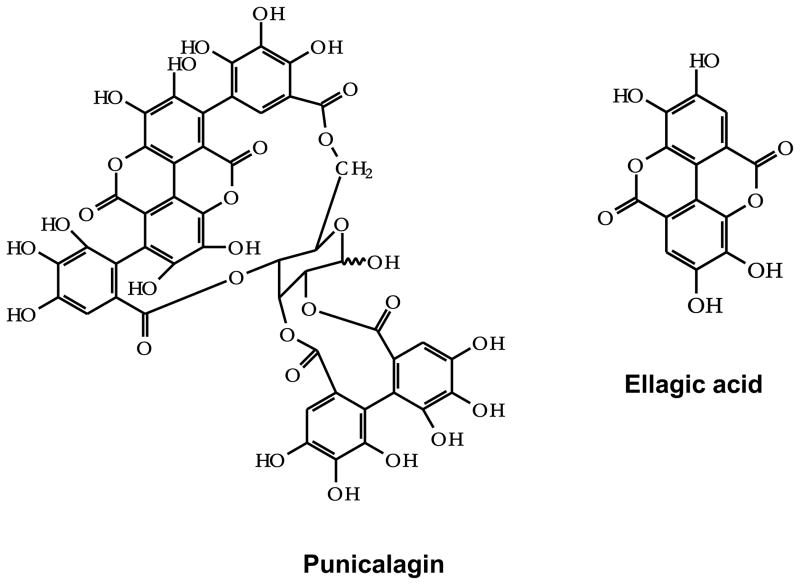
Punicalagins (PC) are ellagitannins in which gallagic and gallic acids are linked to a sugar moiety (Figure 1). They are isomers of 2,3-(S)-hexahydroxydiphenoyl-4,6-(S,S)-gallagyl-D-glucose, hydrolysable tannins with a molecular weight of 1084. Punicalagins are often noted as punicalagin, however, they are found as two reversible α- and β-anomers. The PC isomers have been reported to be mainly responsible for the high antioxidant capacity of pomegranate juice and shown to inhibit the proliferation of human oral, colon, and prostate tumor cells and proapoptotic effects [2, 3]. Little is known about the fate of ellagitannins in animals or humans and only low concentration of PC has been shown in plasma when a bolus dose was provided [4]. In general, these large molecules are not absorbed directly into the body [5], and hydrolyzed in the intestinal tract over several hours leading to sustained blood levels of ellagic acid over 6 h [6].
Figure 1.
Chemical structure of punicalagin and ellagic acid.
PC releases ellagic acid upon hydrolysis, although other metabolites can also be produced and are distinctive of individual ellagitannins (i.e. punicalin, gallagic and tergallagic acid) [7]. Studies have shown that ellagic acid accumulates mostly in intestinal epithelial cells and accumulation in other tissues such as blood, lung and liver is limited [8, 9]. Many chemopreventive compounds, including ellagic acid, have encountered poor bioavailability issues. Therefore, there is an urgent need to develop efficient delivery systems to administer such chemopreventive agents, which can circumvent the problems of bioavailability thus reducing toxicity issues generally associated with high doses.
Subcutaneous implants have been recognized as a useful drug delivery system [10]. They provide better therapeutic outcome than drug therapies administered by the traditional oral route, particularly for chronic use [11–13]. Polycaprolactone (PCL), an FDA-approved biodegradable polymer, has been used for drug delivery in which drug is mixed in the polymer matrix and is gradually released by passive diffusion or bioerosion.
Benzo[a]pyrene (BP) is one of the most potent and widely studied carcinogens. In a cellular system, BP is metabolized to the electrophilic metabolite, benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) which can attach covalently to DNA bases, primarily deoxyguanosine. Inflammatory response to chemical carcinogens and formation of DNA adducts are generally considered a prerequisite in the process of chemical carcinogenesis [14]. Accumulation of DNA adducts resulting from chronic exposure to low-level environmental carcinogens has been used as a possible measure of exposure to carcinogens and cancer risk assessment [15].
In this study, we hypothesized that delivery of PC by PCL implants can circumvent its problem of degradation by GI tract microflora and can provide continuous (“24 hours a day/seven days a week”) systemic delivery. In this study we determined formulation of PC implants, in vitro-in vivo release kinetics, and its effect on BP-induced DNA adducts.
2. Material and Methods
2.1. Chemicals
Polycaprolactone (MW 65,000) (P-65), Glucose-6-phosphate, glucose-6-phosphate dehydrogenase from baker’s yeast (G6PDH), NADP+, BP, and salmon testis (st)-DNA were purchased from Sigma-Aldrich (St. Louis, MO). The pluronic® F68, water soluble copolymers based on ethylene oxide and propylene oxide, was kindly provided by BASF Corporation (Florham Park, NJ, USA). Silastic tubing was purchased from Allied Biomedical (Ventura, CA). st-DNA was made free of contaminating RNA by treatments with RNases and proteinase K and solvent extractions as described previously [16]. Ellagic acid (EA) was purchased from LKT laboratories, Inc. (St. Paul, MN). PC was isolated from pre-enriched Punica husk powder (Pharmenza, India) in our laboratory and was >96% pure, as described elsewhere [17] and was essentially free of EA. Anti-BPDE was kindly provided by Dr. Subodh Kumar, State University of New York College at Buffalo. Chemicals used in 32P-postlabeling DNA adduct analysis were the same as described previously [16]. Other chemicals used in this study were of analytical grade.
2.2. Microsomal BP-DNA Adducts
st-DNA (300 μg/mL) was preincubated with 50 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 2.5 mM glucose-6-phosphate, 1 U/mL G6PDH, 0.5 mM NADP+, and α-naphthoflavone-induced rat liver microsomal proteins (1 mg/mL) in 1 mL for 10 min, in the presence of vehicle alone or PC (1–40 μM). BP dissolved in DMSO was added at a final concentration of 1 μM, and incubation was continued for another 30 min at 37 °C. The reaction was terminated by the addition of EDTA and centrifugation (9,000g; 10 min). DNA was isolated from the supernatant by removal of RNA and proteins by digestions with RNases A and T1 and proteinase K and a series of extractions with phenol, phenol: Sevag (chloroform:isoamyl alcohol, 24:1), and Sevag, followed by precipitation of the DNA with ethanol [16]. The DNA concentration was estimated spectrophotometrically.
