Abstract
The way we are thinking about the kidney filtration barrier is changing from that of a static sieve into a highly dynamic structure regulated through the motility of podocyte foot processes. It has been shown in vitro that inactivation of the small GTPase RhoA causes hypermotility, whereas activation decreases motility. The current article by Wang et al. now shows that both, RhoA over- and underactivation, lead to podocyte foot process effacement and proteinuria in vivo. These data suggest that podocyte health requires a well-controlled balance between the two extremes.
During the last decade, podocytes have emerged as culprit for the vast majority of proteinuric kidney diseases.1 The interdigitating foot processes (FP) of neighbouring podocytes are connected by porous slit diaphragms (SD) and hence form sieve-like structures that prevent the loss of plasma proteins into the urine. The majority of proteinuric kidney diseases share as ultrastructural hallmark the loss of the interdigitating podocyte FP structure, which is called FP effacement. It is generally accepted that FP effacement represents the cause of proteinuria in most cases of glomerular proteinuria. The view of the podocytic filter barrier has changed from static to highly dynamic with podocyte FPs that can rapidly reorganize their actin-based cytoskeleton and change their structure and function: in the classical experimental perfusion of rat kidneys with the polycation protamine sulfate (PS), FP effacement develops within a few minutes and can be fully reversed within 15 minutes of perfusion with the polyanion heparin.2 These rapid changes of podocyte FP architecture require that FPs are able to retract or elongate, hence move, quickly along the glomerular basement membrane (GBM). Reiser and colleagues extended this observation using cultured podocytes to study their motility patterns.3 Indeed, stimuli that lead to the development of FP effacement in vivo (e.g., lipopolysaccharide or puromycin aminonucleoside) cause commonly hypermotility of cultured podocytes in classical cell migration assays.1,3 Based on these observations, the term “podocyte motility” has been coined, referring to dynamic reorganization of the interdigitating FP structure in vivo and for cell migration of cultured podocytes in vitro that is seen as surrogate for the former. Until recently, it has been believed that a stationary podocyte phenotype reflects a stable FP structure with intact slit diaphragms, whereas hypermotility would manifest as FP effacement in vivo.1
Supposing that the reorganization of podocyte FP structure reflects a form of cell motility, it is reasonable to assume similar cellular pathways to be involved as in cell migration, e.g., of tumor cells, one of the most intensely studied forms of cell migration.4 The Rho family of small GTPases (RhoA, Rac1, and Cdc42) are key players in the regulation of actin dynamics and cell migration.4 In the classical view, Rac1 and Cdc42 promote cell motility at the leading edge through the formation of lamellipodia and filopodia, respectively. On the contrary, RhoA promotes the formation of contractile actin–myosin containing stress fibers in the cell body and at the rear of the migrating cell. This simplistic view, though, has been recently challenged by the finding that all three GTPases are activated at the leading edge of migrating cells in a spatiotemporally highly coordinated manner.5
Several studies have suggested an involvement of the small GTPases RhoA, Rac1 and Cdc42 in podocytes during glomerular disease (all reviewed in Ref. 1). Induction of the podocyte urokinase plasminogen activator receptor (uPAR) or of its circulating form suPAR6 leads to increased podocyte motility in vitro and FP effacement and proteinuria in vivo through activation of podocyte β3-integrin which in turn promotes Cdc42 and Rac1 signaling. Furthermore, the podocyte adapter protein synaptopodin has been shown to induce stress fibers by stabilizing RhoA and suppresses filopodia by disrupting Cdc42–IRSp53–Mena signaling complexes. The Mena inhibitor FP(4)-Mito suppresses aberrant filopodia formation in cultured synaptopodin knockdown podocytes and protects against LPS-induced proteinuria in mice in vivo. These data, together with the observation that (1) many proteinuric stimuli that lead to FP effacement in vivo induce a loss of actin stress fibers in cultured podocytes, (2) overexpression of RhoA induces the formation of stress fibers in many cell types and (3) RhoA and Rac1/Cdc42 antagonistically regulate each other, suggested the following concept: RhoA stabilizes the podocyte FP structure and protects from effacement and proteinuria, whereas Rac1 and Cdc42 promote FP motility and promote the development of FP effacement and proteinuria.1
A number of novel in vivo observations are now further developing this model of podocyte FP dynamics. Whereas podocyte-specific ablation of Rac1 protected mice from PS-induced FP effacement as expected from the above, ablation of Cdc42 did not protect the filter barrier yet led to proteinuria.7 Hence, these findings point toward divergent functions of Rac1 and Cdc42. Furthermore, inhibition of Rho-Kinase, a downstream effector of RhoA, ameliorated proteinuria in several models of proteinuria (reviewed in ref. 8). Most recently, Zhu et al.8 have shown that inducible overexpression of a constitutive active variant of RhoA (CA-RhoA) in mice caused podocyte FP effacement and proteinuria. Taken together, these data indicated that although basal levels of RhoA may be necessary to maintain podocyte FP structure, overactivation of RhoA in disease seems to be also detrimental to podocytes and the cellular response is FP effacement.
