Abstract
Food-triggered anaphylaxis can encompass a variety of symptoms that affect multiple organ systems and can be life threatening. The molecular distinction between non–life-threatening and life-threatening modes of such anaphylaxis has not yet been delineated. In this study, we sought to identify the specific immune functions that regulate the severity of oral antigen-induced anaphylaxis. We thus developed an experimental mouse model in which repeated oral challenge of ovalbumin-primed mice induced an FcεRI- and IgE-dependent oral antigen-triggered anaphylaxis that involved multiple organ systems. Strikingly, the severity of the systemic symptoms of anaphylaxis (eg, hypothermia) positively correlated with the levels of intestinal mast cells (r = −0.53; P < 0.009). In addition, transgenic mice with both increased intestinal and normal systemic levels of mast cells showed increased severity of both intestinal and extra-intestinal symptoms of IgE-mediated passive as well as oral antigen- and IgE-triggered anaphylaxis. In conclusion, these observations indicate that the density of intestinal mast cells controls the severity of oral antigen-induced anaphylaxis. Thus, an awareness of intestinal mast cell levels in patients with food allergies may aid in determining their susceptibility to life-threatening anaphylaxis and may eventually aid in the treatment of food-triggered anaphylaxis.
Severe food allergy-related reactions, termed food-triggered anaphylaxis, are serious, life threatening, and responsible for 30,000 to 120,000 emergency department visits, 2000 to 3000 hospitalizations, and ∼150 deaths per year in the United States.1,2 Clinical studies indicate that food reactions account for 30% to 75% of anaphylactic cases in emergency departments in North America, Europe, Asia, and Australia.1,3–5 The onset of symptoms from food-induced anaphylaxis is variable, occurring within seconds to a few hours after exposure to the casual food allergen, and often affects multiple organ systems, including gastrointestinal, cutaneous, respiratory, and cardiovascular systems.6 Cutaneous symptoms (urticaria and angioedema) are the most common, occurring in ∼80% of cases, whereas cardiovascular involvement occurs in 39% of food allergic reactions.6 Notably, 20% of cases do not present with skin findings, particularly cases of food reaction in children.7
At present, the laboratory tests to support the clinical diagnosis of an anaphylactic episode have intrinsic limitations. In addition, no reliable method is currently available to distinguish between atopic persons who do not have increased risk of anaphylaxis and atopic persons who have increased risk of anaphylaxis and possible fatality.8 Furthermore, because patients with food-triggered anaphylaxis do not generally present with a consistent constellation of symptoms, determination of susceptibility and severity of food-induced anaphylaxis cannot necessarily be predicted on the basis of clinical history.9,10
Clinical and experimental analyses have identified a central role for IgE/FcεR/mast cells and mast cell-derived mediators, including histamine, platelet-activating factor, serotonin, proteases (tryptase and chymase), and lipid-derived mediators [prostaglandin D2 and leukotrienes (leukotriene C4, leukotriene D4, and leukotriene E4)] in promoting the clinical manifestations associated with food-triggered anaphylaxis.11–17 However, the relative contribution of tissue mast cell distribution to the intestinal and systemic manifestations of anaphylaxis and how it relates to the clinical presentation of food-triggered life-threatening and non–life-threatening anaphylaxis is not yet fully delineated. The lack of clinical diagnostic tests for anaphylaxis and lack of risk stratification criteria to distinguish life-threatening from non–life-threatening anaphylaxis may reflect this incomplete understanding.
In the present study, we developed an experimental model of oral antigen-induced anaphylaxis in which oral antigen challenge of mice promotes an anaphylactic reaction characterized by multiorgan involvement (gastrointestinal, cutaneous, respiratory, and cardiovascular systems). We used this model to begin identifying influential effector functions important in regulating anaphylaxis severity. We show that gastrointestinal, cutaneous, and cardiovascular symptoms are IgE/mast cell dependent, whereas the induction of airway hyperresponsiveness (AHR) can occur in the absence of IgE/mast cell activation. We show that development of systemic symptoms of anaphylaxis positively correlates with intestinal mast cell levels. Importantly, we show that increased intestinal mast cell number is associated with increases in both intestinal and systemic anaphylaxis severity.
Materials and Methods
Mice
Six- to 8-week-old BALB/c wild-type (WT), FcεRI, and intestinal IL-9 transgenic (iIL9Tg) mice were used as previously described.18,19 BALB/c mice were obtained from the National Cancer Institute (Bethesda, MD). All mice were maintained in a barrier facility, and animals were handled under approved protocols of the Institutional Animal Care and Use Committee from Cincinnati Children's Hospital Medical Center.
Reagents
Hybridomas were obtained from the following sources: 2.4G2 (rat IgG2b anti-mouse FcγRII/III mAb) and anti-trinitrophenyl (TNP)-IgE (mouse IgE mAb clone IGELa2) from ATTC (Rockville, MD),20,21 EM-95 (rat IgG2a anti-mouse IgE mAb)22 from Zelig Eshhar (Rehoveth, Israel), and rat IgG2a (GL117) and rat IgG2b (J1.2) control mAbs from Dr. John Abrams (DNAX Research Institute, Palo Alto, CA). The anti-FcγRII/III antibody (clone 2.4G2) is specific for the extracellular domains of murine FcγRIIb and FcγRIII.20 The anti-IgE antibody (clone EM-95) recognizes a domain of the ε-chain of IgE that is not blocked by the binding of IgE to FcεRI.23 The anti-CD4 depleting antibody (clone GK1.5) binds to the surface molecule L3T4/CD4 and depletes CD4+ T cells.24,25 The monoclonal antibodies were purified from ascites produced by the hybridomas in pristane-primed athymic nude mice by ammonium sulfate precipitation followed by diethylaminoethyl-cellulose ion exchange chromatography as previously described.26 All antibodies were diluted in normal saline to a final volume of 200 μL per mouse, unless otherwise stated.
Passive Anaphylaxis
Mice were injected intravenously (i.v.) with 10 μg of anti-IgE (EM-95) or 500 μg of anti-FcγRII/III (2.4G2) or control Ig (GL117, 10 μg/200 mL saline; or J1.2, 500 μg/200 mL saline, respectively), and the severity of shock was assessed by rectal temperature with a rectal probe.17,27
Passive Oral Antigen-Induced Anaphylaxis
Mice were injected i.v. with 10 μg of anti-TNP-IgE or control Ig (200 mL of saline). Twenty-four hours later, mice were held in the supine position and orally gavaged with 250 μL of TNP-bovine serum albumin (BSA; 50 mg) in saline. Before the intragastric (i.g.) challenge, mice were deprived of food for 3 to 4 hours. Challenges were performed with i.g. feeding needles (01-290-2B; Fisher Scientific Co., Pittsburgh, PA). Rectal temperatures were measured before and 60 minutes after ovalbumin (OVA) challenge. Evidence of secretory diarrhea was assessed by determination of short-circuit current (Isc) of small intestinal segments ex vivo in the Ussing chamber system up to 60 minutes after i.g. challenge. Severity of shock was assessed by rectal temperature with a rectal probe.17,27
Oral Antigen-Induced Intestinal Anaphylaxis
Four- to 8-week-old mice were sensitized with 50 μg of OVA and 1 mg of alum in sterile saline by intraperitoneal (i.p.) injection. Beginning 2 weeks later, mice were held in the supine position three times a week for 3 weeks and orally gavaged with 250 μL of OVA (50 mg) in saline or 250 μL of saline [vehicle (Veh)]. Before each i.g. challenge, mice were deprived of food for 3 to 4 hours. Challenges were performed with i.g. feeding needles (01-290-2B; Fisher Scientific Co.). Rectal temperatures were measured before and 60 minutes after OVA challenge. Diarrhea was assessed by visually monitoring mice for up to 60 minutes after i.g. challenge. Mice showing profuse liquid stool were recorded as diarrhea-positive. Evidence of secretory diarrhea was assessed by determination of Isc of small intestinal segments ex vivo in the Ussing chamber system up to 60 minutes after i.g. challenge. Unless stated, all measurements of parameters of anaphylaxis were measured after the seventh challenge. In some experiments mice were treated with anti-CD4 antibody (GK1.5; 1 mg/200 μL i.p.) 24 hours before the seventh challenge. CD4+ T-cell depletion was analyzed by flow cytometric analyses. Splenic CD4+ T cells were reduced by >95%.