2.3. Reaction of st-DNA with anti-BPDE
st-DNA (200 μg/mL) was pre-incubated for 10 min in the presence of vehicle or PC in 0.5 mL 50 mM Tris-HCl (pH 7.5). Then, anti-BPDE was added at a final concentration of 0.5 μM and incubated at 37°C for another 30 min. The reaction was terminated by precipitating DNA with ethanol, and the DNA concentration was measured spectrophotometrically.
2.4. Preparation of polymeric implants
Polymeric implants of PC [18] and BP [19, 20] were prepared by extrusion method essentially described for cancer chemopreventive agents. Briefly, PCL, and F68 were dissolved in dichloromethane and PC was dissolved in ethanol. After mixing the two solutions, the solvents were evaporated in a water bath by continuous stirring at 70°C for about 45 min, followed by drying under reduced pressure at ~65 °C overnight using Savant Speed-Vac (Thermo-Savant, Holbrook, NY). Molten polymeric material was then extruded through silicon tubing (I.D. 3.2 mm) attached to a disposable syringe. Cylindrical implants were removed from the mold by excision with a scalpel and cut into desired length and stored in dark under argon at −20°C until use. Sham implants were prepared the same way without adding test agent. BP implants were prepared the same way by substituting PC with BP, except that BP was dissolved in dichloromethane instead of ethanol.
2.5. In-vitro release of PC from the polymeric implants
The rate of release of PC was determined by adopting the procedure described for curcumin implants [18]. Briefly, 2-cm implants were shaken in 20 mL amber glass vial containing 10 mL of release medium (phosphate-buffered-saline (PBS), pH 7.4 supplemented with 10% calf serum and 1% penicillin/streptomycin) in a shaker water bath (Julabo SW 23, Seedbach, Germany). The release medium was changed every 24 h. Since PC is stable for 24 h in release medium (unpublished data), PC released was determined spectrophotometrically at 378 nm against standard curve following the addition of ethanol (10% v/v) to ensure complete dissolution.
2.6. Animals, diet and treatment
Five to six-week-old female Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN). Animals received food (4% Teklad diet; Harlan-Teklad, Inc.) and water ad libitum. After a week of acclimation, animals were randomized into three groups with 5 animals each. Two groups received control diet (4% Teklad) and the third group received diet supplemented with 1,500 ppm PC. After one week, all the groups were treated with BP (2-cm; 200 mg/implant with 10% BP). The two group that received control diet were also grafted with PC implants (two 2-cm; 200 mg/implants; 20% load) or sham implants. Implants were grafted subcutaneously at the back of the animals as described elsewhere [21]. The body weight and diet consumption were recorded twice a week. After 10 days of treatment, rats were euthanized by CO2 asphyxiation. Tissues and blood are collected and stored appropriately. Implants were also recovered from the animals, wiped, dried under vacuum and stored at −20°C until analysis.
2.7. Isolation of DNA from lung tissues
DNA from lung tissue was isolated by a solvent extraction procedure as described previously [16]. The procedure involves removal of RNA and proteins by digestion of isolated crude nuclei with RNases and proteinase K, respectively, followed by extractions with phenol, phenol: Sevag and Sevag. DNA was recovered by precipitation with ethanol and its concentration was measured spectrophotometrically.
2.8. Analysis of DNA adducts
DNA adducts were analyzed by 32P-postlabeling as described [16]. Briefly, 10 μg of DNA was digested with micrococcal nuclease and spleen phosphodiesterase (MN/SPD). Before further treatment with nuclease P1 to enrich DNA adducts, an aliquot was removed for evaluation of normal nucleotide levels. DNA adducts and normal nucleotides were labeled with [γ-32P]ATP and T4 polynucleotide kinase. Labeled adducts were separated by multi-directional polyethyleneimine (PEI)-cellulose TLC using the following solvents: D1, 1.0 M sodium phosphate, pH 6.0; D3, 4 M lithium formate/7 M urea, pH 3.5; D4, 4 M ammonium hydroxide/isopropanol (1:0.9); and D5, 1.7 M sodium phosphate, pH 6.0. Normal nucleotides were resolved in 180 mM sodium phosphate, pH 6.0, by one-directional PEI-cellulose TLC. DNA adducts and normal nucleotides were detected and quantified by Packard InstantImager.
2.9. Stability and measurement of residual PC in polymeric implants
Stability and residual amount of the PC in the implants was determined by solvent extractions and HPLC analysis. To extract PC from the implants, implants were dissolved in appropriate volume of dichloromethane and ethanol (1:1). The mixture was then partitioned with water and centrifuged at 12000g. The aqueous layer containing PC was separated, dried under reduced pressure and dissolved in methanol, appropriately diluted and analyzed by HPLC using UV detection.
HPLC analysis was carried out on a Shimadzu HPLC system coupled with photo diodarray detector (Kyoto, Japan). PC (20 μL injection volume) was analyzed on a ShimPack reverse phase column (Shimadzu; 250 × 4.6 mm, 2.2 μm). Gradient of 2% acetic acid in water (solvent A) with 2% acetic acid in methanol (Solvent B) was used, where solvent B was initially 1% for 5 min then increased to 60% in 40 min, the latter ratio was then maintained till 42 min and finally decreased to 1% in 42.1 min at a flow rate of 1 mL/min. The hold time between the injections was 5 min. Spectra was recorded between 200–400 nm and punicalagins anomers were monitored at 378 nm.
The cumulative release from the implant was calculated by subtracting the residual amount from the initial amount. Total PC released in vivo was compared with cumulative release in the same period in vitro.
2.10. Preparation of lung microsomes
Lung tissues (~200 mg) were homogenized in 0.25 M sucrose buffer using Polytron homogenizer. After 20 min centrifugation (Beckmann Coulter, Allegra 25R) at 4000g, 4°C, the supernatant and nuclear pellet were separated. Supernatant was again centrifuged at 11,000g, 4°C, to separate the mitochondrial fraction. Finally, microsomes were prepared by ultracentrifugation of the post-mitochondrial supernatant at 100,000g, 4°C.
2.11. Protein determination
The protein content of lung microsomes was determined by BCA methods using BCA™ Protein Assay kit (Thermo Scientific, Rockford, IL, USA) as described elsewhere [18] with bovine serum albumin as standard.
