Abstract
Background
Although corticosteroids remain a mainstay of treatment for acute lymphoblastic leukemia (ALL), they can cause troublesome neurobehavioral changes during active treatment, especially in young children. We evaluated acute neurobehavioral side effects of corticosteroid therapy in preschool versus school-age children by obtaining structured reports weekly for one month.
Procedure
Parents of 62 children (2 to 17 years at diagnosis) treated on Dana-Farber Cancer Institute (DFCI) ALL Consortium Protocol 00-01 participated during the continuation phase of treatment. Patients received cyclical twice-daily 5-day courses of prednisone (40 mg/m2/day) or dexamethasone (6 mg/m2/day). Parents completed behavior rating scales about their child weekly during one steroid cycle [baseline (Day 0), active steroid (Day 7), post-steroid (Days 14 and 21)].
Results
Behavioral side effects increased significantly (p < .001) during the steroid week for preschool children (< 6 years) on measures of emotional control, mood, behavior regulation, and executive functions, returning to baseline during the two ‘off-steroid’ weeks. In contrast, school-age children (≥ 6 years) did not demonstrate an increase in side effects during the steroid week. Steroid type (prednisone vs. dexamethasone) was not a significant predictor of neurobehavioral side effects.
Conclusions
Preschool children are at greater risk for neurobehavioral side effects during active steroid treatment for ALL than school age children and adolescents. Dexamethasone was not associated with more neurobehavioral side effects than prednisone. Counseling of families about side-effects should be adapted according to age. The observed effects, moreover, were transient, reducing concerns about longer-term neurobehavioral toxicities.
Keywords: Pediatric hematology/oncology, ALL, chemotherapy neurotoxicities, corticosteroids, behavior
INTRODUCTION
Systemic corticosteroids, typically administered in the form of prednisone or dexamethasone, are an essential component of successful leukemia therapy. Despite their effective anti-leukemia properties, their potential impact on brain function has been a long-standing concern. In low concentrations, corticosteroids are necessary for regulation of neuronal metabolism; however, increased levels, especially when prolonged, can have neurotoxic effects [1–3]. This is particularly true for brain regions with increased steroid receptor density (i.e., hippocampal and frontal regions), which are associated with mood, behavior regulation, and memory.
In fact, children treated with corticosteroids for various medical conditions can display behavioral side-effects, including frequent mood swings, increased irritability, depression and anxiety, and problems with behavioral control, aggression and attention during active therapy [4–10]. Various case reports and studies based on small samples suggest that the severity of these side effects depends on the sex and age of the child. Girls may be more vulnerable than boys [5,6], and preschool age children more vulnerable than older children [11–15]. Interpretability of relevant studies is constrained, however, by various methodological limitations, including small sample sizes, lack of repeated measures designs, or insensitivity of instruments.
Steroid preparation may also be relevant. Acute lymphoblastic leukemia (ALL) treatment protocols are increasingly shifting from prednisone (PRED) to dexamethasone (DEX) because of dexamethasone’s greater therapeutic efficacy [16–18], but its superior CNS penetration has raised concerns about increased potential for neurotoxicity. One of the few behavioral studies based on randomization to DEX vs. PRED, did not find differences in health-related QOL or behavior between these two steroid preparations during active treatment [19]; however, literature on steroid comparisons is just emerging.
In the present study, we used standardized behavioral questionnaires to evaluate whether preschool-age children experienced more behavioral symptoms than school-age children during active treatment in the context of a prospective off-on-off steroid repeated measures design implemented during the continuation phase of therapy. The behavioral assessments were focused on children’s regulation of cognition, behavior and emotion (executive functioning). The study design allowed us to query not only whether steroid treatment was associated with acute behavioral disturbance, but also whether cyclic administration of steroids results in persisting adverse effects on behavior. The latter would be indicated by an incomplete return to baseline at the end of a cycle. Finally, a secondary objective of the study was to explore whether DEX was associated with more significant side effects than PRED.
METHODS
Treatment Protocols
This behavioral study was integrated with Dana-Farber Cancer Institute (DFCI) ALL Consortium Protocol 00-01, which consisted of Induction (4 weeks), Central Nervous System therapy (3 weeks), Intensification (30 weeks) and Continuation therapy (74 weeks). The overall DFCI protocol included a randomization to DEX or PRED as the steroid component of Intensification as well as Continuation therapy. A second randomization specified individualized versus conventional asparaginase dosing [20]. Although participants were not recruited for this behavioral study based on the steroid randomization, only patients who were participating in the randomization were eligible; those directly assigned to prednisone were ineligible.
Continuation therapy included 3-week cycles of either PRED (40 mg/m2/day twice a day for 5 days during the first week of the cycle) or DEX (6 mg/m2/day twice a day for 5 days during the first week) for Standard Risk (SR) and High Risk (HR) patients. During the first week of each steroid cycle, additional therapeutic agents included a single dose of vincristine, daily doses of 6-mercaptopurine (6-MP) and methotrexate (MTX), during the second week 6-MP and MTX, and during the third week MTX only. During the first 6 months of Continuation therapy, intrathecal (IT) chemotherapy was administered every 9 weeks to SR patients and every 18 weeks to HR patients. During the last 12 months of Continuation, both groups received IT chemotherapy every 18 weeks.