Solutions and Drugs
The Krebs buffer used on each side of the Ussing chamber contained 4.70 mmol/L KCl, 2.52 mmol/L CaCl2, 118.5 mmol/L NaCl, 1.18 mmol/L NaH2PO4, 1.64 mmol/L MgSO4, and 24.88 mmol/L NaHCO3. The tissues were allowed to equilibrate for 15 minutes in Krebs buffer that contained 5.5 mmol/L glucose. All reagents were obtained from Sigma-Aldrich (St Louis, MO) unless stated otherwise.
Ussing Chambers
One-centimeter, freshly isolated, serosal-stripped segments of jejunum were mounted between the hemi-chambers of an Ussing apparatus (U2500 Dual Ussing chamber; Warner Instruments, Hamden, CT), and 0.112 cm2 of tissue was exposed to 10 mL of Krebs buffer at 37°C. The transepithelial potential difference was detected with two paired electrodes that contained 4% agar in 3 mol/L KCl. The electrodes were connected to a voltage clamp amplifier (EC-800, Epithelial voltage clamp; Warner Instruments). The electrode potential difference and fluid resistance were compensated before mounting tissue segments into the chamber. To establish equilibrium, potential difference was continuously monitored under open-circuit conditions for 15 minutes. Thereafter, the tissues were voltage-clamped at 0 mV while continuously measuring Isc. Voltage pulses (3-mV square waves sustained for 5 seconds) were delivered every 50 seconds to yield a current response for calculation of transepithelial resistance (TER) from Ohm's law. For ion conductance experiments, changes in Isc were determined for the cumulative addition of forskolin and acetylcholine to the serosal reservoir. After the peak response to the final concentration of each agonist was recorded, the Krebs buffer on each side of the chamber was replaced, and the tissue was allowed to equilibrate for 30 minutes. Immediately after re-equilibration, tissue was preincubated with ion channel blockers [4,4′-Diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) 100 μmol/L or CFTRInh172 20 μmol/L to mucosal reservoir], and changes in Isc were measured in response to the addition of forskolin to the mucosal side.
Intestinal Mast Cell Quantification
Jejunum tissue was collected 7 to 10 cm distal to the stomach, whereas ileum and colon samples were collected 1 cm proximal or distal of the cecum. All samples were fixed in 10% formalin and processed by standard histologic techniques. The 5-μm tissue sections were also stained for mucosal mast cells with chloroacetate esterase (CAE) activity as described elsewhere28 and lightly counterstained with hematoxylin. At least four random sections per mouse were analyzed. Quantification of stained cells was performed by counting the number of CAE+ cells from 25 to 50 fields of view (magnification, ×40). In some experiments matched samples were fixed in 4% paraformaldehyde and processed as described above, and mast cells were quantified to confirm accurate mast cell assessment.
Respiratory Status Measurements
Airway resistance was measured with the use of barometric plethysmography and the flexiVent system (Scireq Scientific Respiratory Equipment, Inc., Montreal, QC). For determination of responsiveness to methacholine by barometric plethysmography, mice were placed, unrestrained, in cylindrical Plexiglas plethysmograph chambers that were connected to a nebulized control aerosol delivery system and a Max II apparatus for analyzing barometric plethysmography (Buxco Research Systems, Wilmington, NC). Baseline measurements of enhanced pause29 were made over a 5-minute period. Mice were then challenged for 3 minutes by inhalation of aerosolized β-methacholine in PBS, produced with a nebulizer (model 5500D-030; DeVilbiss Healthcare, Somerset, PA), starting at a methycholine concentration of 0.8 mg/mL. Enhanced pause measurements were made for 5 minutes, starting 2 minutes after completion of exposure to the aerosolized methacholine, and average enhanced pause values for the 5-minute period were calculated. The procedure was then serially repeated with methacholine at concentrations of 1.6, 3.2, 6.4, 12.8, and 25.6 mg/mL. Airway responsiveness to methacholine of anesthetized, intubated mice was also performed with a flexiVent apparatus (Scireq Scientific Respiratory Equipment, Inc.). Briefly, the mice were anesthetized, a tracheotomy was performed, and a cannula was inserted. A positive end-expiratory pressure of 0.2 kPa was established. Saline aerosol followed by β-methylcholine (25 to 100 mg/mL; Sigma-Aldrich) established control baseline. Aerosols were generated with an ultrasonic nebulizer (UltraNeb 2000; DeVilbiss Healthcare) and delivered to the inspiratory line of the FlexiVent. Each aerosol was delivered for 20 seconds during which time regular ventilation was maintained. Five measurements were made at 25-second intervals, and three peak responses were compared with the mean response of the saline aerosol as previously described.30,31
BALF Analysis
OVA- or control (Veh)-challenged mice were sacrificed, the trachea was cannulated after midline neck incision, and the lungs were lavaged twice with 0.8 mL of normal saline containing 0.5 mmol/L EDTA. The recovered broncho-alveolar lavage fluid (BALF) was centrifuged at 400 × g for 10 minutes at 4°C, the supernatant fluid was removed, and mast cell protease (Mcpt-1) levels were assessed by enzyme-linked immunosorbent assay as described by the manufacturer (eBioscience, San Diego, CA). The BALF cells were counted with a hematocytometer. Cytospin preparations were stained with Diff-Quick (Dade Diagnostics of Puerto Rico Inc., Aguada, Puerto Rico). In some experiments lung tissue representing the central (bronchi/bronchiole) and peripheral (alveoli) airways were fixed in 10% phosphate-buffered formalin with standard histologic techniques and stained with H&E for histologic analyses.
Vascular Permeability
Evans blue tissue extravasation was performed as previously described.32 Briefly, mice received Evans blue dye in PBS (20 mg/kg, i.v.); 3.5 hours later, mice were anesthetized with pentobarbital (20 mg/kg, i.p.), and heart perfusion was performed (10 mL of PBS arterial perfusion). Ear and jejunum were harvested, resuspended in 250 μL of formamide, and incubated overnight at 37°C. The tissue was centrifuged at 14,000 rpm for 15 minutes at room temperature. The supernatant fluid was removed, and absorbance was measured at 650 nm. Tissue protein levels were quantified with a BCA protein assay kit per the manufacturer's instructions (Pierce, Rockford, IL). Evans blue extravasation was calculated as microgram of Evans blue per milligram of total protein.
Statistical Analysis
Data are expressed as mean ± SEM, unless otherwise stated. Statistical significance comparing different sets of mice was determined by Student's t-test. In experiments that compared multiple experimental groups, statistical differences between groups were analyzed with the one-way, nonparametric analysis of variance and a Bonferroni posttest. P < 0.05 was considered significant. Spearman's rank coefficients were used to quantify the relations between intestinal mast cell levels and maximum temperature change. All analyses were performed with Prism 5.0 software (GraphPad Software Inc., San Diego, CA).