2.12. Western-blot analysis for cytochrome
Microsomal proteins were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking with 5% non-fat dry milk in blocking solution, the membrane was incubated with the desired primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for overnight at 4°C. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, MA), and the immuno-reactive bands were visualized using the Pierce chemiluminescent substrate kit (Thermo Scientific, Rockford, IL). To ensure equal protein loading, each membrane was stripped and re-probed with β-actin antibody (Sigma-Aldrich, St Louis, MO) to normalize for differences in protein loading.
2.13. 7-Ethoxyresorufin O-deethylase (EROD) activity assay
The measurement of EROD activity allowed us to assess the effect of test agents on the catalytic activity of CYP1A1 [22]. Microsomal protein (20 μg) was pre-incubated with 7-ethoxyresorufin (25 μM) for 10 min at 37°C in the presence of 5 mM MgCl2 and 100 mM potassium phosphate, pH 7.4. Enzymatic reaction was initiated by the addition of NADPH and continued for 1 h at 37°C. Fluorescence of resorufin was measured continuously using a Spectramax with excitation and emission wavelengths set at 530 and 585 nm, respectively.
2.14. Glutathione estimation in lung tissues
The relative levels of glutathione (μM/g) were measured in lung tissues using GSH-Glo Glutathione Assay Kit (Promega Corporation, Medison, WI, USA). GSH concentrations were determined by interpolation from a GSH standard curve generated using the bioluminescent system
2.15. Plasma PC/ellagic acid analysis
PC was first extracted from plasma as described by Seeram et al.[23]. Briefly, 1 mL plasma was acidified to pH 2.5 with 300 μL of 1 M potassium dihydrogen phosphate solution and 30 μL of 50% phosphoric acid. After vortex mixing for 1 min with acetonitrile, the samples were centrifuged at 3,500g for 10 min at 4°C. The supernatant was separated and vacuum-dried in a Speed-Vac and reconstituted in 100 μL of methanol and 25 μL was analyzed by Shimadzu ultra performance liquid chromatography (UPLC) system comprised of two LC-20AD-XR pumps, SIL-20A-XR autosampler, and SPD-M20A photodiode array detector (PDA) controlled by Class VP (ver 7.4, SP3).
A Shim-pack XR-ODS-II column (3.0×150 mm; 2.2 μ) was used for separation at a flow rate of 0.7 mL/min under a binary linear gradient condition using mobile phases, solvent A (100 mM sodium phosphate with 10 mg/L SDS, pH 2.5 with phosphoric acid) and solvent B (60% acetonitrile, 10% methanol, 30% Solvent A). The gradient condition was as follows: 0 – 1.77 min, 6% B; 1.77 – 5.47, 30% B; 5.47 – 7.29, 100% B; 7.29 – 9.12 min, 100% B; 9.12 – 12.0 min, 6% B. The PDA was set to scan from 200–600 nm. Ellagic acid standard was prepared in DMSO (200 μg/mL) and serially diluted in methanol to 1000, 500, 250, 125 and 62.5 ng/mL.
2.16. Statistics
All calculations were performed with GraphPad Prizm statistical software (version 4.03; La Jolla, CA). One way analysis of variance (ANOVA) was done to compare the dose response. Student’s t-test was used to compare for parameters between treatment groups. We chose a p-value of ≤0.05 to declare significant results. Since the sample size is small, results should be interpreted with caution.
3. Results
3.1. Effect of PC on microsomal BP-DNA adducts
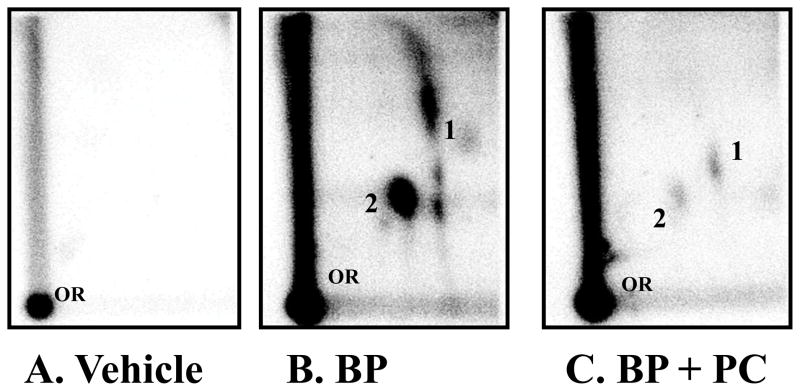
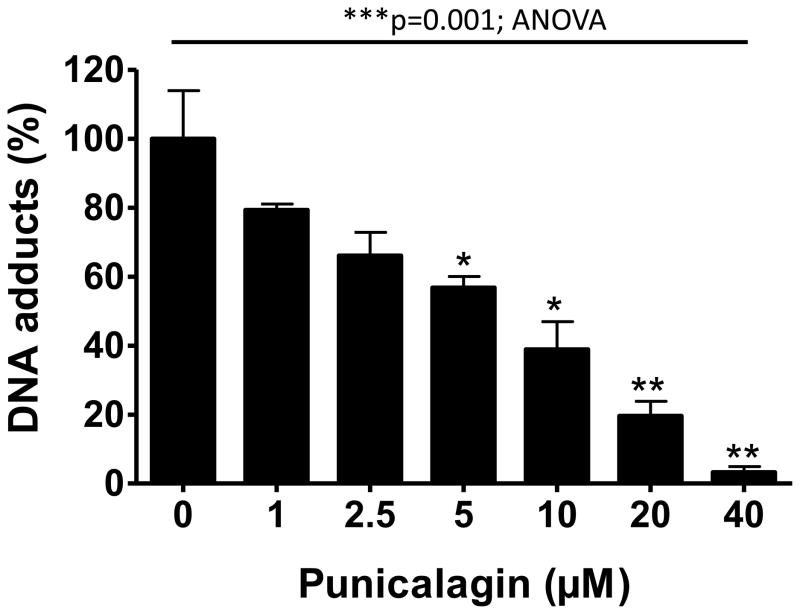
BP (1 μM) when incubated with rat liver microsomes in the presence of st-DNA resulted in the formation of two major adducts (Figure 2). We have previously characterized these adducts as products of the interaction of anti-BPDE and dG (adduct 2) and 9-OH-benzo[a]pyrene-4,5-epoxide and dG (adduct 1) [24]. No adducts spots were detected in DNA incubated with vehicle alone. Incubation of st-DNA with BP (1 μM) in the presence of varying concentrations (1–40 μM) of punicalagin or vehicle produced qualitatively the same DNA adduct profile but the adduct levels varied. PC inhibited BP-DNA adducts in dose-dependent manner. As compared with BP alone (21.9 ± 5.3 DNA-adducts/107 nucleotides; n = 6), PC (≥5 μM) resulted in significant inhibition of BP-induced DNA adducts (Figure 3). The half maximal inhibitory concentration (IC50) values of PC was 8.25 μM, and at 40 μM, it elicited almost complete BP-DNA adducts inhibition (97% inhibition; p=0.001; ANOVA).