Patients
Between February 2003 and March 2006, 62 parents of patients aged 2 to 17 years at diagnosis participated in the study during their child’s Continuation phase of treatment. Parents of children already enrolled in DFCI ALL Consortium Protocol 00-01 were approached about this study by mail prior to their child's routine clinic visit at the end of Intensification or beginning of Continuation treatment to be enrolled for one steroid cycle during Continuation. Interested families were then contacted by phone or during their child’s clinic visit to screen for eligibility, obtain consent, and administer questionnaires. Families were recruited from three out of ten DFCI Consortium sites: the Dana-Farber Cancer Institute, McMaster University Medical Center and Columbia University. The local institutional human investigations committees at each participating site approved the study. Informed consent was obtained from each parent participant prior to starting the study.
Exclusion criteria for this behavioral study included: (1) parent lacking sixth grade literacy in English (determined through screening of highest parental education); (2) patient history of a CNS disorder (e.g., brain tumor, head injury), prolonged steroid use unrelated to ALL therapy (e.g. asthma treatment), and/or pre-existing significant developmental or behavioral/emotional disorder; and (3) patient not participating in the steroid randomization due to parental decline.
Sixty-two families were recruited to the study. Of these, two were subsequently excluded from analysis because the parent had declined the steroid randomization and the child was therefore directly assigned to prednisone, leaving a final N = 60. A total of 424 out of the 498 enrolled families (or 85.9%) had consented to the steroid randomization on DFCI Consortium ALL protocol 00-01.
Neurobehavioral Assessment
Behavioral ratings in the preschool (2 to < 6 years) and school age (≥ 6 to 17 years) groups were assessed four times at weekly intervals during one 3-week steroid cycle. Parents completed two widely used and psychometrically valid child behavior rating scales, the Child Behavior Checklist (CBCL) [21] and the Behavior Rating Inventory of Executive Function (BRIEF) [22]. Both instruments provide separate age-appropriate versions for preschool and school age children [21–24]. Since the item content of the questionnaires differed by age group, the preschool and school age data needed to be analyzed in parallel, rather than combined into a single analysis, even though many of the scales are comparable across versions.
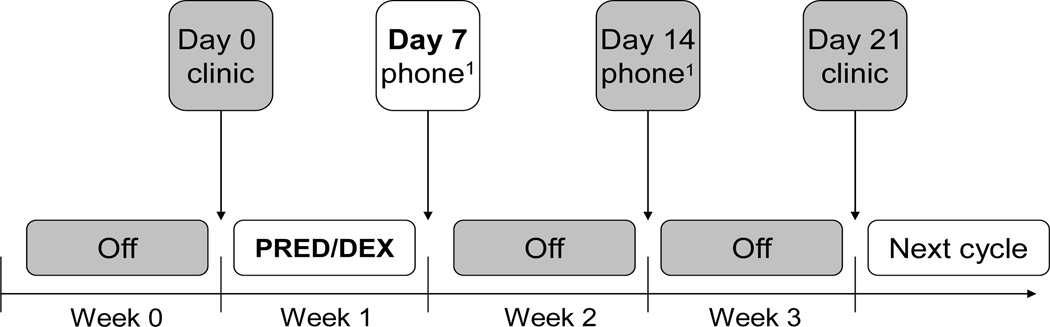
The data collection scheme for one steroid cycle is illustrated in Figure 1. Parents were asked to complete the questionnaires at 4 weekly time points based on the child’s behaviors observed during the week prior to each timepoint as follows: 1) during the clinic visit prior to starting a week of steroid treatment about the previous week off steroids (baseline, Day 0); 2) per phone interview one week later after the child had completed the steroid course about the week on steroids, (steroid week, Day 7); 3) per phone interview one week later about the previous week off steroids (Day 14); and 4) at the return clinic visit two weeks after end of steroid treatment about the previous week off steroids and before the start of the next steroid dose (Day 21). Note that Day 21 represents the end point of one steroid cycle and the beginning of the next steroid cycle. Although every attempt was made to adhere to this schedule, the Day 7 and Day 14 data collections could have been off by one day for some children because of difficulty scheduling phone calls. Regardless of whether assessments occurred during a clinic visit or over the phone, parent ratings at all 4 time points pertained to the child’s behavior in their natural home/school environment and are thus deemed comparable between time points.
Fig. 1.
Assessment Schedule During One Steroid Cycle of Continuation Therapy. Ratings were completed at each time point for behaviors observed during the preceding week.
Statistical Analysis
Potential differences in demographic distributions between the two age groups were tested using Fisher’s exact test. Since families were recruited to the study at various points during Continuation therapy, the children would have been exposed to varying cumulative steroid doses at the time of recruitment. Potential effects of cumulative steroid dose were assessed by correlating (Spearman) duration of the randomized component of steroid therapy (time elapsed in weeks from start of Intensification to first behavioral assessment) with behavioral outcomes.
