Abstract
Specific inhibitors of the production of reactive oxygen species (ROS) by the NADPH oxidases (Nox’s) are potentially important therapeutic agents in the wide range of human diseases that are characterized by excessive ROS production. It has been proposed that VAS2870 (3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine), identified as an inhibitor of Nox2 by small-molecule screening, may serve as an example of such an agent. Here we show that VAS2870 inhibits ROS production in the sarcoplasmic reticulum (SR) of mammalian skeletal muscle, previously identified with Nox4, and thereby abrogates O2-coupled redox regulation of the ryanodine receptor-Ca2+ channel (RyR1). However, we also find that VAS2870 modifies directly identified cysteine thiols within RyR1. Mass spectrometric analysis of RyR1 exposed in situ to VAS2870 and of VAS2870-treated glutathione indicated that thiol modification is through alkylation by the benzyl-triazolopyrimidine moiety of VAS2870. Thus, VAS2870 exerts significant off-target effects, and thiol alkylation by VAS2870 (and closely related Nox inhibitors) may in fact replicate some of the effects of ROS on cellular thiol redox status. In addition, we show that SR-localized Nox4 is inhibited by other thiol alkylating agents, consistent with a causal role for cysteine modification in the inhibition of ROS production by VAS2870.
Keywords: Ryanodine receptor, RyR1, NADPH oxidase, Nox4, VAS2870, thiol modification
Introduction
One or more of the five forms of NADPH oxidase (Nox1–5) and the closely related Duox’s (Duox1–2) are expressed in all mammalian cell types examined [1]. However, understanding of the physiological functions of reactive oxygen species (ROS) generated by the Nox’s remains incomplete, in part because inhibitors that are specific for Nox’s versus other endogenous sources of ROS and that are without off-target effects have not been available. Oxidative stress resulting from overproduction of ROS by endogenous cellular mechanisms has been implicated in a broad spectrum of human diseases, including cardiovascular, neurological and endocrine disorders [2]. Because the ubiquitously expressed Nox’s serve as a principal source of endogenous ROS [1], increasing attention has been focused on the possibility that compounds acting specifically on ROS production by the Nox’s could serve as efficacious therapeutic agents [3–6].
A number of Nox inhibitors have been employed extensively in experimental settings, including diphenylene iodonium (DPI), aminoethyl-benzenesulfono-fluoride (AEBSF) and 4-hydroxy-3-methoxy-acetophenone (apocyanin), but all exhibit significant off-target effects and are therefore neither efficacious agents for the cellular analysis of Nox function nor suitable candidates for use as therapeutic agents. Recently, 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870) was identified as a small molecule inhibitor of Nox2 by means of high-throughput screening [7], and a number of subsequent reports verified the efficient inhibition of ROS production by VAS2870 in several cell types [8–10]. No molecular mechanism has been demonstrated for the inhibitory action of VAS2870.
We reported recently that ROS, in particular hydrogen peroxide (H2O2), are generated by Nox4 in proportion to O2 levels (partial pressure of O2; pO2) within the sarcoplasmic reticulum (SR) of mammalian skeletal muscle [11]. O2-coupled production of H2O2 results in the oxidation of a small set of cysteine thiols within the skeletal muscle ryanodine receptor/Ca2+-release channel (RyR1), which activates RyR1 [11]. These Nox4-mediated effects of pO2 are abrogated by DPI. pO2-dependent H2O2 production and RyR1 activation are also blocked by VAS2870, but we show here that VAS2870 removes free thiols within RyR1 regardless of pO2, and analysis of the reaction of VAS2870 with RyR1 in situ and with glutathione in vitro demonstrates that this compound alkylates Cys residues under physiological conditions.