Figure 2.
Representative autoradiographs of 32P-postlabelling analysis of microsomal-benzo[a]pyrene (BP) DNA adducts in the presence of vehicle alone (2% DMSO) (A), BP (1 μM) + vehicle (B) and BP (1 μM) + punicalagin (PC) (40 μM) (C). Adduct 1, anti-benzo[a]pyrene-7,8-diol-9,10-epoxide-dG and adduct 2, 9-OH-benzo[a]pyrene-4,5-epoxide-dG. PC alone group was not included since we do not expect any background BP_DNA adduct. OR, origin.
Figure 3.
Inhibition of microsomal benzo[a]pyrene-induced DNA adducts by punicalagin. DNA adducts were analyzed by 32P-postlabeling assay. Data represent an average (±SE) of 4–6 animals. ***p<0.001, **p<0.01, *p<0.05.
3.2. Effect of PC on anti-BPDE-DNA adducts
To determine if the inhibition of microsomal BP-induced DNA adducts was due to scavenging of the DNA-reactive metabolite anti-BPDE with PC, we tested the activity of PC in a non-enzymatic reaction using anti-BPDE. The reaction of anti-BPDE with St-DNA produced one predominant and several trace DNA adducts (not shown). Comparison of vehicle- (590 ± 55 adducts per 107 nucleotides) and PC treatment at its highest concentration (40 μM) (591 ± 42 adducts per 107 nucleotides) showed no adduct inhibition. On the other hand, ellagic acid included as a positive control (40 μM) resulted in ~50% inhibition of the adduct formation. These data suggest that while PC contains ellagic acid moiety, the adjacent hydroxyl groups of the ellagic acid present in PC are unavailable to scavenge anti-BPDE.
3.3. Release of PC from the implants in vitro and its stability
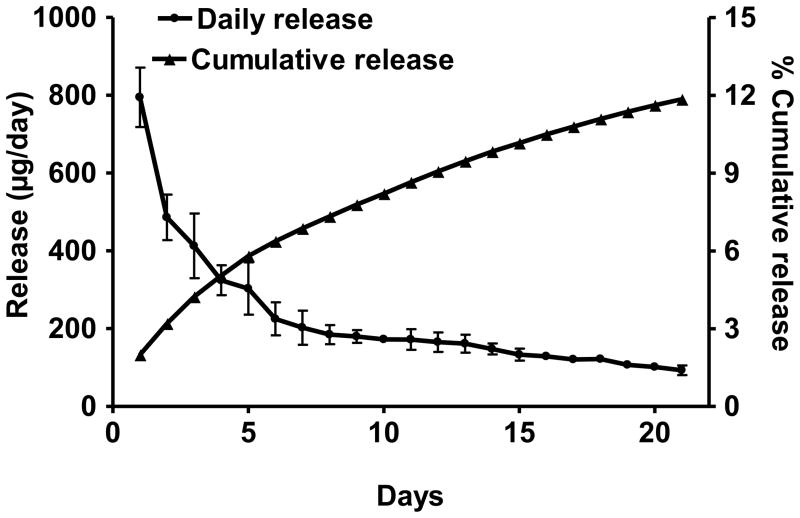
The release of PC from the implants in vitro was measured in PBS supplemented with 10% bovine calf serum to simulate the in vivo conditions. Biphasic release kinetics was observed with a burst release of 795 ± 76 μg on day 1 that decreased almost exponentially to 93 ± 13 μg after 21 days. The cumulative release after three weeks was 4.74 mg (11.85% of the initial amount) (Figure 4).
Figure 4.
Release of punicalagin from the polymeric implants in vitro. Implants (2 cm; 20% drug load) were incubated in 10 mL of PBS containing 10% bovine calf serum at 37°C with constant shaking. The medium was changed daily and the release was measured spectrophotometrically. Data represent average (±SD) of three replicates.
3.4. Effect of PC delivered via the diet and the implants on BP-DNA adducts in vivo
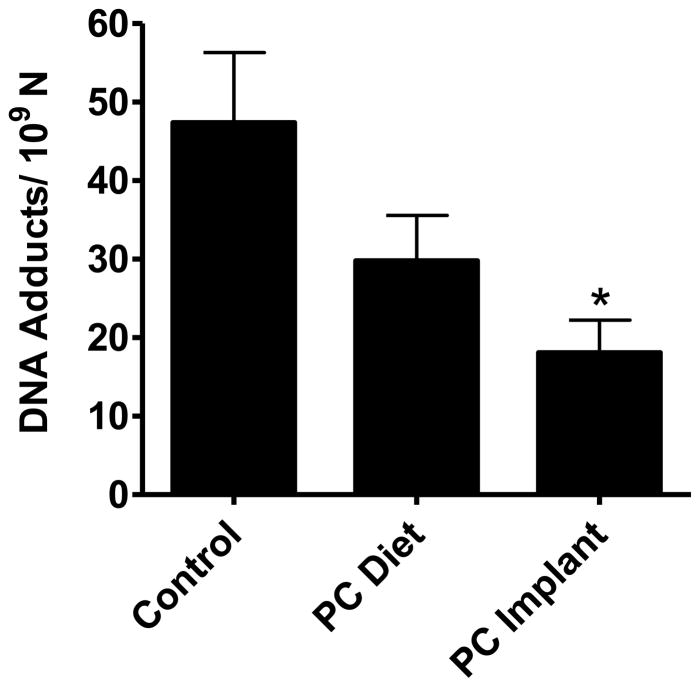
To determine relative biological effects of PC administered via the diet or the implant, female S/D rats were treated with PC either via the implants or diet prior to challenging with low-dose of BP. There was no significant difference in the body weight or in lung and liver weight indicating that PC had no adverse effects during the study period. Analysis of the lung DNA by 32P-postlabeling showed essentially two DNA adducts. These adducts have previously been characterized as dG derivatives of anti-BP,7,8-diol-9,10-epoxide (adduct 1) and 3,4-epoxide of 9-OH-BP (adduct 2) (Figure 2) [25]. There was no qualitative difference in the adduct patterns with or without PC intervention. However, significant quantitative differences were observed in the adduct burden: Animals treated with PC implants showed a significant inhibition of DNA adducts by approximately 60% (p=0.029). The dietary administration of PC also showed a modest reduction by 34% but this was statistically insignificant (Figure 5).