Repeated measures analyses of variance (ANOVA) were fitted to the composite scores of the CBCL and BRIEF questionnaires to assess patterns of change across one steroid cycle. Models were adjusted for Time (the 4 assessment time points) and Risk Group (Standard vs. High Risk). Steroid randomization and sex were included in the initial models, but since neither was a statistically significant predictor for any model, they were removed from further analyses. These models were fitted separately for the preschool and school age groups to the major composite scales provided by the questionnaires. In additional exploratory analyses age was included as a linear covariate in repeated measures ANOVAs within each age group. The 2-sided significance level was set at 0.05, with no adjustment for multiple comparisons.
Secondary post-hoc analyses, based on the same repeated measures models, were carried out for the 12 subscales, across both questionnaires, for the preschool sample only. These results were adjusted for multiple comparisons by a Bonferroni correction (α = 0.05/12 = 0.00417).
RESULTS
Demographic characteristics of the sample are presented in Table I. Forty-five percent were in the “school-age” (≥ 6 years) group, and 55% in the “preschool” (< 6 years) group. Steroid randomization was evenly distributed between and within the two age groups. Although the majority of school-age patients were randomized to dexamethasone, this difference was not statistically significant. All other baseline variables had similar distributions in both age groups. Duration of post-induction randomized steroid therapy between the two age groups was also comparable. For both groups, the median elapsed time from start of randomized steroid therapy (during Intensification) to the first behavioral assessment (during Continuation) was 55 weeks (Preschool range: 38 – 86 weeks, School-age range: 33 – 100 weeks).
Table I.
Patient Characteristics for Preschool and School-Age Samples
| Preschool (< 6 years) |
School-Age (≥ 6 years) |
p-value | |||
|---|---|---|---|---|---|
| Patient Characteristic | No. | % | No. | % | |
| No. of patients | 33 | 55 | 27 | 45 | - |
| Sex | |||||
| Male | 18 | 55 | 14 | 52 | 1.0 |
| Female | 15 | 45 | 13 | 48 | |
| Risk Group | |||||
| Standard Risk (SR) | 25 | 75 | 16 | 59 | 0.26 |
| High Risk (HR) | 8 | 24 | 11 | 41 | |
| Steroid Randomization | |||||
| Prednisone | 15 | 45 | 10 | 37 | 0.60 |
| Dexamethasone | 18 | 55 | 17 | 63 | |
| Age at Steroid Randomization | |||||
| Median (years) | 2 | 7 | - | ||
| Range (years) | 1–5 | 4–16 | |||
| Age at Assessment | |||||
| Median (years) | 4 | 8 | - | ||
| Range (years) | 2–5 | 6–17 | |||
| Race | |||||
| White | 30 | 91 | 23 | 85 | 0.17 |
| Black | 0 | 0 | 3 | 11 | |
| Asian | 1 | 3 | 1 | 4 | |
| More than one | 2 | 6 | 0 | 0 | |
| Ethnicity | |||||
| Hispanic or Latino | 4 | 13 | 1 | 4 | 0.26 |
| Non-Hispanic | 28 | 87 | 24 | 96 | |
| Not specified | 1 | 2 | |||
| Parent Education | 0.76 | ||||
| Associate’s degree or higher | 26 | 79 | 18 | 72 | |
| Less than associate’s degree | 7 | 21 | 7 | 28 | |
| Not specified | 0 | 2 | |||
Table II displays parameter estimates and significance levels for change from baseline in the behavioral outcome scores at each time point for the two age groups (preschool vs. school-age) separately. An explanation of how these parameter estimates can be interpreted is provided in the legend of Tables II and III. For the preschool group, there was a main effect of Time for all the composite scores. More specifically, the parameter estimates indicate significant increases in behavioral problem ratings from Day 0 (baseline) to Day 7 (steroid week) on all main composite scales for both CBCL (ps < 0.0001 to 0.0002) and BRIEF (ps < 0.0001 to 0.013). The estimates on Day 14 or Day 21 did not differ from those on Day 0, however, indicating that the level of behavior problems had returned to baseline post-steroid.
Table II.