Materials and methods
Subcellular fractionation of skeletal muscle, preparation of SR vesicles and purification of RyR1
SR vesicles were prepared essentially as described [12]. Briefly, rabbit hind limb muscle was homogenized in buffer containing 20 mM HEPES, pH 7.4, 2 mM EDTA, 0.2 mM EGTA, 0.3M sucrose and protease inhibitors (100 nM aprotinin, 20 μM leupeptin, 1 μM pepstatin, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine). Homogenates were then subjected to differential centrifugation: 100 × g, 10 min (to remove unbroken cells and debris); 1000 × g, 10 min (to pellet nuclei); 10k × g, 20 min (to pellet mitochondria); 100k × g, 1 hr (to generate a membrane-enriched microsomal pellet and cytosol-enriched supernatant).
To isolate SR vesicles, the 100k × g pellet (microsomal fraction) was re-suspended and fractionated on a continuous 20%–45% sucrose gradient in 0.4 M KCl by centrifugation at 100k × g for 14 hr. Heavy and light SR vesicle fractions were eluted separately and, after collection by centrifugation at 120k × g, resuspended, aliquoted and stored in liquid nitrogen. RyR1 was purified from SR vesicles solubilized with CHAPS by sucrose density gradient centrifugation as described [12, 13]. Protein concentrations were determined with a bicinchoninic acid-based assay.
Assay of RyR1 activity by 3H-ryanodine binding
RyR1 activity was assayed essentially as described [13]. Isolated SR vesicles were incubated overnight with 5 nM [3H]-ryanodine at room temperature in medium containing 20 mM imidazole/125 mM KCl, pH 7.0, 0.3 mM pefabloc, 30 μM leupeptin, 10 μM free Ca2+. The medium was bubbled continuously with a gas mixture containing a fixed concentration of O2, 5% CO2, remainder N2. Non-specific binding was determined using a 1000-fold excess of unlabeled ryanodine. After incubation, samples were diluted with 20 vol H2O at 4°C and placed on Whatman GF/B filters soaked with 2% (w/w) polyethyleneimine. Filters were washed three times by vacuum with 5 ml buffer per wash (1 mM K-Pipes, 0.1 M KCl, pH 7.0) and the radioactivity remaining on the filters was quantified by liquid scintillation counting.
Quantification of free protein thiols (sulfhydryls)
The free thiol content of RyR1 was quantified by monobromobimane fluorescence (MBB; Molecular Probes) as described [13]. MBB labeling was carried out either in SR vesicle preparations in the presence of 10μM Ca2+ prior to purification of RyR1, or after purification of RyR1.
Assay of ROS production by dihydroethidium (DHE) or 1′,2′-dichlorofluorescein (DCF) conversion
Isolated SR vesicles were incubated with 10 μM DHE or DCF [14] (Molecular Probes) for 20 min at room temperature and controlled pO2 (glove box) as described [15] and fluorescence was quantified with a fluorescence microplate reader. When employed, DPI or VAS2870 was added 30 min before DHE or DCF.
Mass spectrometric analysis of adduct formation by VAS2870 (RyR1 and GSH)
SR vesicles (1 mg protein/ml) were exposed to VAS2870 (20 μM) at ambient pO2 for 20 min in PBS. SR vesicles were solubilized in 1% SDS and unbound VAS2870 was removed by size-exclusion filtration (P6). SR protein thiols were then reduced with Tris(2-carboxyethyl)phosphine hydrochloride (TCEP; 2 mM) and alkylated with iodoacetamide (10 mM). RyR1 was isolated by SDS-PAGE and the band containing RyR1 was excised followed by in-gel digestion with trypsin or chymoptrypsin. Eluted peptides were analyzed by LC-MS/MS. GSH (Sigma) was incubated with a two-fold-excess of VAS2870 at room temperature in PBS (pH 7.4) and the complete reaction mixture was analyzed by LC-ESI-MS.
Statistical analysis
All data were analyzed with Student’s t-test or one-way ANOVA with appropriate post-hoc analysis, and p-values are given in the figure legends.