Results
Intestinal and Systemic Involvement in Oral Antigen-Induced Anaphylaxis
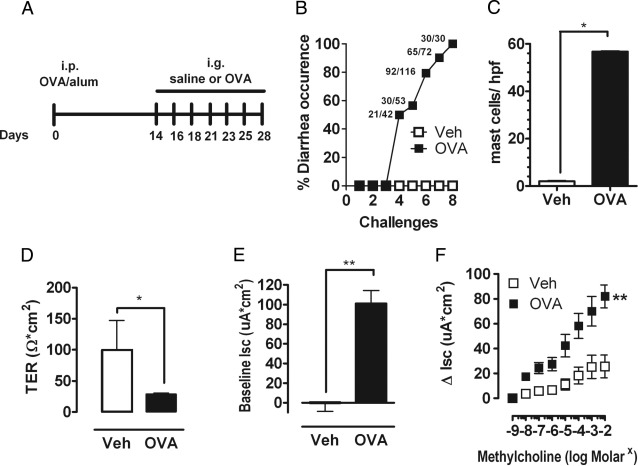
To begin deciphering the molecular pathways that modulate severity of food-triggered anaphylaxis, we developed a murine model of oral antigen-triggered anaphylaxis that has both intestinal and systemic manifestations. BALB/c WT mice were primed i.p. with OVA/alum and subsequently challenged by repeated oral gavage with OVA or with saline (Figure 1A). Repeated oral gavage induced evidence of anaphylaxis by the fourth challenge, whereby ∼50% of the mice had diarrhea within 60 minutes of oral gavage. The occurrence of diarrhea increased after the subsequent fifth to seventh challenges, and by the eighth challenge 100% showed diarrhea (Figure 1B). We chose to focus our analyses for evidence of intestinal and systemic anaphylaxis after the seventh challenge because we observed a robust anaphylactic response (diarrhea). This was the first challenge whereby >90% of mice experienced diarrhea (Figure 1B). Assessment of other features of intestinal symptoms of anaphylaxis after the seventh challenge showed a pronounced intestinal mastocytosis (Figure 1C). Oral antigen-induced secretory diarrhea is caused by net chloride (Cl−) secretion (basolateral → lumen flux) and is indicated by an increased transmural Isc.33–35 To ascertain whether oral antigen challenge induced secretory diarrhea, the jejunum of Veh- and OVA-challenged mice was excised after the seventh oral challenge, and TER and Isc were determined. TER was significantly decreased in jejunum from OVA-challenged WT mice compared with Veh-treated WT mice, indicating increased intestinal permeability (Figure 1D). Furthermore, OVA-challenged WT mice had a significant increase in the basal Isc and ΔIsc after cholinergic (β-methylcholine) stimulation compared with Veh-treated WT mice (Figure 1, E and F). Collectively, these results indicate that oral antigen challenge promotes an intestinal mastocytosis and secretory diarrhea.
Figure 1.
Intestinal symptoms in oral antigen-triggered anaphylaxis. A: Oral antigen-triggered anaphylaxis experimental regime. Diarrhea occurrence (B) and mean number of mast cells per high-power field (hpf) (C) in the small intestine of OVA-sensitized, i.g. Veh- or OVA-challenged BALB/c WT mice. The common fractions in panel B represent the number of mice that experienced diarrhea over the number of total mice challenged. TER (D), Isc (E), and β-methylcholine-stimulated (F) changes in Isc (ΔIsc) in jejunum segments of OVA-sensitized, i.g. Veh- or OVA-challenged (seventh challenge) BALB/c WT mice. Values represent mean ± SEM; n = 6 to 10 mice per group. Statistical significance is *P < 0.05 or **P < 0.01.
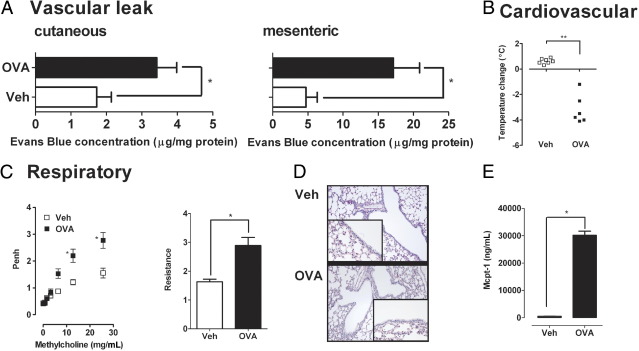
Assessment of systemic signs and symptoms after the seventh OVA challenge to OVA-primed BALB/c WT mice showed induction of cutaneous and mesenteric vascular leak as evidenced by increased Evans blue extravasation and associated hypothermia (Figure 2, A and B). Assessment of respiratory status found increased airway responsiveness to cholinergic (β-methacholine) stimulation in the OVA-gavaged OVA-primed WT mice compared with saline-gavaged OVA-primed animals as measured by barometric plethysmography and by an invasive technique (Figure 2C). Histologic analyses of the lung and BALF analyses showed that the AHR response occurred independently of a pulmonary cellular infiltrate (Veh versus OVA, 16.0 ± 1.6 BALF cells ×104 cells/mL versus 11.0 ± 3.4 BALF cells ×104 cells/mL; mean ± SE; n = 4 mice per group; Figure 2D). Notably, although no pulmonary cellular infiltrate was observed, levels of the mast cell activation marker, Mcpt-1, were significantly elevated in the BALF fluid of OVA-gavaged OVA-primed WT mice compared with saline-gavaged OVA-primed animals (Figure 2E). These data show that oral antigen-triggered anaphylaxis is associated with the development of systemic disease.
Figure 2.
Systemic manifestations in oral antigen-triggered anaphylaxis. A: Before the seventh OVA challenge, mice received i.v. injection of 2% Evans Blue (200 μL/PBS) and were subsequently challenged with OVA. Sixty minutes after OVA challenge, the mice were sacrificed, and Evans blue concentration was determined in the ear (cutaneous) and intestine (mesenteric). Temperature change from 0 to 60 minutes (B), lung function (C), histology (D), and BALF Mcpt-1 levels (E) after the seventh i.g. Veh or OVA challenge in OVA-sensitized WT mice. C: Airway reactivity to methacholine was measured 30 minutes after the seventh OVA challenge with the use of whole-body plethysmography and invasive technique (flexiVent system). D: Original magnification, ×40. Data in A and C are represented as mean ± SEM; n = 4 to 8 mice per group from triplicate experiments. *P < 0.05; **P < 0.01.
FcεRI/IgE Involvement in Intestinal and Systemic Manifestations of Oral Antigen-Induced Anaphylaxis
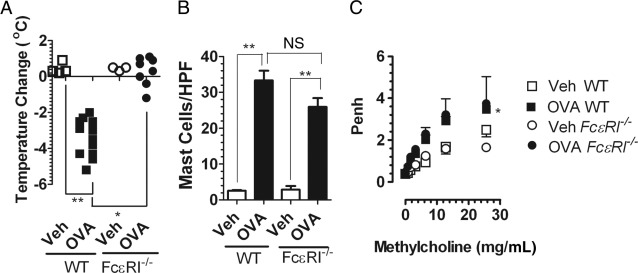
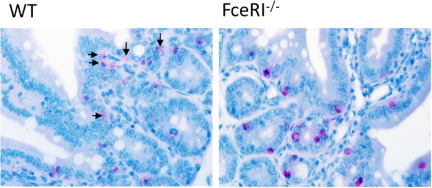
Clinical evidence indicates that food-triggered anaphylaxis is mediated by an IgE/mast cell-mediated pathway.11–17 Previous investigations by us and others indicate that systemic anaphylaxis in mice can be mediated by either an IgE pathway that involves mast cells or a pathway that involves IgG, FcγRIII, macrophages, basophils, and platelet-activating factor.36,37 To further delineate the role of IgE in the intestinal and systemic symptoms of oral antigen-induced anaphylaxis, WT and FceRI−/− mice were sensitized i.p. with OVA/alum and challenged repeatedly with OVA or saline by oral gavage. Notably, WT, but not FceRI−/−, mice had diarrhea and were hypothermic 1 hour after the seventh oral gavage, indicating that oral antigen-induced intestinal and cardiovascular involvement depends on FcεRI/IgE signaling (Figure 3A). Importantly, the level of intestinal mast cells between WT and FceRI−/− mice was equivalent, indicating that the abrogation of intestinal and cardiovascular symptoms was not because of mast cell deficiency (Figure 3B). To confirm lack of mast cell activation in FceRI−/− mice we assessed serum mcpt-1 levels. We observed no significant increase in serum mcpt-1 in FceRI−/− mice after oral gavage challenge (WT control, 74.0 ± 10.0 ng/mL; WT OVA, 25,858.0 ± 2736.0 ng/mL*; FceRI−/− OVA, 69.3 ± 12.6 ng/mL; mean ± SEM; n = 3 to 12 mice per group; *P < 0.001). Histologic assessment of intestinal mast cells showed remnants of extracellular mast cell granules (CAE+ granules) in the lamina propria of the small intestine of WT OVA mice, whereas mast cells in FceRI−/− mice remained intact and granules were confined within the cytoplasm (see Supplemental Figure S1 at http://ajp.amjpathol.org). These data indicate that oral gavage challenge induces local intestinal mast cell degranulation that depends on FcεRI/IgE-signaling.
Figure 3.