Figure 5.
Effect of punicalagin (PC) administered via the diet and polymeric implants by different routes of delivery on total DNA adducts in lung tissues of rats treated with continuous low-dose exposure to benzo[a]pyrene by polymeric implants. Animals received subcutaneous implants of PC (two 2–cm implants; 20% load) or diet supplemented with 1,500 ppm PC. DNA adducts were analyzed by 32P-postlabeling assay. Data represent mean (±SE) of 5 replicates.
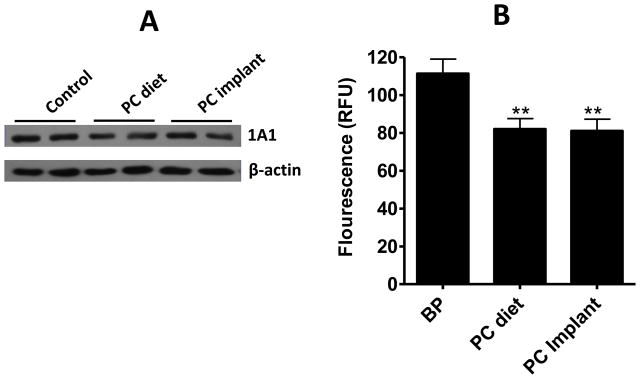
3.5. PC inhibits CYP1A1 expression and activity
To determine the mechanism by which PC inhibits BP-induced DNA adducts in lung tissue, we analyzed the expression of CYP1A1, CYP1A2, CYP1B1 and GSTM, the major enzymes involved in the metabolism of BP. PC treatment irrespective of the route of administration was found to lower CYP1A1 expression (20–30% reduction) as analyzed by Versa-Doc imaging system (Bio-Rad, Hercules, CA) (Figure 6A); CYP1A2, CYP1B1 and GSTM were undetectable (not shown). The activity of CYP1A isozymes with and without PC treatment was determined by EROD assay. PC treatment significantly (p=0.0058) lowered CYP1A activity irrespective of the route of treatment – the dietary route (82 ± 5.6 RFU) or the implant route (81 ± 6.24 RFU) versus vehicle treatment (111.3 ± 7.6 RFU) (Figure 6B).
Figure 6.
Effect of punicalagin (PC) on CYP1A1 expression and CYP1A activity. (A) Western-blot analysis of microsomal protein from lung tissues of rats treated with benzo[a]pyrene (BP) in the absence and presence of punicalagin administered via the diet and implants. (B) Inhibition of CYP1A activity by PC in the lung tissue of BP-treated rats. Data represent mean (±SE) of 5 replicates. **p<0.01.
3.6. Glutathione levels in lung tissues
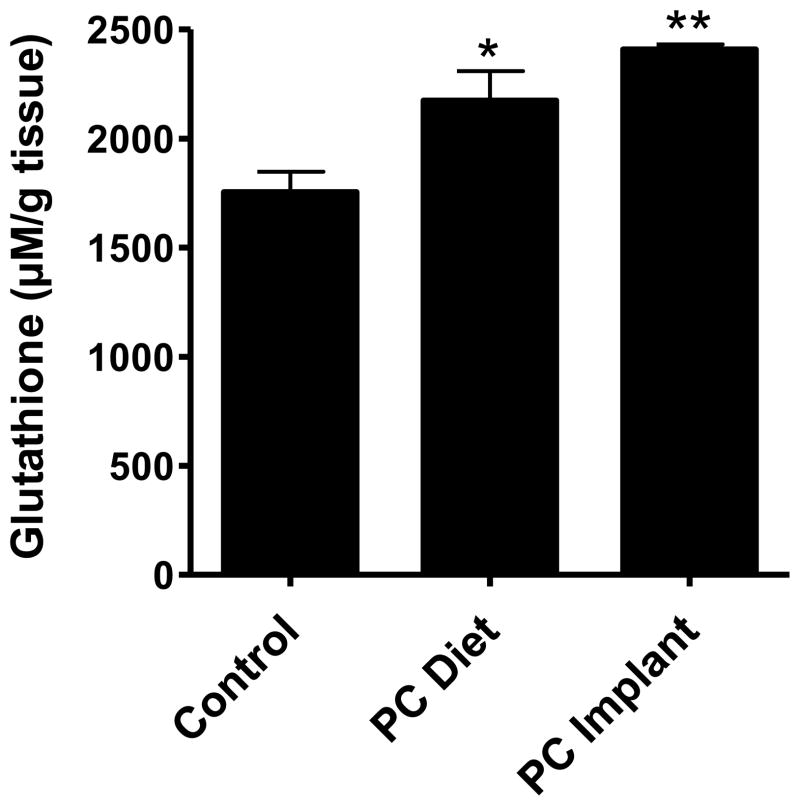
Glutathione was measured in the target (lung) tissue. PC by dietary route significantly increased the glutathione levels compared to vehicle treatment (2174 ± 234 versus 1754 ± 188 μM/g of lung tissue; p=0.045). However, when delivered by implant route, the increase was more pronounced (2409 ± 38 μM/g; p=0.002). Overall the increase in the glutathione by PC was 24% and 37% by dietary and implant routes, respectively (Figure 7).
Figure 7.
The levels of glutathione in lung tissues of rats treated with benzo[a]pyrene in the absence and presence of punicalagin (PC) delivered via the diet and polymeric implants. Glutathione was measured with GSH-Glo assay kit. Data were obtained from 5 different rats and represented as mean (±SE). **p<0.01, *p<0.05.