Results
Inhibition of sarcoplasmic reticular H2O2 production by VAS2870
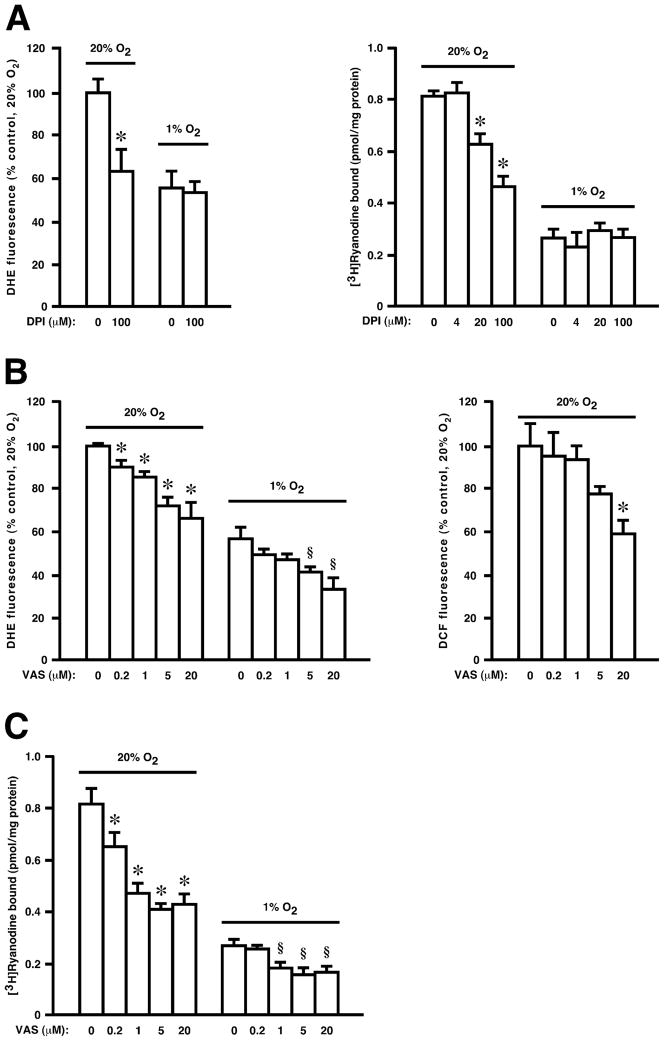
We have shown previously that exposure to ambient pO2 (21% O2, referred to subsequently as high pO2) versus low, physiological pO2 (1% O2) results in both enhanced production of H2O2 and activation of RyR1 within RyR1-enriched subcellular fractions isolated from mammalian skeletal muscle (SR vesicles), as assessed by DHE or DCF fluorescence and by [3H]-ryanodine binding respectively [11]. These effects could be ascribed to Nox4 (with no role for either Nox2 or mitochondria) [11]. As reported [11], the Nox inhibitor DPI suppressed pO2-coupled H2O2 production by SR vesicles and activation of RyR1 (Fig. 1A). VAS2870 also suppressed H2O2 production as assessed by both DHE and DCF fluorescence (Fig. 1B). Both DPI and VAS2870 essentially eliminated the increase in H2O2 production at high versus low pO2, but VAS2870 appeared to be both more potent than DPI on a molar basis and better able to suppress Nox4 activity at low pO2 where substrate (O2) availability would become limiting (Fig. 1B). Finally, VAS2870 largely eliminated activation of RyR1 at high versus low pO2 (Fig. 1C).
Fig. 1.