Intestinal and systemic manifestations of food-triggered anaphylaxis is FcεRI/IgE dependent. Temperature change from 0 to 60 minutes and diarrhea (A) and mast cells per high-power field (HPF) in small bowel in WT and FceRI−/− mice (B) after the seventh OVA challenge. Solid symbols indicate mice that developed diarrhea. C: Lung function in OVA-sensitized, i.g. Veh- or OVA-challenged WT and FceRI−/− mice. Airway reactivity to methacholine was measured 30 minutes after the seventh Veh or OVA challenge with the use of whole-body plethysmography. Data in B and C are represented as mean ± SEM; n = 4 to 8 mice per group from duplicate experiments. *P < 0.05; **P < 0.01. NS, not significant.
Surprisingly, although the diarrhea and hypothermia were attenuated in FceRI−/− mice, we observed no differences in AHR between OVA-challenged WT and FceRI−/− mice as measured by barometric plethysmography and by an invasive technique (Figure 3C; see also Supplemental Figure S2 at http://ajp.amjpathol.org). Thus, the FcεRI/IgE pathway is important in intestinal and cardiovascular symptoms; however, it is not required for the AHR response associated with oral antigen-triggered anaphylaxis. Studies with mouse models of allergic airway disease indicate that the development and maintenance of AHR depends on CD4+ T-cell-derived IL-13 and to a lesser extent IL-4.38 To assess the contribution of CD4+ T cells to the AHR response in FceRI−/− mice, we treated FceRI−/− mice with either isotype control or CD4+ T-cell-depleting antibody (GK1.5) 24 hours before the seventh OVA challenge and assessed AHR response 60 minutes after oral OVA gavage. We show that anti-CD4 treatment abrogated the AHR response in oral OVA-gavaged FceRI−/− mice compared with isotype control-treated mice (see Supplemental Figure S2 at http://ajp.amjpathol.org). These data indicate that the OVA challenge-induced AHR response in FceRI−/− mice depends on CD4+ T cells.
Relations between Intestinal Mast Cell Levels and Severity of Systemic Symptoms
We were next interested in identifying immune pathways that modulate the severity of oral antigen-induced anaphylaxis. Because we have demonstrated a requirement for the IgE/FceRI/mast cell pathway in oral antigen-induced anaphylaxis, we initially assessed for a relation between levels of IgE and mast cells and the systemic parameter of oral antigen-induced anaphylaxis severity, hypothermia (maximum temperature change), after the seventh oral challenge. Spearman's rank coefficient correlation analysis showed no correlation between oral antigen-specific IgE levels and maximum temperature change (results not shown); however, we did observe a significant negative correlation between intestinal mast cell levels and maximum temperature change (r = −0.5377, P = 0.0098), indicating a relation between intestinal mast cell levels and the systemic manifestations of oral antigen-induced anaphylaxis. These data indicate that intestinal mast cell levels may influence severity of food-induced anaphylaxis.
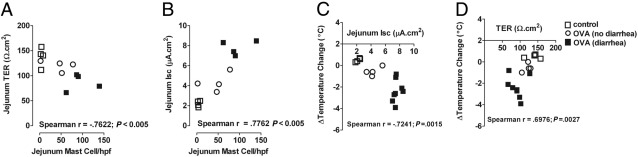
To begin to assess the relation between intestinal mast cell levels and severity of anaphylaxis in WT mice we assessed systemic (hypothermia) and intestinal (TER and secretory diarrhea) symptoms of anaphylaxis after the fourth oral gavage challenge. We specifically chose the fourth oral gavage challenge because we observed an ∼50% penetrance of oral antigen-induced anaphylaxis in WT mice after this challenge (see Supplemental Figure S3 at http://ajp.amjpathol.org), and mice that do develop anaphylaxis experience an anaphylactic reaction that ranged from mild to moderate severity (see Supplemental Figure S3 at http://ajp.amjpathol.org). Quantification of systemic (hypothermia) and intestinal (TER and secretory diarrhea) symptoms of anaphylaxis after the fourth oral gavage challenge showed a strong correlation between intestinal mast cell density and intestinal involvement (TER and Isc) (Figure 4, A and B; P < 0.005 and P < 0.005), which in turn correlated with cardiovascular (systemic) involvement (Figure 4, C and D; P < 0.005). Similarly, levels of the mast cell activation marker mcpt-1 in serum negatively correlated with intestinal involvement (TER; r = −0.7, P = 0.04; n = 9). These data indicate a strong relation between intestinal mast cell density and activation and the severity of oral antigen-induced anaphylaxis.
Figure 4.
Correlation between intestinal mast cell levels and intestinal and systemic symptoms of oral antigen-induced anaphylaxis. Spearman's rank correlation coefficient between mean number of mast cells per high-power field (hpf) in the small intestine and small intestine epithelial permeability (TER) (A), or intestinal epithelial chloride secretion (B), and between mean temperature change from 0 to 60 minutes and intestinal epithelial chloride secretion (C), or intestinal epithelial permeability change (D) after the fourth i.g. Veh or OVA challenge in OVA-sensitized WT mice.
Intestinal Mast Cell Load Increases Severity of Passive and Oral Antigen-Triggered IgE-Mediated Anaphylaxis
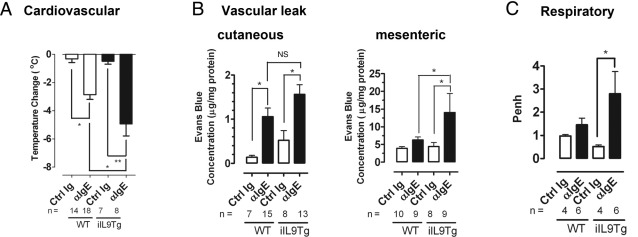
The finding that intestinal mast cell density correlated with increasing severity of systemic symptoms of anaphylaxis led us to speculate that mice with increased intestinal mast cell levels would experience more severe intestinal and systemic symptoms of oral antigen-induced anaphylaxis. To directly test this hypothesis, we examined severity of IgE-mediated anaphylaxis in BALB/c WT and iIL9Tg mice. iIL9Tg mice have increased numbers of mast cells in their intestines but not in other tissues.19 Furthermore, we observed no differences in levels of total IgE or CD4+ T-cell and B-cell function in iIL9Tg mice compared with WT mice.19 First, we examined severity of passive IgE-mediated systemic anaphylaxis in BALB/c WT and iIL9Tg mice. WT and iIL9Tg mice were injected i.v. with 10 μg of anti-IgE or isotype control antibody, and development of shock, a systemic anaphylactic symptom, was assessed by rectal thermometry. The level of hypothermia that developed in iIL9Tg mice was significantly elevated (ie, greater negative change in temperature) compared with WT mice (Figure 5A). Consistent with this observation, other systemic features, including cutaneous and mesenteric vascular leaks and AHR, were also significantly elevated in comparison to WT mice treated with anti-IgE (Figure 5, B and C). The exacerbated anaphylaxis was specific to IgE-mediated responses because we observed comparable development of hypothermia in response to i.v. anti-FcγRII/III mAb injection in these strains (Figure 6). We have previously demonstrated that there are no differences in serum IgG levels between strain- and littermate-matched WT and iIL9Tg mice.19 These data indicate that increased intestinal IL-9 and mast cell numbers increase the severity of passive IgE-mediated systemic anaphylaxis.
Figure 5.
Systemic and intestinal manifestations of anti-IgE-treated WT and iIL9Tg mice. Maximum temperature change (A), cutaneous (ear) and mesenteric leak (B), and lung function (C) in BALB/c WT and iIL9Tg mice treated with isotype control (Ig, clone βGL117) or anti-IgE (clone EM95) antibody. B: Before the anti-IgE treatment, mice received i.v. injection of 2% Evans blue (200 μL/PBS) and were subsequently challenged with anti-IgE. C: WT and iIL9Tg mice received an i.v. anti-IgE injection, and airway reactivity to methacholine was measured by whole-body plethysmography. Number of mice are indicated. Data are presented as mean ± SEM *P < 0.05; **P < 0.01. Ctrl Ig, isotype control; NS, not significant.
Figure 6.