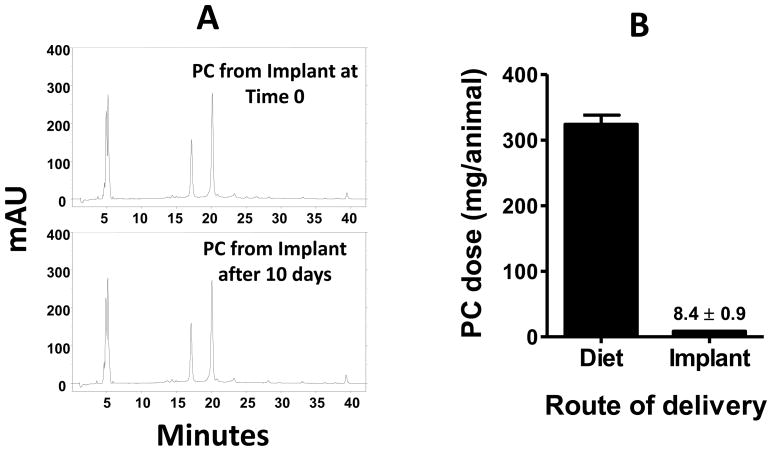
3.7. Release of PC in vivo
The solvent extraction procedure described under Materials and Methods showed analytical recovery of PC from the implants was >88%. Further, PC extracted from the implants at time zero versus 10 days after grafting in the animals showed qualitatively identical HPLC profile (Figure 8A), indicating that PC was stable in vivo. Measurement of the residual amount in the implants revealed that 4.2 mg/implant (a total of 8.4 mg from two implants) PC was released over duration of the study, corresponding to on an average 840 ± 88 μg daily release (Figure 8B).
Figure 8.
Stability and release of punicalagin (PC) from the implants in vivo. Chromatograms in panel A represent stability of PC following preparation of implants and after 10 days of grafting in the animals. The implants were dissolved in dichloromethane and extracted with water and analyzed by HPLC and monitored at 378 nm. Panel B demonstrates total PC delivered during the study via the diet and implants in vivo. mAU, milli absorbance unit.
3.8. Plasma PC and ellagic acid analysis
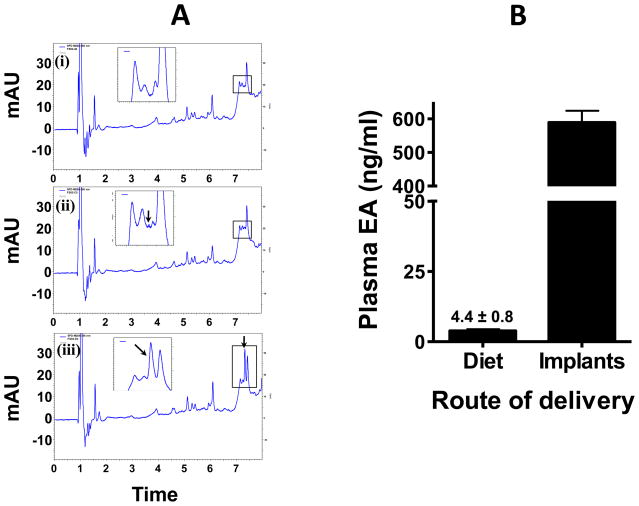
Analysis of the plasma collected from vehicle- and PC-treated animals by UPLC showed clear differences both qualitatively and quantitatively (Figure 9A). PC in intact forms was not detected in any of the plasma samples irrespective of the route of the treatment. However, ellagic acid (a hydrolysis product of PC) was detected in the same groups. Control plasma showed no detectable peak of ellagic acid. For quantification, plasma collected from untreated animals was spiked with different concentrations of ellagic acid standard and processed accordingly. With an average intake of nearly 19 mg PC in diet, based on daily diet consumption (12.7 ± 0.95 g/day), the plasma ellagic acid was only 4.36 ± 0.83 ng/mL. However, with PC implants, the plasma PC levels were substantially higher (588.9 ± 77.6 ng/mL) (Figure 9B).
Figure 9.
Representative UPLC chromatograms of ellagic acid (EA) extracted from plasma of rats treated with benzo[a]pyrene in the absence and presence of punicalagin delivered via the diet and implants. (i) Control plasma, (ii) plasma from punicalagin diet, and (iii) plasma of punicalagin implants. Panel B demonstrates the amount of EA (hydrolyzed product of punicalagin) detected in animal plasma of punicalagin diet and punicalagin implants. Insets show the absence and presence of EA. Punicalagin was below the detection limit. mAU, milli absorbance unit.
4. Discussion
DNA adduct formation represents a net effect of activation and detoxification processes and can be used to determine efficacy of chemopreventive agents. In this study, efficacy of PC was determined by its ability in reducing BP induced DNA adducts levels in vitro as well as in vivo. PC was found to inhibit both anti-BPDE- and 9-OH-BP-derived DNA adducts in vitro as well as in vivo. There are at least two major pathways by which PC can inhibit BP-induced DNA adducts in vivo: i) by inhibiting the P450 activity and/or enhancement of phase II enzymes, and ii) by direct conjugation with anti-BPDE. The possibility that adduct inhibition occurred due to scavenging of anti-BPDE directly by PC can be ruled out since no inhibition of anti-BPDE-induced adducts was observed in the presence of PC in vitro. However, ellagic acid, which resulted by hydrolysis of PC in vivo, has been shown to scavenge anti-BPDE in which the catechol moieties of ellagic acid covalently interact with anti-BPDE as determined by HPLC [26]. We have demonstrated previously that different hydrolysable tannins inhibit the DNA adducts by conjugating with anti-BPDE [27, 28]. In another study, we have shown the ellagic acid does not inhibit the CYP 1A1 activity but significantly inhibits the activity of 1B1 and directly conjugate with the DNA reactive intermediates of BP [29]. Therefore, it is likely that the inhibition of BP-induced DNA adducts in vivo by PC occurred due to inhibition of CYP1A1, or scavenging of anti-BPDE by ellagic acid, a principal metabolite of PC, or both; apparently, the catechol moieties in PC constituents were protected in the conjugated PC complex.
To assess the efficacy of agents delivered via implants, BP was delivered via the implant route to obtain low-dose continuous exposure. Unlike most published studies in which bolus doses (50–100 mg/kg, b.wt.) have been used, the implant route delivered 10 – 20 μg daily dose of BP [20]. The low-dose continuous exposure to BP induced substantial over-expression of CYP1A1, consistent with the original findings of Jeyabalan et al. [20]. This CYP is the primary cytochrome P450 involved in the conversion of BP to the electrophilic metabolite, anti-BPDE. The CYP1A1 and 1B1 are known to be induced by treatment with a bolus dose of BP in the lung [20, 30]. Significant inhibition of CYP1A1 by PC administered via the implant route as well as the diet accompanied by significant enhancement of glutathione can also account, at least, in part, to the inhibition of BP-induced DNA adducts. The greater degree of inhibition of the DNA adducts by PC implants compared with dietary route could be due to poor absorption of PC by the gut, or due to lack of hydrolysis of PC to ellagic acid in the gut and/or the liver, or both, in addition to more pronounced upregulation of glutathione by the implant delivery compared to the dietary route.