Both DPI (A) and VAS2870 (B) inhibit ROS production (as assessed by DHE or DCF fluorescence), and (A,C) RyR1 activity (as assessed by [3H]ryanodine binding), in isolated SR vesicles. Means ± SEM, n = 3 for A–C. In A left panel, *p < 0.03 re 0 μM DPI at 20% O2. In A right panel, *p < 0.01 re 0 μM DPI at 20% O2. In B left panel, *p < 0.025 re 0 μM VAS at 20% O2; § p < 0.017 re 0 μM VAS at 1% O2. In B right panel, *p < 0.04 re 0 μM VAS. In C, *p < 0.02 re 0 μM VAS at 20% O2; § p < 0.01 re 0 μM VAS at 1% O2.
Direct modification of Cys thiols by VAS2870
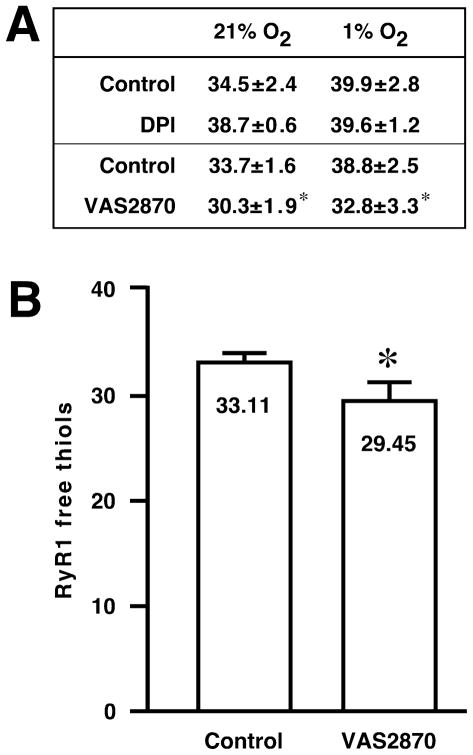
We reported previously that the enhancement of RyR1 activity at high versus low pO2 was associated causally with oxidation of a small set of RyR1 Cys thiols RyR1 (an average of ~ 5 of a total of ~ 40 free thiols) [13] and that scavenging H2O2 prevented both thiol oxidation within and activation of RyR1 at high pO2 [11]. In SR vesicles, as shown in [11] and in the table of free thiol number presented in Fig. 2A, DPI also largely prevented the loss of free thiols at high versus low pO2, but had no apparent effect on thiol number at low pO2 (34.5 ± 2.4 versus 38.7 ± 0.6 at 21% in the absence versus presence of DPI and 1% O2 in the absence of DPI and 39.9 ± 2.8 versus 39.6 ± 1.2 at 1% O2 in the absence versus presence of DPI). In contrast, thiol number was diminished at both high and low pO2 by VAS2870 (33.7 ± 1.6 versus 30.3 ± 1.9 at 21% in the absence versus presence of VAS2870 and 38.8 ± 2.5 versus 32.8 ± 3.3 at 1% O2 in the absence versus presence of VAS2870) (Fig. 2A). To eliminate the possibility that the loss of free thiols within RyR1 was a downstream effect of VAS2870 in SR vesicles, we also determined that exposure to VAS2870 of isolated RyR1 (purified from CHAPS-solubilized SR vesicles by density gradient centrifugation prior to treatment with VAS2870) resulted in a loss of free thiols (Fig. 2B) comparable to that seen when SR vesicles were treated with VAS2870 prior to solubilization and purification of RyR1 (Fig. 2A). Thus, VAS2870 suppresses H2O2 production by Nox4 and the consequent activation of RyR1, but exposure to this compound also appears to result directly in the modification of a set of thiols within RyR1. Because VAS2870 does not activate RyR1 at either low or high pO2 (Fig. 1B), the directly modified thiols apparently comprise a set distinct from those that are oxidized by Nox4-dependent H2O2 or, alternatively, the effects of VAS2870-derived modification differ functionally from those elicited by H2O2-derived modification.
Fig. 2.