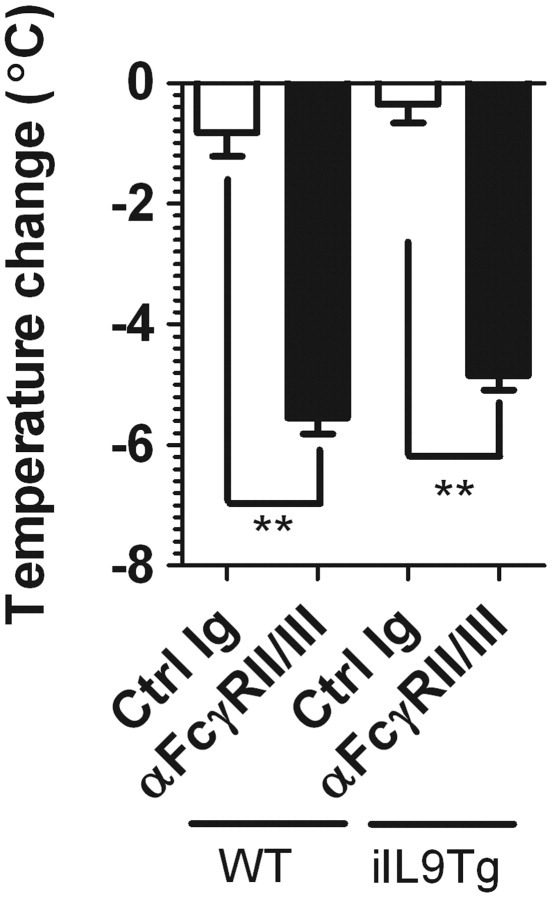
Anti-FcγRII/III-mediated passive systemic anaphylaxis in WT and iIL9Tg mice. Maximum temperature change of WT and iIL9Tg mice to i.v. injection of anti-FcγRII/III mAb (clone 2.4G2) or isotype control (Ig, clone J1.2) antibody. Data represent mean ± SEM; n = 4 mice per group from duplicate experiments. **P < 0.01 compared with WT control (Ctrl) Ig. Ctrl Ig, isotype control.
We next assessed whether intestinal mast cell load can influence severity of oral antigen-triggered anaphylaxis. WT and iIL9Tg mice were primed i.p. with OVA/alum and subsequently challenged by repeated oral gavage with OVA or with saline (Figure 7). Repeated administration of OVA induced anaphylaxis with intestinal and systemic involvement (Figures 1 and 7). Notably, the severity of the hypothermia was significantly greater in iIL9Tg mice than in WT mice (Figure 7). These studies indicate that elevated intestinal IL-9 increases mast cell number and severity of oral antigen-triggered anaphylaxis.
Figure 7.
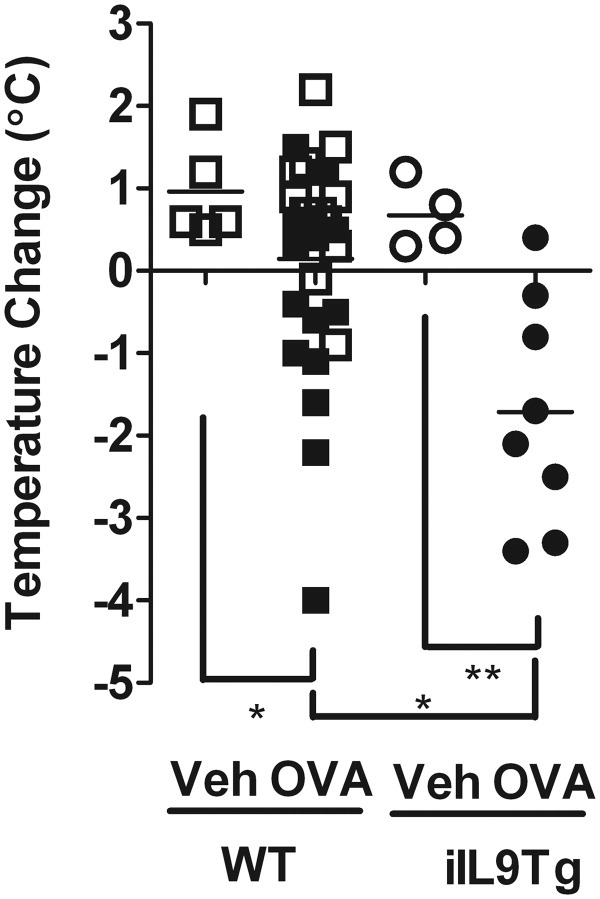
Intestinal and systemic manifestations of oral antigen-triggered anaphylaxis in WT and iIL9Tg mice. Temperature change from 0 to 60 minutes and diarrhea in OVA-sensitized, i.g. Veh- or OVA-challenged BALB/c WT and iIL9Tg mice after the seventh OVA challenge. Solid symbols indicate mice that developed diarrhea. *P < 0.05; **P < 0.01.
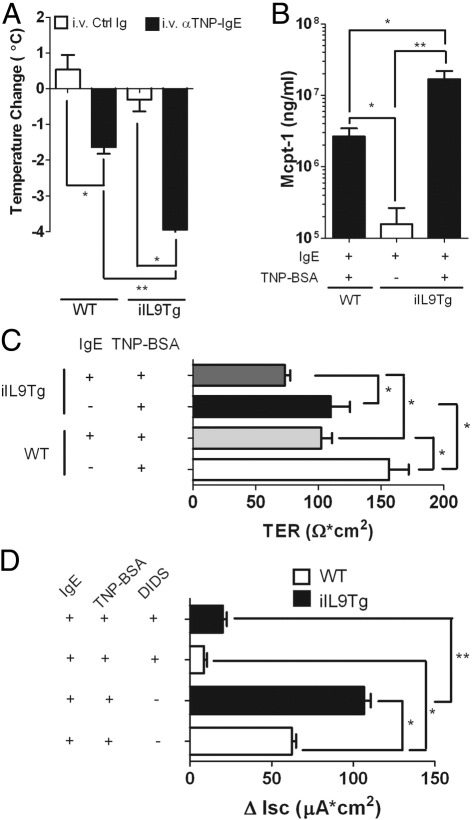
We have previously reported that oral antigen-triggered anaphylaxis in WT mice is associated with increased T helper type 2 (Th2) cytokine production and that Th2 cytokines can exacerbate severity of anaphylaxis.39 To exclude the contribution of Th2 cytokines and altered immune repertoire in IgE-mediated reactions in iIL9Tg mice, we developed a passive oral antigen-triggered anaphylaxis model to assess whether increased intestinal mast cell levels are sufficient to increase severity of oral antigen-triggered anaphylaxis. WT and iIL9Tg mice were injected i.v. with isotype control Ig or 10 μg of anti-TNP IgE antibody and were administered TNP-BSA (50 mg/mL; 250 μL; i.g.) 24 hours later. Oral gavage of TNP-BSA induced hypothermia in anti-TNP-IgE-treated WT mice ∼10 to 15 minutes after antigen challenge (Figure 8A), which was resolved by 60 minutes (not shown). Importantly, the kinetics of the hypothermia were comparable to those observed in the active oral antigen-triggered anaphylaxis model (Figure 2; results not shown). Similarly, TNP-BSA administration to IgE anti-TNP mAb-primed iIL9Tg mice induced hypothermia; however, the hypothermia in these mice was significantly greater than that observed in WT mice (Figure 8A), as was mast cell protease production (Figure 8B). Assessment of intestinal symptoms found that neither the WT nor iIL9Tg mice developed observable external diarrhea (results not shown). We speculated that, although external diarrhea in the oral antigen-triggered anaphylaxis depends on IgE/mast cells, it may also require CD4+-derived Th2 cytokine involvement to promote exaggerated peristaltic activity.40 We therefore directly assessed basolateral → apical Cl− ion conductance of the small intestine of WT and iIL9Tg mice ex vivo because Cl− conductance drives small intestine hydration and secretory diarrhea (Figure 8). Oral gavage of TNP-BSA induced a decrease in TER in anti-TNP-IgE-treated WT mice compared with control mice, indicating changes in intestinal epithelial permeability (Figure 8C). Consistent with this observation, carbachol-induced ΔIsc of the small intestine of WT mice administered TNP-BSA was significantly increased compared with control-treated WT mice (Figure 8D). Notably, the TER and ΔIsc of the small intestine of iIL9Tg mice after carbachol stimulation was significantly greater than that observed in WT mice administered TNP-BSA (Figure 8, C and D). To confirm that the ΔIsc is because of increased Cl− conductance, we assessed Isc after pharmacologic blockade of the apical Cl− channel by Ca2+-activated Cl− channel inhibitor DIDS.41,42 We show that the carbachol-induced ΔIsc was attenuated in the presence of DIDS, showing that oral administration of TNP-BSA induced basolateral → apical Cl− ion conductance. These studies indicate that increased intestinal IL-9 and mast cells predispose to increased severity to IgE-mediated oral antigen-triggered anaphylaxis.