Wang et al.9 (this issue) confirm the findings by Zhu et al.8 (overexpression of CA-RhoA causes proteinuria) and extend them in an important way, showing that overexpression of dominant negative RhoA (DN-RhoA) also leads to podocyte FP effacement and proteinuria. Importantly, although as expected the downstream pathways that were altered in the two transgenic mouse models differed, both mouse lines exhibited a remarkable similarity at the ultrastructural level, i.e., both, podocyte-specific overactivation and inactivation of RhoA leads to podocyte FP effacement.
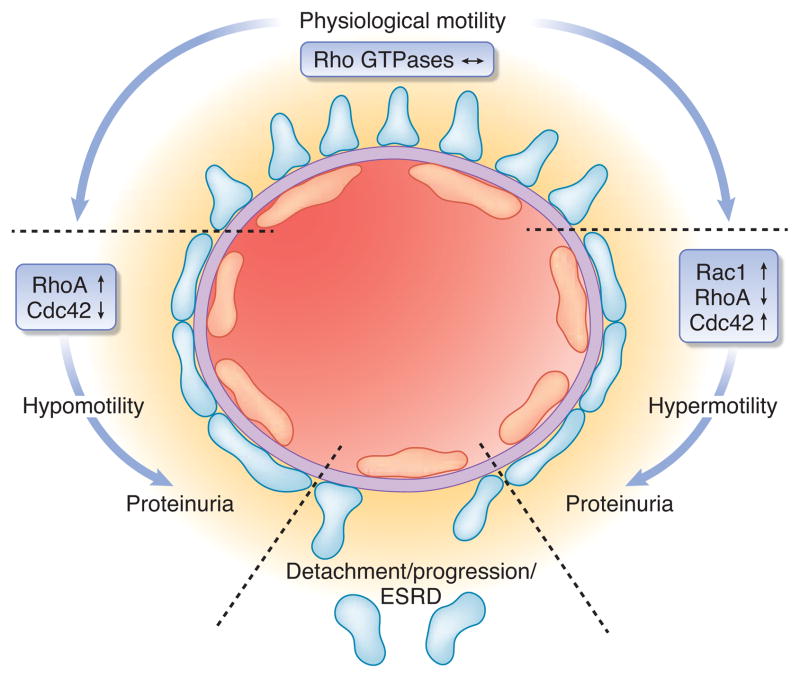
What do we learn from these studies? First, the study shows that both too much and too little podocyte FP motility may be detrimental to glomerular health (Fig. 1). Although the motility of podocytes overexpressing CA- or DN-RhoA has not been directly assessed in the study by Wang et al.,9 a recent study has shown that DN-RhoA induced, whereas CA-RhoA abrogated motility of cultured podocytes.9 Hence, some basal podocyte motility may be required to maintain a functioning glomerular filtration barrier whereas hypermotility may disrupt the podocyte SD structure and promote FP effacement. Podocyte health thus requires a healthy balance between the two extremes. Second, and more importantly, the study demonstrates that too much and too little podocyte motility both result in podocyte FP effacement. Both, the podocyte FP morphology as well as the time course at which proteinuria developed was remarkably similar between the two mouse strains overexpressing either CA- or DN-RhoA.9 Thus, continuous FP motility may be required to maintain the interdigitating FP structure, with hypermotility leading as quickly and directly to FP effacement as hypomotility. This differs somewhat from the idea of too much RhoA activity causing chronic podocyte damage and ultimately detachment due to failure adapt to changes in their environment. More generally spoken, the study supports the hypothesis that podocyte FP effacement is the first and probably universal morphological sign of podocyte injury. Third, the study questions the simplified analogy that prominent stress fibers in cultured podocytes reflect intact FPs in vivo, whereas the loss of actin stress fibers and reorganization into cortical actin in vitro equals FP effacement in vivo. As expected, expression of CA-RhoA in cultured podocytes induced more actin stress fibers, whereas expression of DN-RhoA abrogated stress fibers.9 However, since both morphological phenotypes of cultured podocytes translated into proteinuria and FP effacement in vivo, we may in the future have to more critically assess in vitro podocyte structure upon treatment with drugs or genetic manipulation since an increase in actin stress fibers may be as much be a sign of podocyte damage as a loss of stress fibers. All the above being said about podocyte motility and its influence on FP structure in vivo, one needs to keep in mind a caveat: in light of recent findings that both RhoA as well as Rac1 and Cdc42 are active at the leading edge of migrating cells, although at a highly coordinated and mutually antagonistic way,5 we need to be prepared to once again redefine the current view that RhoA activity equals a stationary podocyte phenotype