Estimated Baseline Scores and Parameter Estimates of Change from Baseline in CBCL and BRIEF Composite T Scores at Each Time Point for Preschool and School Age Groups.
| Rating Scale | Age Group | Day 0 Baseline |
Day 7 Steroid |
Day 14 Off Steroid |
Day 21 Off Steroid |
HR |
|---|---|---|---|---|---|---|
| CBCL Composites | ||||||
| Total Problems | Preschool | 49.42 | 7.04*** | −0.08 | −2.07 | 1.73 |
| School Age | 52.54 | 2.84 | −5.72** | −7.28*** | −6.56t | |
| Total Internalizing | Preschool | 50.30 | 6.79** | −0.15 | −1.75 | 2.99 |
| School Age | 57.72 | 1.91 | −6.80** | −8.83*** | −7.98+ | |
| Total Externalizing | Preschool | 49.24 | 6.96*** | 0.42 | −1.36 | 2.67 |
| School Age | 52.74 | 2.02 | −3.24t | −3.47* | −8.95* | |
| BRIEF Indices | ||||||
| General Executive | Preschool | 46.73 | 7.34** | −0.83 | −2.06 | 2.45 |
| School Age | 50.07 | 0.11 | −4.11* | −4.79** | −6.34+ | |
| Metacognition | Preschool | 45.95 | 3.99+ | −1.30 | −1.59 | 1.81 |
| School Age | 49.13 | −0.33 | −4.56** | −5.13** | −5.67+ | |
| Inhibitory Self Controla |
Preschool | 47.62 | 10.05*** | 0.70 | −0.67 | 3.46 |
| Flexibilitya | Preschool | 49.37 | 8.63*** | −0.24 | −1.92 | 1.12 |
| Behavior Regulationb |
School Age | 50.65 | 0.96 | −3.00t | −3.75* | −4.59 |
HR, High Risk; CBCL, Child Behavior Checklist; BRIEF, Behavior Rating Inventory of Executive Functions.
p ≤ 0.0001;
p ≤ 0.001;
p ≤ 0.01;
p ≤ 0.05;
p ≤0.10.
Scale only in preschool version of questionnaire;
Scale only in school age version. Standard Risk is reference group; estimated difference (collapsed across time points) for High Risk displayed at right. Note: The parameter estimates can be used to estimate scores of an individual with specific characteristics. For example, for a preschool-age Standard Risk patient, the baseline CBCL Total Problem score is estimated to be 49.42. This score is estimated to increase by 7.04 points to 56.46 at Day 7; at Day 14, it is estimated to decrease 0.08 points from baseline to 49.34. If the child is in the High Risk group, scores at each time point are estimated to increase by 1.73 points.
Table III.
Estimated Baseline Scores and Parameter Estimates of Change from Baseline in CBCL and BRIEF Subscale T-Scores at Each Time Point for the Preschool Group.
| Day 0 Baseline |
Day 7 Steroid |
Day 14 Off- Steroid |
Day 21 Off Steroid |
HR | |
|---|---|---|---|---|---|
| CBCL Subscales | |||||
| Emotionally Reactive | 56.11 | 4.89* | 0.16 | −1.06 | −0.21 |
| Anxious/Depressed | 53.40 | 3.89+ | 0.38 | −0.08 | 1.93 |
| Somatic Complaints | 55.94 | 2.39 | 0.71 | −1.02 | 1.44 |
| Withdrawn | 55.01 | 2.15 | −0.20 | −0.87 | −2.00 |
| Sleep Problems | 54.79 | 3.61 | 0.42 | −0.50 | −2.18 |
| Attention Problems | 51.91 | 2.37+ | 0.33 | −0.39 | 0.88 |
| Aggressive Behavior | 55.58 | 5.47** | −1.13 | −0.37 | 2.17 |
| BRIEF Subscales | |||||
| Inhibit | 46.14 | 6.81** | 0.31 | −0.90 | 3.67 |
| Shift | 48.57 | 2.43 | −2.15 | −3.42 | −0.11 |
| Emotional Control | 50.12 | 13.66*** | 2.01 | −0.17 | 3.54 |
| Working Memory | 45.99 | 4.65+ | −1.13 | −0.96 | 1.67 |
| Plan | 47.51 | 2.59 | −1.40 | −2.99 | −0.97 |
HR, High Risk; CBCL, Child Behavior Checklist; BRIEF, Behavior Rating Inventory of Executive Functions. Bonferroni Adjusted Significance-Level: p = 0.00417;
p ≤ 0.0001,
p ≤ 0.001,
p ≤ 0.004,
p ≤ 0.01.
Standard Risk is reference group; estimated difference (collapsed across time points) for High Risk displayed at right. Note: For example, for a preschool-age Standard Risk patient, the baseline BRIEF Emotional Control problem score is estimated to be 50.12. This score is estimated to increase by 13.66 points to 63.78 at Day 7; at Day 14, it is estimated to increase 2.01 points from baseline to 52.13 and decrease 0.17 points from baseline to 49.95 at Day 21. If the child is in the High Risk group, scores at each time point are estimated to increase by 3.54 points.
Secondary analysis of subscales for the preschool group (Table III) documented significant increases of behavioral problems during the steroid week (Day 7) on the Emotionally Reactive (p = 0.0013) and Aggressive Behavior (p = 0.0002) subscales of the CBCL and the Emotional Control (p < 0.0001) and Inhibition (p = 0.0007) subscales of the BRIEF, with marginally significant increases of problems in Working Memory (p < 0.005). Elevations in problem scores at Day 7 reached clinical significance for the Emotional Control and Aggressive Behavior subscales, with estimated T scores falling in the “At Risk” range (T scores > 60) (see Table III). Similar elevations were noted for Anxiety/Depression, although only at trend-level. Again, no significant differences were found between scores at Day 0 and at Day 14 or Day 21, indicating a return to baseline when the children were off steroids.