Figure 8.
Passive oral antigen-triggered IgE-mediated anaphylaxis in WT and iIL9Tg mice. Maximum temperature change (A), serum Mcpt-1 levels (B), TER (C), and carbachol-induced change in Isc (ΔIsc) (D) of the small intestine of anti-TNP-IgE- (10 μg) or control Ig- (10 μg) treated WT and iIL9Tg mice orally gavaged with TNP-BSA (50 mg/mL; 500 μL). Values represent mean ± SEM; n = 4 to 8 mice per group. *P < 0.05; **P < 0.01. Ctrl Ig, isotype control.
Discussion
In the present study, we have developed a murine model of oral antigen-induced anaphylaxis with intestinal and systemic symptoms to assess the relative contribution of intestinal mast cell levels to the severity of the anaphylactic reaction. We show that OVA oral gavage of OVA-primed BALB/c mice induces intestinal and systemic symptoms of anaphylaxis. We show that the oral antigen-triggered anaphylaxis depends on IgE-mast cell activity; however, AHR occurred independently of IgE mast cell activation (Figure 3C; see also Supplemental Figure S2 at http://ajp.amjpathol.org). Notably, we show that intestinal mast cell levels positively correlated with the severity of systemic symptoms of anaphylaxis induced by oral antigen challenge. With the use of mice with increased intestinal mast cells, we show that increased intestinal mast cell load is sufficient to increase severity of both passive and oral active IgE-mediated anaphylaxis. These data indicate that intestinal mast cell levels may be an important factor in the regulation of severity of the clinical symptoms of food-induced anaphylaxis.
Intestinal and systemic signs and symptoms of food-induced anaphylaxis in humans include diarrhea (intestinal), urticaria (cutaneous), shock (cardiovascular), and wheezing and bronchospasm (respiratory).6 We show that oral gavage with OVA induced intestinal (diarrhea) and systemic (cutaneous and mesenteric vascular leak, AHR, and hypothermia) signs and symptoms of anaphylaxis within 60 minutes of antigen challenge. The diarrhea was associated with increased basal Isc and carbachol-induced ΔIsc and was sensitive to Cl− channel blockade, suggesting increased basolateral → luminal Cl− secretion. Notably, the diarrheal response was abrogated in FceRI−/− mice, indicating that this symptom depends on IgE/FcεRI signaling and mast cells. This finding is consistent with the demonstration of ablation of oral antigen-induced ΔIsc in sensitized, mast cell-deficient mice.43–45 A number of mast cell-derived mediators, including histamine, prostaglandin D2, and leukotrienes, have been shown to modulate intestinal epithelial ion transport.44,46,47 Systemic anaphylaxis in mice is predominantly a consequence of vascular leak, which causes hypovolemic hypotension that leads to hypothermia12,17 and can be mediated by a mast cell/IgE-dependent pathway or by an IgG-dependent pathway that involves IgG, FcγRIII, macrophages, and basophils.36,37 Notably, the systemic symptoms could be induced in passive IgE-induced anaphylaxis and were abrogated in FceRI−/− mice, indicating that systemic responses depend on IgE/FcεRI signaling. We have previously reported no effect of neutralization of FcγRIIb and FcγRIII pathway or IgG1 deficiency on oral antigen-induced anaphylaxis in IgG1-deficient mice, indicating no role for the IgG pathway in oral antigen-triggered anaphylaxis.18 These results are consistent with human food-induced anaphylactic reactions that occur within seconds to a few hours after exposure to the causal food allergen7 and are associated with IgE/mast cell activity and mast cell-derived mediator release.8 Furthermore, anti-IgE antibodies can effectively decrease symptoms in humans after peanut challenge.48
We show that administration of anti-TNP-IgE and subsequent oral gavage with TNP-BSA in iIL9Tg mice induced AHR, suggesting that IgE/mast cell activation can promote an AHR response. The IgE-mast cell-mediated AHR response may be mediated by mast cell-derived mediator-induced smooth muscle contraction of the airway, or, alternatively, by a consequence of increased interstitial fluid in the lung. Mast cells release a number of mediators, including histamine, prostaglandins, leukotriene C4, and IL-4, which are potent agonists for smooth muscle contraction of airways and vascular leak.49,50,17 Previously, active systemic anaphylaxis in mice has been associated with altered pulmonary function, including decreased pulmonary conductance and dynamic compliance.51 Notably, the decreased pulmonary conductance, an indicator of pulmonary obstruction from bronchospasm, partially depended on IgE signaling, suggesting that the IgE-mediated AHR response associated with anaphylaxis in mice is a consequence of bronchospasm. Note that antihistamines have been shown to be effective for the treatment of the cutaneous symptoms, but not the respiratory symptoms, associated with human food-induced anaphylaxis,6 suggesting the presence of parallel but independent immune pathways that regulate different systemic manifestations of food-induced anaphylaxis.
Although our studies indicate IgE/mast cell-dependent pathways in the AHR response, the observation of AHR in OVA-primed FcεRI−/− mice after repeated oral gavage of OVA indicates that AHR can also occur independently of the IgE/FcεRI/mast cell pathway. Significant experimental evidence indicates an important contribution of CD4+ T cells in the onset of AHR associated with the asthmatic phenotype.52–54 Consistent with this, we showed that AHR in FcεRI−/− mice depended on CD4+ T cells. However, we observed no effect of oral gavage of OVA on pulmonary IL-4 and IL-13 levels or IL-4- and IL-13-driven processes such as mucus hyperplasia between Veh- and OVA-gavaged WT mice, suggesting that the CD4+ T-cell effect on the AHR response was independent of these cytokines (results not shown). Interestingly, the AHR induced by repeated oral gavage of OVA occurred in the absence of any significant pulmonary cellular infiltrate. Consistent with this observation, previous clinical studies describing double-blinded, placebo-controlled food challenge of patients with food allergies have reported the development of airway reactivity changes in the absence of bronchopulmonary obstruction.55,56 Because asthma and atopy are considered risk factors for severe anaphylaxis,6 we are currently investigating the contribution of a preexisting Th2 phenotype to the IgE-independent AHR response in oral antigen-triggered anaphylaxis.
We provide experimental evidence that increased IL-9 and intestinal mast cell levels are sufficient to increase severity of oral antigen-triggered anaphylaxis. We speculate that elevated intestinal mast cell load amplifies the severity of oral antigen-triggered anaphylaxis by modulation of oral antigen absorption and production of IgE-induced potent autacoid mediators. We have previously reported that iIL9Tg mice have increased intestinal paracellular permeability.19 Altered intestinal permeability can promote increased systemic antigen absorption and dissemination, which may lead to increased IgE/FcεRI mast cell degranulation and increased severity of anaphylaxis. In support of this hypothesis, we have previously reported a positive correlation between systemic antigen dose and severity of systemic anaphylaxis.12 In addition, ablation of susceptibility to oral antigen-triggered anaphylaxis was achieved through abrogation of the altered intestinal barrier function in ilL9Tg mice.12,19 Furthermore, wheat challenge tests on patients with wheat-dependent, exercise-induced anaphylaxis have shown a positive correlation between blood gliadin levels and clinical symptoms of exercise- and aspirin-induced anaphylaxis.57 Intriguingly, physical exercise or intake of aspirin promotes increases in gastrointestinal permeability,58,59 suggesting that altered intestinal permeability may contribute to the increased severity of food-induced anaphylaxis.