Ellagitannins are not absorbed directly into the body instead hydrolyzed in the intestinal tract over several hours prior to absorption [5, 31]. Polymeric implantable delivery systems provide a viable alternative for compounds with poor bioavailability, bioaccessibility and first-pass metabolism. PCL was chosen because of its propensity to form compatible blend with a variety of compounds and make it suitable for delivery of such chemopreventives due to its biodegradability at slow rate [32]. PC from these implants followed biphasic release kinetics in vitro starting with a burst release and then declining gradually over a period of three weeks (Figure 4). This type of short-time release kinetics might be a result of simple diffusion process [33]. Burst release could be due to the release of surface bound drug, followed by a more sustained release of PC from the inner part of the polymeric matrix. Because of slow erosion of PCL-based polymeric matrix, the release of PC from implants was apparently controlled primarily by the passive diffusion under a concentration gradient. Furthermore, additives can change drug release significantly and it depends on solubility, hydrophilic nature and interaction with polymer. Pluronic F68 is a FDA-approved excipient and it is soluble in water as well as in organic-solvents [34] and makes it favorable to forming a molecular dispersion in the lipophilic PCL matrix. It is our understanding that the F68 will first diffuse out from the implants leading to microchannels that will allow the release of the chemopreventives [35].
Studies published so far for anticarcinogenicity evaluation of natural compounds have used bolus doses of BP. Chemopreventive agents given orally are destroyed in the gut, either by the low pH of the stomach and/or by the intestinal microflora and the fraction absorbed in the gut then undergoes liver first-phase effect [36]. The use of PCL implants is well established for the delivery of contraceptives [37], however their use in the delivery of carcinogen and/or chemopreventives such as PC is novel. Notably, in this study, the systemic delivery by subcutaneous polymeric implants lowered the effective dose of PC by 38-fold compared with the traditional dietary route. Resveratrol has been shown to be effective in reducing BP-induced lung DNA adducts only when it was administered by subcutaneous multiple doses and not by the oral route [30], highlighting the importance of exploring alternate routes of administration for chemopreventive agents that have poor bioavailability.
The amount of PC released in vivo was 1.3-fold higher compared with the in vitro release. This difference might be due to continuous circulation of extracellular fluid, and distribution of PC to various organs/tissues. Moreover the composition of body fluid and presence of additional proteins, lipids and distribution of PC in tissues and plasma can also enhance the release [32]. One of the main events in ellagitannin metabolism and bioavailability is the microbial transformation into a series of hydroxylated dibenzopyranone derivatives. Studies suggest that only 6% of PC is excreted in feces or in urine, suggesting absorption or transformation of remaining PC into the metabolites [4]. In our study, we could not find any detectable PC in the plasma samples. A plausible reason for this could be the low extraction efficiency from plasma and limit of detection by UPLC-UV system. However, the presence of ellagic acid was evident, which was found to be two orders of magnitude higher in the implant group than the dietary group. The experimental data available indicate that dietary PC is generally not detected in plasma [23], except when the dose given was rather high (60,000 ppm) which showed very low concentration of the these compounds in plasma and urine [4].
In summary, we have demonstrated that PC is able to abrogate BP-induced DNA adducts most likely by offsetting CYP1A1 expression and its activity and possibly by scavenging of the electrophilic metabolite, anti-BPDE by ellagic acid formed by hydrolysis of PC. The results of this study also suggest that the approach of using polymeric implants can significantly lower the effective dose of test agents compared to the traditional oral dosing and that it can provide higher biological effects by enhancing the bioavailability. This novel delivery system will allow assessment of efficacy of minor constituents or metabolites which otherwise remain uninvestigated in vivo because of they are required in large quantities. The therapeutic application of implants is already in practice for long acting reversible contraception [38], testosterone delivery [39] and for delivering histrimine, gonadotropin releasing hormone agonist for prostate cancer patient [40]. Hence, we do not anticipate any major issue in adopting the implant system to deliver chemopreventive agents in the high-risk population like heavy smokers, secondary prevention involving subjects with early-stage disease, and tertiary prevention involving patients after therapy for recurrence.
Highlights.
Punicalagin from pomegranate abrogate BaP-induced DNA adducts by offsetting CYP1A1 activity.
The PC metabolite, ellagic acid scavenges the electrophilic metabolite of BP, anti-BPDE.
Systemic delivery by implants enhances the bioavailability of test agents vs. oral route.
This delivery system allows in vivo assessment of minor constituents or metabolites.