For the school-age sample, the results were less clear. Although problem ratings did not increase significantly between Day 1 and Day 7, there was an unanticipated and statistically significant decrease in problem behaviors relative to baseline at Day 14 and Day 21 for all composite scales (Table II). Another unexpected finding was that parents of standard-risk patients endorsed significantly more problem behaviors than did parents of high-risk patients of the same age (p < 0.05).
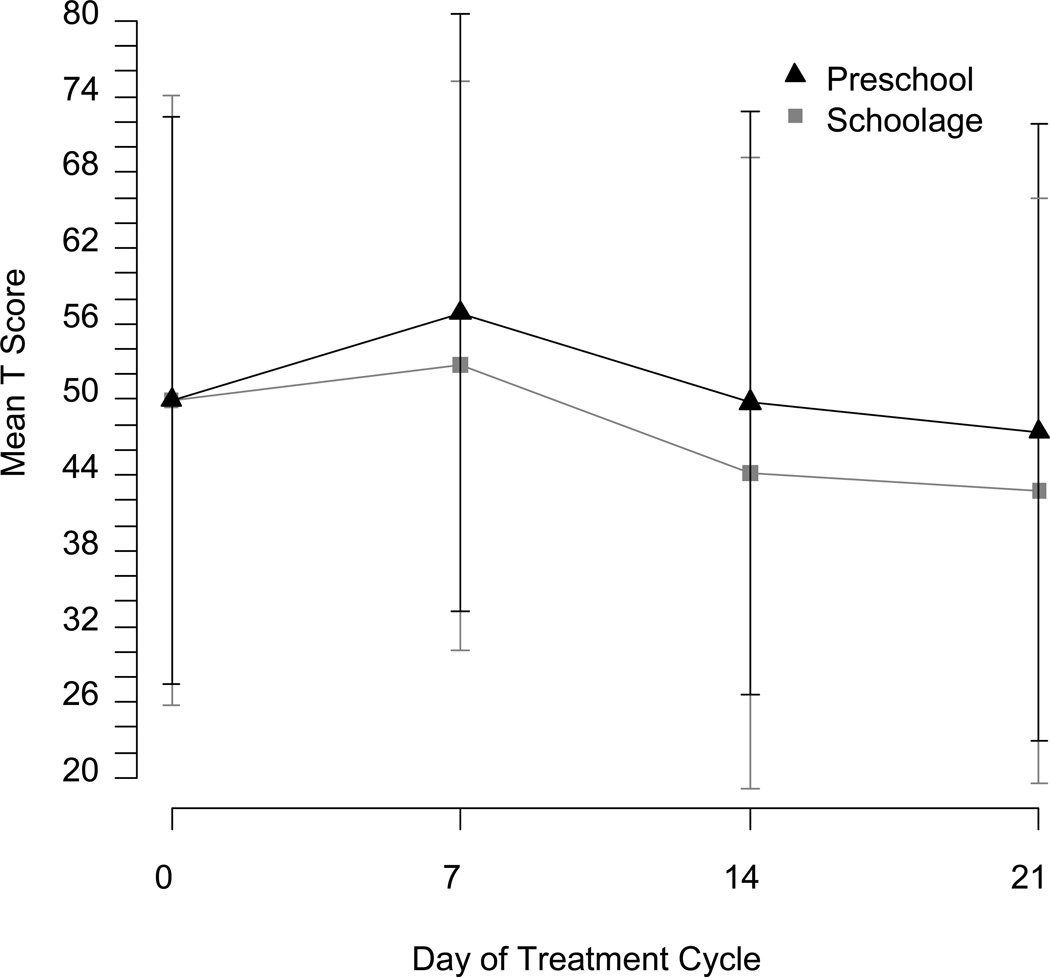
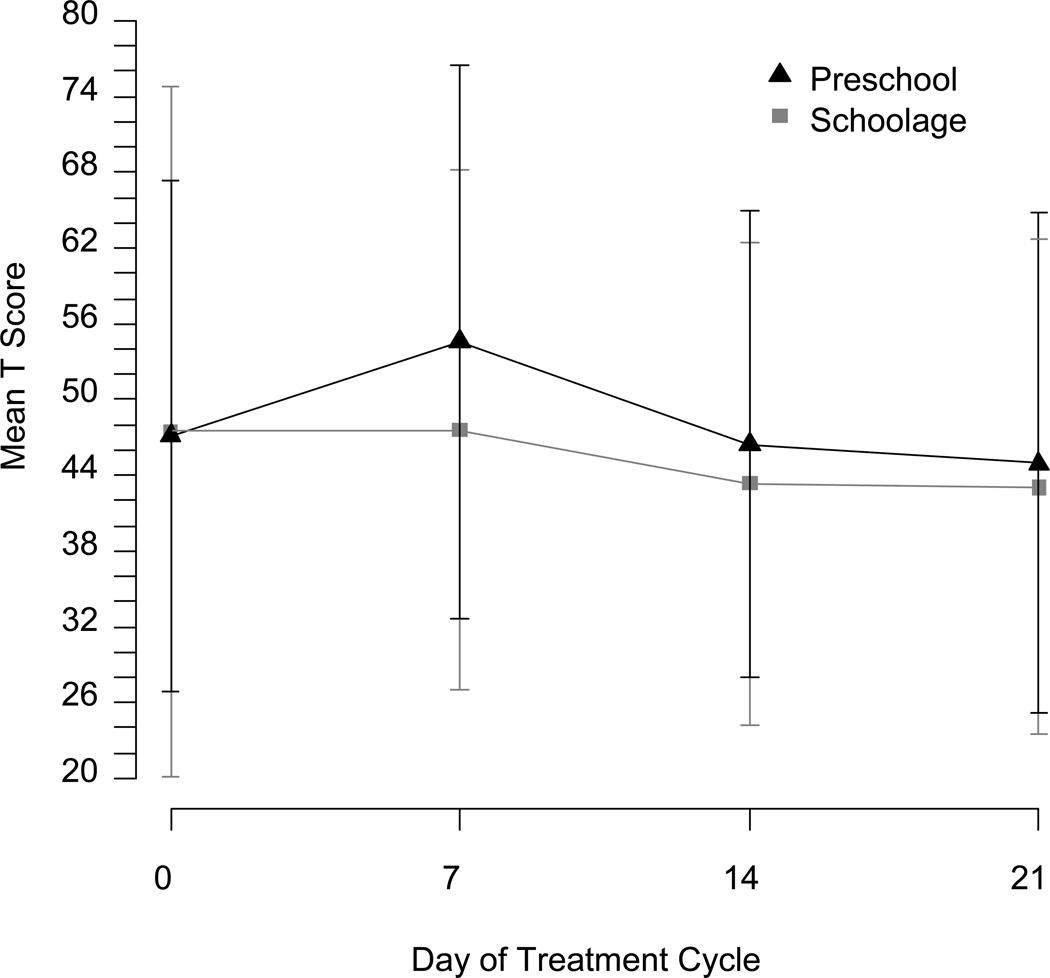
Although direct comparison between age groups was not possible, standardized problem scores were consistently higher for preschool than school age children (see Figures 2 and 3). Age as a linear covariate within each age group was a marginally significant predictor for Internalizing Problems on the CBCL in the preschool group (p = 0.07) and for Externalizing Problems in the school age group (p = 0.05) in uncorrected analyses, but otherwise not a significant predictor.
Fig. 2.
1)Day 7 and Day 14 ratings were completed in clinic for the majority of patients enrolled at McMaster University Medical Center.
Fig. 3.
Childhood Behavior Checklist (CBCL) Total Problem Mean T Score and Confidence Interval Across 4 Weekly Timepoints of One Steroid Cycle (Day 7 = steroid week).
Duration of steroid therapy (time elapsed from start of randomized steroid therapy to behavioral assessment) was not correlated with behavior composite scores at baseline within either age group (correlation coefficients ranged from −0.2 to 0.2). Similarly, duration of steroid therapy was not associated with baseline behavior ratings within either risk group (correlation coefficients ranged between -0.31 and 0.24). Thus, cumulative steroid dose did not appear to influence the findings.
DISCUSSION