(A) In isolated SR vesicles, the number of free thiols within RyR1 (as assessed by monbromobimane labeling) is reduced at 21% O2 versus 1% O2. The pO2 –dependent loss of thiols is prevented by DPI (100 μM). In contrast, exposure to VAS2870 (20 μM) reduces the number of free thiols regardless of pO2. Means ± SEM, n = 5; *p < 0.18 re control. (B) Exposure of purified RyR1 to VAS2870 (10 μM) results in a loss of free thiols (as assessed by monobromobimane labeling). Note that SR vesicles were prepared at ambient pO2 (21% O2) prior to purification of RyR1. Means ± SEM, n = 4; *p = 0.031.
VAS2870 alkylates Cys thiols within RyR1
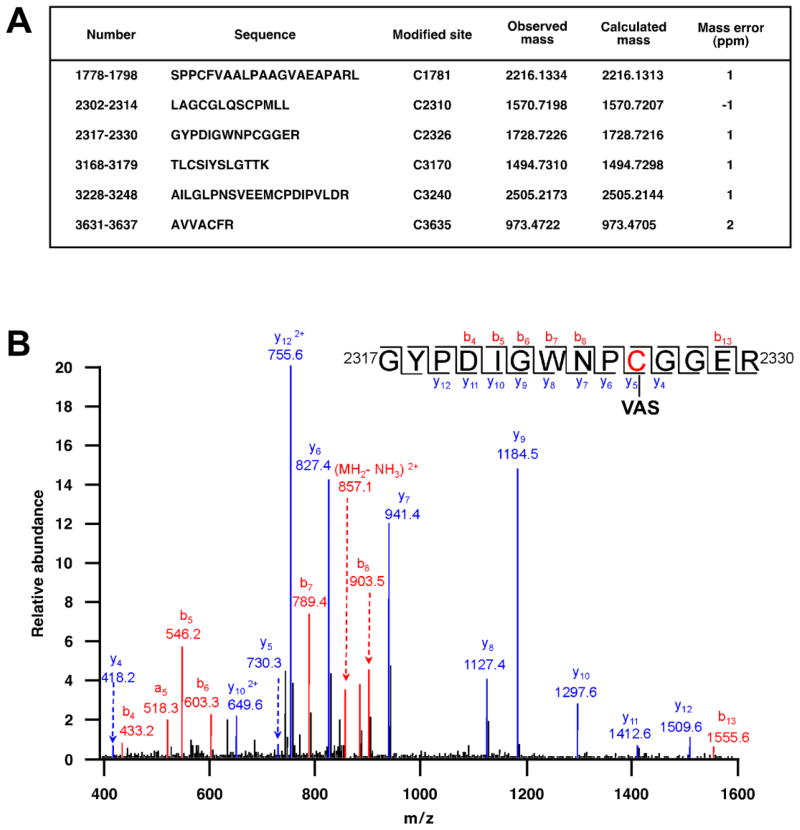
We employed mass spectrometric analysis to examine further the modification of RyR1 Cys thiols following treatment with VAS2870. Following treatment of SR vesicles with VAS2870, RyR1 was isolated by SDS-PAGE and the gel segment containing RyR1 was excised and digested with trypsin or chymotrypsin followed by LC-MS/MS. Six non-overlapping peptides were identified, each of which contained a single Cys that was modified by a group of 210.2 amu, consistent with adduction by the benzyl-triazolopyrimidine moiety of VAS2870 (Fig. 3A,B). Among the Cys residues identified as sites of modification was Cys3635 (Fig. 3A), previously identified as a principal site of regulation of RyR1 function by S-nitrosylation [16].
Fig. 3.
(A) Following treatment of SR vesicles with VAS2870, mass spectrometric analysis (LC-MS/MS) of trypsin-digested RyR1 identified six peptides containing a Cys residue bearing a modification of 209.2 amu, consistent with adduction by the benzyl-triazolopyrimidine moiety of VAS2870 (see Fig. 5 for further analysis). (B) By example, the spectrum shown identifies Cys2326 as a site of modification by VAS2870.