Acknowledgments
Supported from the USPHS grants CA-118114 and CA-125152, Kentucky Lung Cancer Research Program Cycle 7 and Agnes Brown Duggan Endowment. R.C.G. holds the Agnes Brown Duggan Chair in Oncological Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 3.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- 5.Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 6.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 7.Tomas-Barberan FA, Espin JC, Casillas MT Garcia. Bioavailabilty and metabolism of ellagic acid and ellagitannins. In: Quideau S, editor. Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols. World Scientific Publishing Co. Pte. Ltd; Singapore: 2009. pp. 273–297. [Google Scholar]
- 8.Smart RC, Huang MT, Chang RL, Sayer JM, Jerina DM, Conney AH. Disposition of the naturally occurring antimutagenic plant phenol, ellagic acid, and its synthetic derivatives, 3-O-decylellagic acid and 3,3′-di-O-methylellagic acid in mice. Carcinogenesis. 1986;7:1663–1667. doi: 10.1093/carcin/7.10.1663. [DOI] [PubMed] [Google Scholar]
- 9.Whitley AC, Stoner GD, Darby MV, Walle T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid--extensive binding to protein and DNA. Biochem Pharmacol. 2003;66:907–915. doi: 10.1016/s0006-2952(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 10.Coukell AJ, Balfour JA. Levonorgestrel subdermal implants. A review of contraceptive efficacy and acceptability. Drugs. 1998;55:861–887. doi: 10.2165/00003495-199855060-00019. [DOI] [PubMed] [Google Scholar]
- 11.Dash AK, Cudworth GC. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 12.Torres AJ, Zhu C, Shuler ML, Pannullo S. Paclitaxel Delivery to Brain Tumors from Hydrogels: A Computational Study. Biotechnol Progr. 2011;27:1478–1487. doi: 10.1002/btpr.665. [DOI] [PubMed] [Google Scholar]
- 13.Salmaggi A, Duri S, Silvani A, Gaviani P, Milanesi I, Casali C, Di Meco F. Loco-regional treatments in first-diagnosis glioblastoma: literature review on association between Stupp protocol and Gliadel. Neurological Sciences. 2011;32:241–245. doi: 10.1007/s10072-011-0797-8. [DOI] [PubMed] [Google Scholar]
- 14.Loeb LA, Harris CC. Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res. 2008;68:6863–6872. doi: 10.1158/0008-5472.CAN-08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godschalk RW, Van Schooten FJ, Bartsch H. A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J Biochem Mol Biol. 2003;36:1–11. doi: 10.5483/bmbrep.2003.36.1.001. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RC. 32P-postlabeling for detection of DNA adducts. In: Pfeifer GP, editor. Technologies for Detection of DNA Damage and Mutations. Plenum Press; New York: 1996. pp. 45–61. [Google Scholar]
- 17.Aqil F, Munagala R, Jeyabalan J, Vadhanam MV, Gupta R. Enhanced bioactivity of punicalagins by polymeric implants against benzo[a]pyrene-induced DNA adducts in vivo. Ann Meet Am Ass Can Res; Orlando, Florida. 2011. p. 888. [Google Scholar]
- 18.Bansal SS, Vadhanam MV, Gupta RC. Development and In Vitro-In Vivo Evaluation of Polymeric Implants for Continuous Systemic Delivery of Curcumin. Pharm Res. 2011;28:1121–1130. doi: 10.1007/s11095-011-0375-z. [DOI] [PubMed] [Google Scholar]
- 19.Cao P, Vadhanam MV, Spencer WA, Cai J, Gupta RC. Sustained Systemic Delivery of Green Tea Polyphenols by Polymeric Implants Significantly Diminishes Benzo[a]pyrene-Induced DNA Adducts. Chem Res Toxicol. 2011;30:877–886. doi: 10.1021/tx2000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyabalan J, Vadhanam MV, Ravoori S, Gupta R. Sustained overexpression of 1A1 and 1B1 and and steady accumulation of DNA adducts by low-dose, continuous exposure to benzo[a]pyrene by polymeric implants. Chem Res Toxicol. 2011;24:1937–1943. doi: 10.1021/tx2002788. [DOI] [PubMed] [Google Scholar]
- 21.Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007;31:113–120. [PubMed] [Google Scholar]
- 22.Burke MD, Thompson S, Weaver RJ, Wolf CR, Mayer RT. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem Pharmacol. 1994;48:923–936. doi: 10.1016/0006-2952(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 23.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–68. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Fang AH, Smith WA, Vouros P, Gupta RC. Identification and characterization of a novel benzo[a]pyrene-derived DNA adduct. Biochem Biophys Res Commun. 2001;281:383–389. doi: 10.1006/bbrc.2000.4161. [DOI] [PubMed] [Google Scholar]
- 25.Ross J, Nelson G, Kligerman A, Erexson G, Bryant M, Earley K, Gupta R, Nesnow S. Formation and persistence of novel benzo(a)pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res. 1990;50:5088–5094. [PubMed] [Google Scholar]
- 26.Sayer JM, Yagi H, Wood AW, Conney AH, Jerina DM. Extremely Facile Reaction between the Ultimate Carcinogen Benzo[a)Pyrene-7,8-Diol 9,10-Epoxide and Ellagic Acid. J Am Chem Soc. 1982;104:5562–5564. [Google Scholar]
- 27.Smith WA, Gupta RC. Use of a microsome-mediated test system to assess efficacy and mechanisms of cancer chemopreventive agents. Carcinogenesis. 1996;17:1285–1290. doi: 10.1093/carcin/17.6.1285. [DOI] [PubMed] [Google Scholar]
- 28.Cao P, Cai J, Gupta RC. Effect of green tea catechins and hydrolyzable tannins on benzo[a]pyrene-induced DNA adducts and structure-activity relationship. Chem Res Toxicol. 2010;23:771–777. doi: 10.1021/tx900412a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell GK, Vadhanam MV, Gupta RC. Identification of chemopreventive agents against dibenzo[a,l]pyrene-induced DNA adducts and potential mechanism. Ann Meet Am Ass Can Res. 2010:Abst 5667. [Google Scholar]
- 30.Revel A, Raanani H, Younglai E, Xu J, Rogers I, Han R, Savouret JF, Casper RF. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 31.Cerda B, Periago P, Espin JC, Tomas-Barberan FA. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53:5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg BD, Blanco E, Gao J. Polymer implants for intratumoral drug delivery and cancer therapy. J Pharm Sci. 2008;97:1681–1702. doi: 10.1002/jps.21038. [DOI] [PubMed] [Google Scholar]
- 33.Lin S, Chao PY, Chien YW, Sayani S, Kuma S, Mason M, Wes T, Yang A, Monkhouse D. In vitro and in vivo evaluations of biodegradable implants for hormone replacement therapy: effect of system design and PK-PD relationship. AAPS PharmSciTech. 2001;2:E16. doi: 10.1208/pt020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma G, Song C, Sun H, Yang J, Leng X. A biodegradable levonorgestrel-releasing implant made of PCL/F68 compound as tested in rats and dogs. Contraception. 2006;74:141–147. doi: 10.1016/j.contraception.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int J Food Microbiol. 2010;140:175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 37.Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-epsilon-caprolactone microspheres and nanospheres: an overview. Int J Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 38.Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71:969–980. doi: 10.2165/11591290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelleher S, Howe C, Conway AJ, Handelsman DJ. Testosterone release rate and duration of action of testosterone pellet implants. Clin Endocrinol (Oxf) 2004;60:420–428. doi: 10.1111/j.1365-2265.2004.01994.x. [DOI] [PubMed] [Google Scholar]
- 40.Schlegel PN. Efficacy and safety of histrelin subdermal implant in patients with advanced prostate cancer. J Urol. 2006;175:1353–1358. doi: 10.1016/S0022-5347(05)00649-X. [DOI] [PubMed] [Google Scholar]