This multi-site, prospective, repeated-measures study investigated whether the behavioral response to active steroid treatment differs for preschool and school-age children during treatment for ALL. Parents rated their children’s behavior using structured behavioral questionnaires four times at weekly intervals spanning a single steroid cycle during continuation therapy. Significant neurobehavioral changes were found during active steroid treatment for the preschool children only. Their scores increased markedly relative to baseline during the steroid week on measures of emotional control, mood, and behavior regulation, with reported problems in a range of clinical significance, and to a lesser extent emergent cognitive aspects of executive functions. The behavioral ratings returned to baseline levels during off-steroid weeks. In contrast, these pronounced effects were not detected among school-age children (≥ 6 years). The behavioral effects also did not differ between children who had been randomized to dexamethasone or prednisone.
An earlier behavioral pilot study integrated with DFCI ALL Protocol 00-01, on a separate sample of patients (N = 18) and based on selected subscales of the full behavioral questionnaires, demonstrated the same pattern, that is, an elevation in behavioral symptoms during the active treatment week and a pronounced response to steroids among preschool but not school-age children [25]. The consistency of these findings on successive samples provides confidence in the reliability of the age-related patterns reported here and is consistent with prior accounts of greater behavioral vulnerability to steroids in younger children during treatment [4,7,14,15]. Despite other treatment agents administered concurrently with steroid therapy (i.e., 6 MP, MTX), the observed behavioral changes can be confidently attributed to steroids, since these concurrent treatments were also administered during off-steroid weeks, when behavior problems returned to baseline.