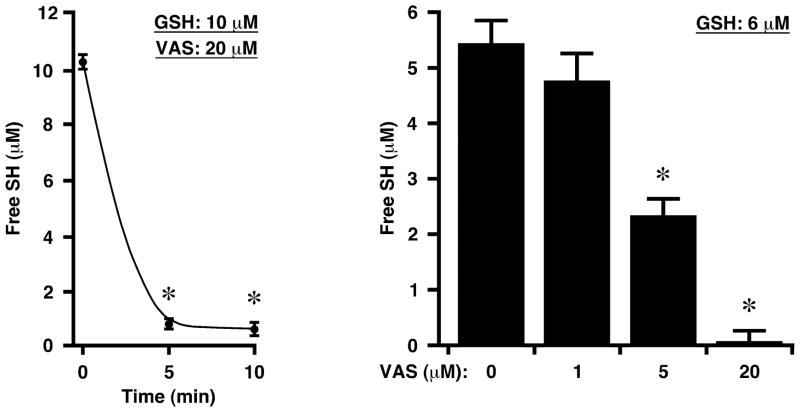
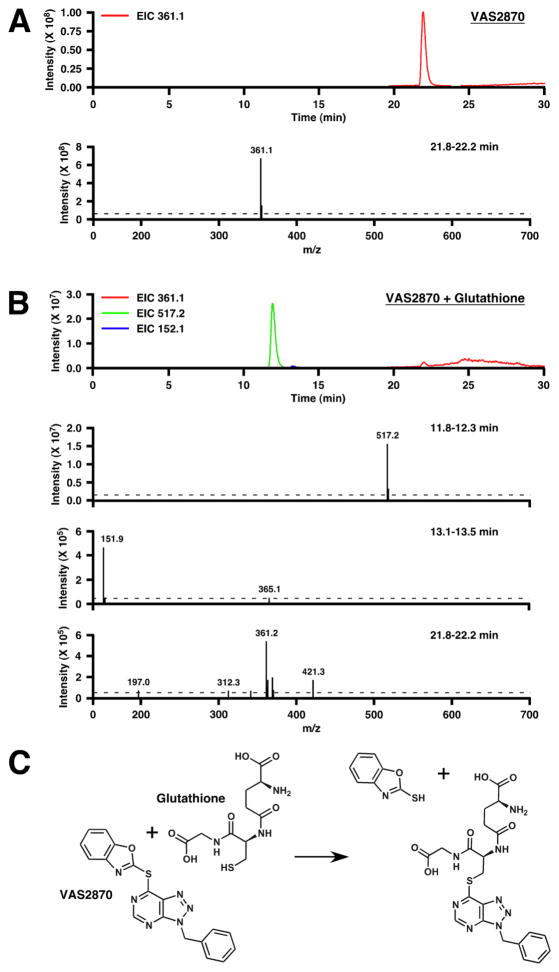
To verify the ability of VAS2870 to modify directly Cys thiols and the identity of the modifying group, we employed mass spectrometry (LC-ESI-MS) to assess the results of adding VAS2870 to the Cys-containing tripeptide, glutathione (GSH). Admixture of VAS2870 (20 μM) and GSH (10 μM) resulted in the rapid and essentially complete loss of GSH thiols as assessed by thiol labeling (Fig. 4). Analysis of reaction mixtures by mass spectrometry revealed loss of the 361.1 amu species corresponding to VAS2870 and the appearance of two new species of 517.2 amu and 151.9 amu, which may be interpreted to represent respectively the product of alkylation of GSH (307 amu) by the benzyl-triazolopyrimidine moiety (210.2 amu) of VAS2870, and the thio-benzoxazole leaving group (151.9 amu) (Fig. 5).
Fig. 4.
Exposure of GSH to VAS2870 results in the rapid loss of free thiols. Means ± SEM, n = 3; *p < 0.01.
Fig. 5.