The increased severity of anaphylaxis in the iIL9Tg mice may not necessarily pertain to mast cell-dependent physiological effects but could simply be because of the increased intestinal mast cell load. Moreover, iIL9Tg mice have increased total number of intestinal mast cells; thus, after IgE-mediated activation, they release a greater level of mast cell-derived mediators that disseminate and induce increased systemic anaphylactic symptoms. The serum level of Mcpt-1 in the iIL9Tg mice was significantly increased compared with WT mice, indicating increased mast cell degranulation. Furthermore, i.v. administration of anti-IgE antibody to iIL9Tg mice induced a greater anaphylactic response than that observed in WT mice, indicating that the observed increase in anaphylactic reaction severity can occur independently from the mast cell-induced decrease in intestinal epithelial barrier function. The increased anaphylactic response is probably a consequence of a specific intestinal mast cell degranulation because mast cell levels in other tissues, including skin, tongue, heart, and spleen are normal (WT, 8.7 ± 2.9 C-kit+ FcεRI+ cells × 103 per 106 splenocytes; iIL9Tg, 7.7 ± 0.4 C-kit+ FcεRI+ cells × 103 per 106 splenocytes; n = 4 mice per group19). A clinical correlate to the iIL9Tg mouse may be patients with mastocytosis, a proliferative disorder of the hematopoietic mast cell progenitor lineage resulting in excessive numbers of tissue mast cells.60 Two recent, large studies consisting of a total of 320 cases of mastocytosis have reported increased risk of developing anaphylaxis.61,62 Anaphylactic reactions have been reported in 22% to 49% of adult and 6% to 9% of pediatric patients with mastocytosis, whereas the lifetime prevalence in the general population is ∼1%.63
Development of risk assessment criteria for differentiation between life-threatening and non–life-threatening anaphylaxis is hindered by the inconsistent constellation of symptoms present in clinical histories. Furthermore, skin prick test and food antigen-specific IgE levels are not necessarily predictive of susceptibility to or severity of anaphylaxis.64 We present experimental data showing that increased intestinal mast cell levels can increase susceptibility and severity to oral antigen-triggered anaphylaxis in mice. On the basis of this evidence and similarities between clinical anaphylaxis and our experimental model, investigation of the intestinal mast cell levels and phenotypes in patients with and without a clinical history of food-triggered anaphylaxis is warranted to assess the potential predictive value of intestinal mast cells for susceptibility to life-threatening and non–life-threatening anaphylaxis.
Acknowledgments
We thank Drs. Pablo Abonia, Ariel Munitz, and Marc Rothenberg for helpful discussions and Shawna Hottinger for editorial assistance.
Footnotes
Supported in part by Food Allergy Anaphylaxis Network, American Heart Foundation Midwest Affiliate, the Academy of Allergy and Asthma and Immunology Interest Section Award 2007 (S.P.H.), and NIH grants (R01AI073553-01 to S.P.H. and P30DK078392 to R.S.).
R.A. and H.O. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.12.036.
Supplementary data
Supplemental Figure S1.
Histologic assessment of intestinal mast cells in anaphylactic WT and FceRI−/− mice. Histologic assessment of chloroacetate esterase (CAE)-stained small intestine of OVA-sensitized, i.g. OVA-challenged WT and FceRI−/− mice. Arrows indicate remnants of extracellular mast cell granules (CAE+ granules) in the lamina propria of the small intestine of WT OVA mice, whereas mast cells in FceRI−/− mice remained intact, and granules were confined within the cytoplasm.
The role of CD4+ T cells in airway resistance in anaphylactic FceRI−/− mice. Airway resistance in OVA-sensitized, i.g. Veh- or OVA-challenged FceR−/− mice treated with isotype control (control Ig) or anti-CD4-depleting antibody. Airway resistance was measured 30 minutes after the seventh Veh or OVA challenge with the use of invasive technique (flexiVent system). Data represent mean ± SEM; n = 4 to 7 mice per group from duplicate experiments. *P < 0.05.
Systemic manifestations in oral antigen-triggered anaphylaxis. Temperature change from 0 to 60 minutes after the fourth i.g. Veh or OVA challenge in OVA-sensitized WT mice. Individual symbols represent one mouse. Dash indicates mean temperature change (°C).
References
- 1.Sampson H.A. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–1608. [PubMed] [Google Scholar]
- 2.Ross M.P., Ferguson M., Street D., Klontz K., Schroeder T., Luccioli S. Analysis of food-allergic and anaphylactic events in the national electronic injury surveillance system. J Allergy Clin Immunol. 2008;121:166–171. doi: 10.1016/j.jaci.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 3.De Smit V., Cameron P.A., Rainer T.H. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–388. doi: 10.1016/j.jemermed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Brown A.F., McKinnon D., Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–866. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 5.Simons E.R., Chad Z.H., Gold M. Anaphylaxis in children: realtime reporting from a national network. Allergy Clin Immunol Int J World Allergy Org. 2004;(Supplement 1):242. [Google Scholar]
- 6.Wang J., Sampson H.A. Food anaphylaxis. Clin Exp Allergy. 2007;37:651–660. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 7.Sampson H.A., Mendelson L., Rosen J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–384. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 8.Simons F.E., Frew A.J., Ansotegui I.J., Bochner B.S., Golden D.B., Finkelman F.D., Leung D.Y., Lotvall J., Marone G., Metcalfe D.D., Muller U., Rosenwasser L.J., Sampson H.A., Schwartz L.B., van Hage M., Walls A.F. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120:S2–S24. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Sampson H.A., Munoz-Furlong A., Campbell R.L., Adkinson N.F., Jr, Bock S.A., Branum A., Brown S.G., Camargo C.A., Jr, Cydulka R., Galli S.J., Gidudu J., Gruchalla R.S., Harlor A.D., Jr, Hepner D.L., Lewis L.M., Lieberman P.L., Metcalfe D.D., O'Connor R., Muraro A., Rudman A., Schmitt C., Scherrer D., Simons F.E., Thomas S., Wood J.P., Decker W.W. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373–380. doi: 10.1016/j.annemergmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A., Assa'ad A.H., Bahna S.L., Bock S.A., Sicherer S.H., Teuber S.S. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 11.Galli S.J., Kalesnikoff J., Grimbaldeston M.A., Piliponsky A.M., Williams C.M.M., Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 12.Strait R.T., Morris S.C., Yang M., Qu X.W., Finkelman F.D. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 13.Dombrowicz D., Flamand V., Brigman K.K., Koller B.H., Kinet J.P. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 14.Lorentz A., Schwengberg S., Mierke C., Manns M.P., Bischoff S.C. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. 1999;29:1496–1503. doi: 10.1002/(SICI)1521-4141(199905)29:05<1496::AID-IMMU1496>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Santos J., Benjamin M., Yang P.C., Prior T., Perdue M.H. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G847–G854. doi: 10.1152/ajpgi.2000.278.6.G847. [DOI] [PubMed] [Google Scholar]
- 16.Kelefiotis D., Vakirtzi-Lemonias C. In vivo responses of mouse blood cells to platelet-activating factor (PAF): role of the mediators of anaphylaxis. Agents Actions. 1993;40:150–156. doi: 10.1007/BF01984054. [DOI] [PubMed] [Google Scholar]
- 17.Strait R.T., Morris S.C., Smiley K., Urban J.F., Jr, Finkelman F.D. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 18.Osterfeld H.N., Ahrens R.E., Wu D.X., Forbes E., Finkelman F.D., Renauld J., Hogan S.P. Dissection of the role of IL-9/IL-9r-pathway in murine systemic and intestinal anaphylaxis. J Allergy Clin Immunol. 2010;125:469–476. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes E.E., Groschwitz K., Abonia J.P., Brandt E.B., Cohen E., Blanchard C., Ahrens R., Seidu L., McKenzie A., Strait R., Finkelman F.D., Foster P.S., Matthaei K.I., Rothenberg M.E., Hogan S.P. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ujike A., Ishikawa Y., Ono M., Yuasa T., Yoshino T., Fukumoto M., Ravetch J.V., Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudolph A.K., Burrows P.D., Wabl M.R. Thirteen hybridomas secreting hapten-specific immunoglobulin E from mice with Iga or Igb heavy chain haplotype. Eur J Immunol. 1981;11:527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- 22.Baniyash M., Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- 23.Baniyash M., Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- 24.Dialynas D.P., Wilde D.B., Marrack P., Pierres A., Wall K.A., Havran W., Otten G., Loken M.R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 25.Titus R.G., Ceredig R., Cerottini J.C., Louis J.A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985;135:2108–2114. [PubMed] [Google Scholar]