and Rac1/Cdc42 a motile phenotype (see above). Although CA-RhoA and DN Rac1 inhibited podocyte motility whereas DN-RhoA and CA-Rac1 increased podocyte motility in vitro,10 these findings may not readily translate into the in vivo situation of podocyte FPs on the glomerular basement membrane. It seems more probable that all three small Rho family GTPases are active at the tip of the extending or retracting FPs in a highly coordinated manner as shown for cultured mouse embryonic fibroblasts.5 Furthermore, all three small Rho family GTPases have been implicated in a broad variety of cellular processes other than cell motility, including adhesion, proliferation and apoptosis.4 Thus, the effects of CA- and DN-RhoA overexpression in podocytes may not only be mediated by podocyte FP motility. Further studies will be required to unravel the detailed downstream signaling cascades of the small GTPases in podocytes and the exact mechanisms leading to FP effacement and proteinuria in RhoA over- and under-activity as well as how they are functionally interacting with the large GTPase dynamin that is heavily expressed in podocyte FP to also regulate F-actin organization and filter operation1.
Figure 1.
Schematic illustration outlining the tuning of podocyte foot process structure and function by Rho GTPases. Both, too much and too little RhoA activity causes podocyte foot process effacement and proteinuria. The overall dynamic operation of podocyte foot processes is regulated by an interplay of RhoGTPase family members, associated proteins such as RhoA-activated Rac1 GTPase-activating protein (Arhgap24) as well as the large GTPase dynamin. Foot process effacement might be reversible yet failure to restore balance of GTPase signaling will eventually lead to loss of podocytes and progression of glomerular disease.
Given the complexity of the functional role of small GTPases in podocyte FPs, the study by Wang et al.9 puts forward valuable novel in vivo information for a fascinating and expanding topic in glomerular biology, i.e., the tuning of the kidney filter function by means of podocyte dynamics.
Footnotes
Disclosures:
J.R. is an inventor on issued and pending patents related to the diagnostics and treatment of proteinuric renal diseases. He stands to gain royalties from their future commercialization.
References
- 1.Mundel P, Reiser J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 2010;77:571–580. doi: 10.1038/ki.2009.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seiler MW, Rennke HG, Venkatachalam MA, et al. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Invest. 1977;36:48–61. [PubMed] [Google Scholar]
- 3.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 5.Machacek M, Hodgson L, Welch C, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgin JB, Blattner SM, Vining CM, et al. Divergent Functions of Rho GTPases in the Podocyte: Deletion of Cdc42 Results in Podocyte Failure, but Loss of Rac1 Protects Against Podocyte Injury. J Am Soc Nephrol. 2010;21:90A. [Google Scholar]
- 8.Zhu L, Jiang R, Aoudjit L, et al. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1621–1630. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, et al. [Google Scholar]
- 10.Tian D, Jacobo SM, Billing D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]