Mass spectrometric analysis (LC-ESI-MS) of the modification of GSH by VAS2870. (A and B) Extracted ion current (EIC) chromatograms and corresponding mass spectra are shown for, respectively, VAS2870 and a mixture of VAS2870 and GSH (20 min incubation). Note different scales for ordinates. (C) The mass spectrometric analysis indicates that the thiol of GSH is alkylated by the benzyl-triazolopyrimidine moiety of VAS2870, with the thio-benzoxazoyl moiety of VAS2870 serving as leaving group.
Thiol alkylation inhibits Nox4
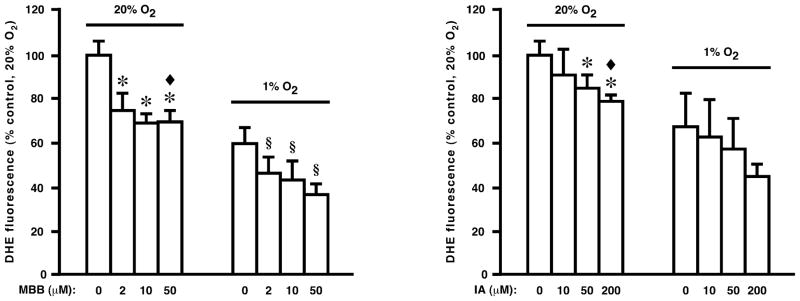
Although no molecular mechanism has been proposed for inhibition by VAS2870 of Nox activity, our results suggest the possibility that Cys thiol alkylation may play a role. We found that exposure to either of two disparate thiol alkylating agents, monobromobimane and iodoacetamide, inhibited H2O2 production by SR vesicles, consistent with a mechanistic role for thiol modification in the inhibitory action of VAS2870 (Fig. 6).
Fig. 6.
Production of ROS by isolated SR vesicles (as assessed by DHE fluorescence) is inhibited by the thiol alkylating agents monobromobimane (MBB), at left, and iodoacetamide (IA), at right. Means ± SEM, n = 4. In left panel, *p < 0.01 re 0 μM MBB at 20% O2; ◆ p = 0.23 re 0 μM DPI at 1% O2 (i.e., MBB eliminates pO2-dependent DHE fluorescence); § p < 0.05 re 0 μM MBB at 1% O2. In right panel, *p < 0.05 re 0 μM IA at 20% O2; ◆ p = 0.2 re 0 μM IA at 1% O2 (i.e., IA eliminates pO2-dependent DHE fluorescence).
Discussion
VAS2870 was identified by small molecule screening as an inhibitor of the prototypical Nox, Nox2. Our results suggest that VAS2870 inhibits Nox4 as well. Recent reports have provided evidence that VAS2870 inhibits Nox4 [17], Nox1 [18] and DUOX [19]. Taken together, these findings suggest that VAS2870 is not isoform specific.
Our mass spectrometric analysis indicates that VAS2870 directly modifies thiols by way of nucleophilic substitution, consistent with known thiol-exchange chemistry involving triazine-like structures that has been extended to other ring systems [20]. It should be noted that oxidation of target thiols (formation of a sulfone, O=S=O), which might be facilitated by Nox-derived H2O2, would be expected to potentiate this reaction (see Fig. 5). Inasmuch as the pathophysiological consequences of overproduction of ROS by a Nox or Nox’s will be exerted in significant part through thiol oxidation, alkylation of Cys thiols by VAS2870 may be expected to replicate at least in part severe oxidative stress. For example, we show that VAS2870 alkylates Cys3635 within RyR1, which will abrogate physiological regulation of RyR1 by NO [16].