- 26.Finkelman F., Kessler S.W., Mushinski J.F., Potter M. IgD-secreting murine plasmacytomas: identification and partial characterization of two IgD myeloma proteins. J Immunol. 1981;126:680–687. [PubMed] [Google Scholar]
- 27.Strait R.T., Morris S.C., Finkelman F.D. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandt E.B., Strait R.T., Hershko D., Wang Q., Muntel E.E., Scribner T.A., Zimmermann N., Finkelman F.D., Rothenberg M.E. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G.L., Irvin C.G., Gelfand E.W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography [see comments] Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 30.Yang M., Rangasamy D., Matthaei K.I., Frew A.J., Zimmmermann N., Mahalingam S., Webb D.C., Tremethick D.J., Thompson P.J., Hogan S.P., Rothenberg M.E., Cowden W.B., Foster P.S. Inhibition of arginase I activity by RNA interference attenuates IL-13-induced airways hyperresponsiveness. J Immunol. 2006;177:5595–5603. doi: 10.4049/jimmunol.177.8.5595. [DOI] [PubMed] [Google Scholar]
- 31.Munitz A., Brandt E.B., Mingler M., Finkelman F.D., Rothenberg M.E. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green T.P., Johnson D.E., Marchessault R.P., Gatto C.W. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J Lab Clin Med. 1988;111:173–183. [PubMed] [Google Scholar]
- 33.Perdue M.H., Gall D.G. Transport abnormalities during intestinal anaphylaxis in the rat: effect of antiallergic agents. J Allergy Clin Immunol. 1985;76:498–503. doi: 10.1016/0091-6749(85)90733-x. [DOI] [PubMed] [Google Scholar]
- 34.Perdue M.H., Gall D.G. Transport abnormalities during intestinal anaphylaxis in the rat: effect of antiallergic agents. J Clin Invest. 1983;76:498–503. doi: 10.1016/0091-6749(85)90733-x. [DOI] [PubMed] [Google Scholar]
- 35.Castro G.A., Harari Y., Russell D.A. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987;253:540–548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- 36.Finkelman F.D. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimura Y., Obata K., Mukai K., Shindou H., Yoshida M., Nishikado H., Kawano Y., Minegishi Y., Shimizu T., Karasuyama H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 39.Strait R., Morrist S.C., Finkelman F.D. Cytokine enhancement of anaphylaxis. Novartis Found Symp. 2004;257:80–91. discussion 91–100, 276–185. [PubMed] [Google Scholar]
- 40.Zhao A., McDermott J., Urban J.F., Jr, Gause W., Madden K.B., Yeung K.A., Morris S.C., Finkelman F.D., Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 41.Kartner N., Hanrahan J.W., Jensen T.J., Naismith A.L., Sun S., Ackerley C.A., Reyes E.F., Tsui L.C., Rommens J.M., Bear C.E., Riordan J.R. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991;64:681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- 42.Anderson M.P., Sheppard D.N., Berger H.A., Welsh M.J. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol. 1992;7:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- 43.Crowe S.E., Perdue M.H. Anti-immunoglobulin E-stimulated ion transport in human large and small intestine. Gastroenterology. 1993;105:764–772. doi: 10.1016/0016-5085(93)90894-i. [DOI] [PubMed] [Google Scholar]
- 44.Perdue M.H., Masson S., Wershil B.K., Galli S.J. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis: Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berin M.C., Kiliaan A.J., Yang P.C., Groot J.A., Taminiau J.A., Perdue M.H. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997;113:856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- 46.Crowe S.E., Sestini P., Perdue M.H. Allergic reactions of rat jejunal mucosa: Ion transport responses to luminal antigen and inflammatory mediators. Gastroenterology. 1990;99:74–82. doi: 10.1016/0016-5085(90)91232-u. [DOI] [PubMed] [Google Scholar]
- 47.Matuchansky C., Bernier J.J. Effect of prostaglandin E1 on glucose, water and electrolyte absorption in the human jejunum. Gastroenterology. 1973;64:1111–1118. [PubMed] [Google Scholar]
- 48.Leung D.Y., Sampson H.A., Yunginger J.W., Burks A.W., Jr, Schneider L.C., Wortel C.H., Davis F.M., Hyun J.D., Shanahan W.R., Jr Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 49.Jancar S., Sirois M.G., Carrier J., Braquet P., Sirois P. PAF induces rat plasma extravasation and releases eicosanoids during anaphylaxis. Inflammation. 1991;15:347–354. doi: 10.1007/BF00917351. [DOI] [PubMed] [Google Scholar]
- 50.Bradding P. Mast cell regulation of airways smooth muscle function in asthma. Eur Respir J. 2007;29:827–830. doi: 10.1183/09031936.00017707. [DOI] [PubMed] [Google Scholar]
- 51.Oettgen H.C., Martin T.R., Wynshaw-Boris A., Deng C., Drazen J.M., Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 52.Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L., Donaldson D.D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 53.Grunig G., Warnock M., Wakil A.E., Venkayya R., Brombacher F., Rennick D.M., Sheppard D., Mohrs M., Donaldson D.D., Locksley R.M., Corry D.B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavett S.H., O'Hearn D.J., Karp C.L., Patel E.A., Schofield B.H., Finkelman F.D., Wills-Karp M. Interleukin-4 receptor blockade prevents airway responses induced by antigen challenge in mice. Am J Physiol. 1997;272:L253–L261. doi: 10.1152/ajplung.1997.272.2.L253. [DOI] [PubMed] [Google Scholar]
- 55.James J.M., Eigenmann P.A., Eggleston P.A., Sampson H.A. Airway reactivity changes in asthmatic patients undergoing blinded food challenges. Am J Respir Crit Care Med. 1996;153:597–603. doi: 10.1164/ajrccm.153.2.8564104. [DOI] [PubMed] [Google Scholar]
- 56.James J.M., Bernhisel-Broadbent J., Sampson H.A. Respiratory reactions provoked by double-blind food challenges in children. Am J Respir Crit Care Med. 1994;149:59–64. doi: 10.1164/ajrccm.149.1.8111598. [DOI] [PubMed] [Google Scholar]
- 57.Matsuo H., Morimoto K., Akaki T., Kaneko S., Kusatake K., Kuroda T., Niihara H., Hide M., Morita E. Exercise and aspirin increase levels of circulating gliadin peptides in patients with wheat-dependent exercise-induced anaphylaxis. Clin Exp Allergy. 2005;35:461–466. doi: 10.1111/j.1365-2222.2005.02213.x. [DOI] [PubMed] [Google Scholar]
- 58.Lambert G.P., Broussard L.J., Mason B.L., Mauermann W.J., Gisolfi C.V. Gastrointestinal permeability during exercise: effects of aspirin and energy-containing beverages. J Appl Physiol. 2001;90:2075–2080. doi: 10.1152/jappl.2001.90.6.2075. [DOI] [PubMed] [Google Scholar]
- 59.Ryan A.J., Chang R.T., Gisolfi C.V. Gastrointestinal permeability following aspirin intake and prolonged running. Med Sci Sports Exerc. 1996;28:698–705. doi: 10.1097/00005768-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Akin C., Metcalfe D.D. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 61.Brockow K., Jofer C., Behrendt H., Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez de Olano D., de la Hoz Caballer B., Nunez Lopez R., Sanchez Munoz L., Cuevas Agustin M., Dieguez M.C., Alvarez Twose I., Castells M.C., Escribano Mora L. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish Network on Mastocytosis (REMA) Clin Exp Allergy. 2007;37:1547–1555. doi: 10.1111/j.1365-2222.2007.02804.x. [DOI] [PubMed] [Google Scholar]
- 63.Muller U., Haeberli G. The problem of anaphylaxis and mastocytosis. Curr Allergy Asthma Rep. 2009;9:64–70. doi: 10.1007/s11882-009-0010-9. [DOI] [PubMed] [Google Scholar]
- 64.Sicherer S.H., Morrow E.H., Sampson H.A. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582–586. doi: 10.1067/mai.2000.104941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The role of CD4+ T cells in airway resistance in anaphylactic FceRI−/− mice. Airway resistance in OVA-sensitized, i.g. Veh- or OVA-challenged FceR−/− mice treated with isotype control (control Ig) or anti-CD4-depleting antibody. Airway resistance was measured 30 minutes after the seventh Veh or OVA challenge with the use of invasive technique (flexiVent system). Data represent mean ± SEM; n = 4 to 7 mice per group from duplicate experiments. *P < 0.05.
Systemic manifestations in oral antigen-triggered anaphylaxis. Temperature change from 0 to 60 minutes after the fourth i.g. Veh or OVA challenge in OVA-sensitized WT mice. Individual symbols represent one mouse. Dash indicates mean temperature change (°C).