Although the school-age group did not exhibit the pronounced steroid effect seen in preschool children, they did exhibit an unexpected finding: levels of problem behaviors observed one to two weeks after the steroid week were significantly lower than baseline. This finding is difficult to interpret and possibly random. Both age groups, however, demonstrated a similar pattern of steroid response, with a higher level of behavioral problems during the steroid week, albeit of a small magnitude and not statistically significant in the school age group, and a decrease of symptoms to baseline level or lower after treatment. These findings suggest that behavioral side-effects are less of a concern for older children, whose problem ratings in general are lower than those of younger patients.
Our data provided no indication that children treated with dexamethasone experience more significant behavioral side-effects than those treated with prednisone. These findings are consistent with those of Eiser et al. [19], who also found no evidence of behavioral or quality of life differences during treatment in children randomized to prednisone or dexamethasone. Whether longer-term neuropsychological effects associated with dexamethasone or prednisone exist remains to be determined. One recent late effects study, however, found minimal or no cognitive differences between children randomized to dexamethasone or prednisone [26]. We also did not find evidence for a cumulative effect of steroids on behavior, with only small correlations between length of prior steroid treatment and behavioral effects at time of assessment.
In contrast to some other studies [5,6], we found no evidence that behavioral side effects were related to the sex of the child. Also, the data demonstrated somewhat paradoxically that children treated for Standard Risk ALL showed more problem behaviors than those treated for High Risk ALL for the school age children only. Because this result was unexpected, particularly in the context of a 3-fold higher steroid dose for High Risk patients, it must be considered inconclusive; however, more frequent delivery of IT chemotherapy to Standard Risk (every 9 weeks) than High Risk patients (every 18 weeks) during the first six months of Continuation treatment could be contributory.
Our findings should be interpreted in the context of several potential considerations. First, since parents could not be blinded to steroid therapy, they could have been biased to over-report symptoms during the steroid week. If this were the case, however, parents of both age groups should have exhibited this bias. Since steroid effects were minimal among school-age children, it would be hard to attribute our findings for the preschool group primarily to bias or expectation, although such bias could certainly have played a role. In addition, because age-appropriate versions of behavior questionnaires naturally differ in the number and content of items, we could not compare age groups directly, but were limited to observing response patterns within age groups. Although age as linear covariate within each age group suggested a trend for differential steroid effects on some behavioral variables, this finding must be viewed tentative due to uneven age distributions within groups.
A major advantage of our study was the repeated measures design over the course of one steroid cycle. More extended administration of repeated measures over several steroid cycles would have been preferable, especially relative to the unexpected findings for the older children. Yet weekly measurement during even one cycle provided a more reliable understanding than data available from studies that did not use such a design (i.e., those where repeated measures were intermittent over a long time period). In addition, since baseline and end of cycle data points can be considered identical (the latter marking the beginning of the next steroid cycle), measurement of change across the cycle serves as a limited indicator of the potential for an increase of behavior problems over cumulative cycles. Behavioral problems, however, returned to baseline post-steroid week. Moreover, there was no apparent relationship between duration of randomized steroid treatment (used as a proxy for cumulative steroids) and baseline ratings. These findings suggest that the observed effects, while significant, are transient in nature. Our sample size was large enough to reliably detect treatment related variation, given the magnitude of observed effects, further contributing to confidence in the findings
Finally, to more clearly delineate any specific effects of steroids, we restricted the study to the Continuation phase of treatment, when children were medically more stable and there would be fewer side-effects of other components of therapy than during the Intensification phase.
CONCLUSIONS
Our findings corroborate prior suggestions from the literature that steroid therapies are associated with distinct neurobehavioral side-effects in children receiving therapy for ALL. Moreover, we confirmed that young children are more vulnerable to these side-effects than school age children. Why this age effect exists is unclear, but it most likely occurs because regulatory brain systems are less well developed in younger children. Absent the regulatory capacities mediated by these systems, young children might be more vulnerable to the adverse effects of steroids on behavior and emotion
These findings have direct implications for counseling parents, which should take into account the age of the child. Potential synergistic effects of corticosteroids administered in combination with other treatment agents (i.e., methotrexate) [15] need to be more carefully studied, especially for young children. Our data strongly suggest that the behavioral side-effects of steroids are transient and likely to disappear post-treatment, decreasing any concerns about longer-term or more permanent behavioral changes.
ACKNOWLEDGMENTS
We thank the Michael J. Garil Fund for Leukemia Research for supporting this research. We also thank Richard Gelber, Ph.D. and Donna Levy, Ph.D. for their conceptual and statistical advice at earlier stages of this research.
Funding was in part provided by NIH F32 HD046245 (Mrakotsky) and K23 HD058466 (Mrakotsky)
Grant sponsor: Michael J. Garil Fund for Leukemia Research.
Footnotes
CONFLICT OF INTEREST STATEMENT
No potential conflict of interest to disclose.