Our results also suggest that alkylation of thiols may underly at least in part inhibition by VAS2870 of Nox4. Inhibition by VAS2870 of multiple Nox/Duox isoforms suggests that VAS2870 may target the core flavocytochrome, because the composition of additional subunits varies substantially between Nox/Duox isoforms. More generally, our finding that thiol alkylation may inhibit Nox4 activity would be consistent with the possibility that Nox’s contain regulatory Cys thiols, which may be subject to redox modification by endogenous ligands. In this context, it is of interest that inhibitory effects on Nox activity of nitric oxide were reported, which may result from direct, NO-based modification of the enzyme [21] but which are independent of modification of known, non-thiol redox centers (heme, FAD) [22], consistent with a role for S-nitrosylation. Recently, S-nitrosylation in vitro of a single Cys conserved in Nox from plants through humans (Nox2) was shown to suppress Nox activity [23], and this residue is conserved in all members of the Nox/Duox family in mammals.
The development of specific Nox inhibitors would greatly facilitate the elucidation of Nox function and would potentially provide clinically useful agents. High-throughput screening of small molecules was used to identify VAS2870 and the closely related triazolopyrimidine VAS3947 [24], and the structurally related pyrazolopyrimidines (acting on Nox1/Nox4) [25] as well as a subset of phenothiazines (acting on Nox1) [26] have also been identified by screening as potential, specific Nox inhibitors, all acting through unknown mechanisms. Our findings indicate that the utility of VAS2870 (and therefore possibly other related compounds) is compromised by significant off-target effects, and emphasize the importance of careful evaluation of potential specific inhibitors of the Nox’s beyond the criteria that they act to inhibit ROS production by a Nox(s) without affecting ROS production from other enzymatic (and non-enzymatic) sources [6]. The possibility that modification of thiols within Nox4 and other Nox’s may inhibit enzyme activity might help inform rational inhibitor design.
Highlights.
VAS2870 suppresses production of ROS in skeletal muscle sarcoplasmic reticulum.
O2-coupled redox regulation of RyR1 by Nox4 is thereby abrogated.
VAS2870 directly modifies Cys thiols within RyR1.
Mass spectrometric analysis demonstrates thiol alkylation by VAS2870.
Off-target effects of VAS2870 may replicate redox effects of ROS.
Acknowledgments
Supported by National Heart, Lung and Blood Institute Grant RO1 HL0591130. The authors thank Dr. George R. Dubay, Duke University, for LC-ESI-MS analysis and Dr. Weimin Wang (Merck) for advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 2.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cave A. Selective targeting of NADPH oxidase for cardiovascular protection. Curr Opin Pharmacol. 2009;9:208–213. doi: 10.1016/j.coph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 5.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 6.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Lange S, Heger J, Euler G, Wartenberg M, Piper HM, Sauer H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc Res. 2009;81:159–168. doi: 10.1093/cvr/cvn258. [DOI] [PubMed] [Google Scholar]
- 9.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun. 2006;344:200–205. doi: 10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 10.Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res. 2008;80:271–279. doi: 10.1093/cvr/cvn185. [DOI] [PubMed] [Google Scholar]
- 11.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A. 2011;108:16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987;262:3065–3073. [PubMed] [Google Scholar]
- 13.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 14.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Yamaguchi N, Xu L, Eu JP, Stamler JS, Meissner G. Regulation of the cardiac muscle ryanodine receptor by O2 tension and S-nitrosoglutathione. Biochemistry. 2008;47:13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Xin C, Eu JP, Stamler JS, Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci U S A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:e1000479. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sancho P, Fabregat I. The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-β-induced apoptosis of liver tumor cells. Biochem Pharmacol. 2011;81:917–924. doi: 10.1016/j.bcp.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackman DE, Westphal DB. A new method for the synthesis of heterocyclic S-alkyl thiolactams. Synthesis-Stuttgart. 1987:1134–1136. [Google Scholar]
- 21.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii H, Ichimori K, Hoshiai K, Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997;272:32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- 23.Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA, Loake GJ. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature. 2011 doi: 10.1038/nature10427. [DOI] [PubMed] [Google Scholar]
- 24.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 26.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]