REFERENCES
- 1.Packan DR, Sapolsky RM. Glucocorticoid endangerment of the hippocampus: Tissue, steroid and receptor specificity. Neuroendocrinology. 1990;51:613–618. doi: 10.1159/000125400. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 3.Conrad CD, McLaughlin KJ, Harman JS, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JC, Carel CA, Rosenberg LA, et al. Intermittent high dose corticosteroid treatment in childhood cancer: Behavioral and emotional consequences. J Am Acad Child Psychiatry. 1986;25:120–124. doi: 10.1016/s0002-7138(09)60608-7. [DOI] [PubMed] [Google Scholar]
- 5.Bender BG, Lerner JA, Poland JE. Association between corticosteroids and psychologic change in hospitalized asthmatic children. Ann Allergy. 1991;66:414–419. [PubMed] [Google Scholar]
- 6.Drigan R, Spirito A, Gelber RD. Behavioral side effects of corticosteroids in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1992;20:13–21. doi: 10.1002/mpo.2950200104. [DOI] [PubMed] [Google Scholar]
- 7.Soliday E, Grey S, Lande MB. Behavioral effects of corticosteroids in steroid-sensitive nephrotic syndrome. Pediatrics. 1999;104:e51. doi: 10.1542/peds.104.4.e51. [DOI] [PubMed] [Google Scholar]
- 8.Klein-Gitelman MS, Pachman LM. Intravenous corticosteroids: adverse reactions are more variable than expected in children. J Rheumatol. 1998;25:1995–2002. [PubMed] [Google Scholar]
- 9.Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. Chest. 2002;122:624–628. doi: 10.1378/chest.122.2.624. [DOI] [PubMed] [Google Scholar]
- 10.Escher JC European Collaborative Research Group on Budesonide in Paediatric IBD. Budesonide versus prednisolone for the treatment of active Crohn's disease in children: a randomized, double-blind, controlled, multicentre trial. Eur J Gastroenterol Hepatol. 2004;16:47–54. doi: 10.1097/00042737-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Copeland DR, Moore BD, Francis DJ, et al. Neuropsychologic effects of chemotherapy on children with cancer: a longitudinal study. J Clin Oncol. 1996;14:2826–2835. doi: 10.1200/JCO.1996.14.10.2826. [DOI] [PubMed] [Google Scholar]
- 12.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 13.Lewis LD, Cochrane GM. Psychosis in a child inhaling budesonide. Lancet. 1983;2:634. doi: 10.1016/s0140-6736(83)90725-0. [DOI] [PubMed] [Google Scholar]
- 14.Connett G, Lenny W. Inhaled budesonide and behavioural disturbances. Lancet. 1991;338:634–635. doi: 10.1016/0140-6736(91)90646-7. [DOI] [PubMed] [Google Scholar]
- 15.Hochhauser CJ, Lewis M, Kamen BA, et al. Steroid-induced alterations of mood and behavior in children during treatment for acute lymphoblastic leukemia. Support Care Cancer. 2005;13:967–974. doi: 10.1007/s00520-005-0882-8. [DOI] [PubMed] [Google Scholar]
- 16.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 17.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129:734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 19.Eiser C, Davies H, Jenney M, et al. HRQOL implications of treatment with dexamethasone for children with acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2006;46:35–39. doi: 10.1002/pbc.20432. [DOI] [PubMed] [Google Scholar]
- 20.Vrooman LM, Neuberg DS, Stevenson KE, et al. Dexamethasone and individualized asparaginase dosing are each associated with superior event-free survival in childhood acute lymphoblastic leukemia: Results from DFCI-ALL Consortium Protocol 00-01. Blood (ASH Annual Meeting Abstracts) 2009 Dec; (abstr 321) [Google Scholar]
- 21.Achenbach TA, Rescorla L. Child Behavior Checklist for Ages 1.5-5. Burlington, VT: ASEBA, University of Vermont; 2000. [Google Scholar]
- 22.Gioia GA, Isquith PK, Guy SC, et al. Behavior Rating Inventory of Executive Function (BRIEF™) Odessa, FL: Psychological Assessment Resources Inc.; 2000. [Google Scholar]
- 23.Achenbach TA. Child Behavior Checklist for Ages 6–18. Burlington, VT: ASEBA, University of Vermont; 2000. [Google Scholar]
- 24.Gioia GA, Espy KA, Isquith PK. Behavior Rating Inventory of Executive Function-Preschool Version (BRIEF-P™) Odessa, FL: Psychological Assessment Resources Inc.; 2003. [Google Scholar]
- 25.Mrakotsky C, Silverman L, Levy D, et al. Neurobehavioral side effects of steroid treatment in children with leukemia. J Int Neuropsychol Soc. 2004;10(suppl 1):58s. [Google Scholar]
- 26.Kadan-Lottick NS, Brouwers P, Breiger D, et al. A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood. 2009;114:1746–1752. doi: 10.1182/blood-2008-12-186502. [DOI] [PMC free article] [PubMed] [Google Scholar]