Abstract
Individuals with attention deficit hyperactivity disorder (ADHD) smoke at rates significantly higher than the general population and have more difficulty quitting than nondiagnosed individuals. Currently, there are no evidence-based approaches for reducing smoking specifically in individuals with ADHD. Adult regular smokers with or without ADHD participated in a study of extended smoking withdrawal where monetary incentives were used to promote abstinence. Participants were paid according to an escalating schedule for maintaining abstinence measured as self-report of no smoking and an expired air carbon monoxide (CO) level of ≤4 parts per million. Sixty-four percent (14/22) of smokers with ADHD and 50% (11/22) of smokers without ADHD maintained complete abstinence for the 2-week duration of the study. Twenty-two percent (5/22) and 9% (2/22) of smokers with ADHD and without ADHD, respectively, maintained continued abstinence for up to 10 days following the removal of the contingencies. Though abstinence rates were higher for the smokers with ADHD, the group differences were not statistically significant. Results suggest that monetary incentives may be a useful approach for promoting abstinence in adult smokers with ADHD, perhaps owing to altered reinforcement processes in these individuals.
Keywords: ADHD, smoking, nicotine dependence, contingency management
Individuals with attention deficit hyperactivity disorder (ADHD) smoke at rates significantly higher than the general population and/or nondiagnosed controls among both adults and adolescents (Lambert & Hartsough, 1998; Milberger, Biederman, Faraone, Chen, & Jones, 1997; Molina & Pelham, 2003; Pomerleau, Downey, Stelson, & Pomerleau, 1995). Moreover, compared to nondiagnosed individuals, those with ADHD start smoking at an earlier age, are more likely to progress to regular use, and have higher levels of nicotine dependence (Milberger et al., 1997; Rohde, Kahler, Lewinsohn, & Brown, 2004; Wilens et al., 2008).
Studies have reported that individuals with ADHD also have a harder time quitting and demonstrate worse outcomes in smoking cessation trials than their undiagnosed peers. For example, Pomerleau et al. (1995) reported that the ratio of current nonsmokers to ever smokers was much lower in a sample of adults with ADHD versus adults without ADHD, indicating that fewer adults with ADHD had successfully quit. More recently, it was shown that subjects enrolled in smoking cessation trials who had a childhood history of ADHD fared significantly worse than those without such a history (Humfleet et al., 2005). Adult smokers with high levels of current ADHD symptoms also demonstrated poorer cessation outcomes compared to those with lower levels of symptoms, independent of clinical diagnosis (Covey, Manubay, Jiang, Nortick, & Palumbo, 2008). Finally, adult smokers with ADHD exhibit greater abstinence-induced disruptions in inhibitory control and attention, which may explain higher relapse rates (McClernon et al., 2008).
To our knowledge, only one study has been conducted that has specifically targeted smoking cessation in individuals with ADHD. Preliminary reports from this study indicate that individuals receiving pharmacological intervention (extended-release methylphenidate) + transdermal NRT for smoking cessation did no better than a group receiving placebo + NRT on the primary outcome measure of 4-week continuous abstinence (Winhusen et al., 2009).
Individuals with ADHD are, by definition, more impulsive than those without the disorder (American Psychiatric Association, 1994), and this may contribute to less favorable smoking cessation outcomes. Whether measured as a personality-based construct or in laboratory measures of impulsive behavior, those with ADHD have a harder time inhibiting responses that may lead to negative consequences (Kollins, Lane, & Shapiro, 1997). Moreover, as noted previously, these deficits in inhibitory control are exacerbated relative to smokers without ADHD following acute withdrawal (McClernon et al., 2008). Thus it may be harder for individuals with ADHD to consider the potential longer term benefits of quitting when faced with the immediate opportunity to smoke, especially under conditions of withdrawal.
Clinical and laboratory research suggests that inhibitory control/impulsivity deficits among those with ADHD are amenable to behavioral modification. First, individuals with ADHD show behavioral improvements following delivery of contingent reinforcement, often times to a greater degree than individuals without ADHD (Luman, Oosterlaan, & Sergeant, 2005). Second, compared to delayed consequences, more immediate delivery of reinforcement has also been shown to exert greater control over behavior in individuals with ADHD compared to those without the disorder (Bitsakou, Psychogiou, Thompson, & Sonuga-Barke, 2009; Marco et al., 2009; Rapport, Tucker, DuPaul, Merlo, & Stoner, 1986; Sonuga-Barke, Taylor, Sembi, & Smith, 1992; Tripp & Alsop, 2001). Finally, among children with ADHD contingent reinforcement increases motivation—defined as task persistence or self-report of motivation—for task-relevant behavior (Carlson, Mann, & Alexander, 2000; Carlson & Tamm, 2000; McInerney & Kerns, 2003; Scheres, Oosterlaan, & Sergeant, 2001).
Collectively, this body of literature documenting the relative effects of contingent reinforcement in ADHD versus non-ADHD individuals is grounded in both behavioral and neurobiological theories of ADHD (Brennan & Arnsten, 2008; Brocki, Fan, & Fossella, 2008; Johansen et al., 2009; Sagvolden, Johansen, Aase, & Russell, 2005; Tripp & Wickens, 2008). Though, this research has largely been conducted in children, it provides a conceptual framework in which to explain why studies have shown more failed quit attempts among smokers with ADHD and why an intervention involving only a pharmacological manipulation (Winhusen et al., 2009) was no more effective than placebo for promoting cessation. Specifically, a typical quit attempt or a pharmacological intervention is unlikely to provide the kinds of immediate consequences necessary to reinforce abstinence-related behavior, especially when competing against the potentially negatively reinforcing effects of smoking to reduce withdrawal symptoms.
Given the high rates of smoking comorbidity in individuals with ADHD and the lack of efficacious treatment strategies for smoking cessation, the present analysis reports on the initial viability of a monetary incentive program for promoting smoking abstinence in individuals with ADHD. We reasoned that providing monetary incentives contingent on smoking abstinence would provide reinforcement in an immediate and salient fashion, and would therefore increase smokers’ with ADHD motivation to persist with not smoking and would be associated with longer term quit rates comparable to smokers without ADHD. Based on previous studies that have used incentive based approaches to reduce smoking in difficult to treat populations (Roll, Higgins, Steingard, & McGinley, 1998; Tidey, O'Neill, & Higgins, 2002), we hypothesized that such an approach would be useful in smokers with ADHD. The present study was initially designed to assess the effects of prolonged smoking withdrawal in smokers with ADHD and smokers without ADHD. As a result, we also were able to compare the relative effectiveness of the incentive-based approach for smoking abstinence between smokers with and without ADHD.
Method
Data for this study were collected as part of an investigation of the effects of prolonged smoking withdrawal in smokers with ADHD and without ADHD. As such, we specifically sought to recruit age-, sex- and smoking history-matched groups of adult smokers with and without ADHD. This study was not designed as a controlled intervention trial. Rather, monetary incentives were provided to participants as described below to promote abstinence across the study period so as to measure withdrawal symptoms between the two groups. Similar procedures have been used in previous studies to examine prolonged abstinence (Gilbert, Crauthers, Mooney, McClernon, & Jensen, 1999; Gilbert, McClernon, et al., 1999). Data describing baseline differences between smokers with ADHD and without ADHD with respect to personality traits, smoking motivations, and the effects of prolonged abstinence on withdrawal, are reported separately.
Participants
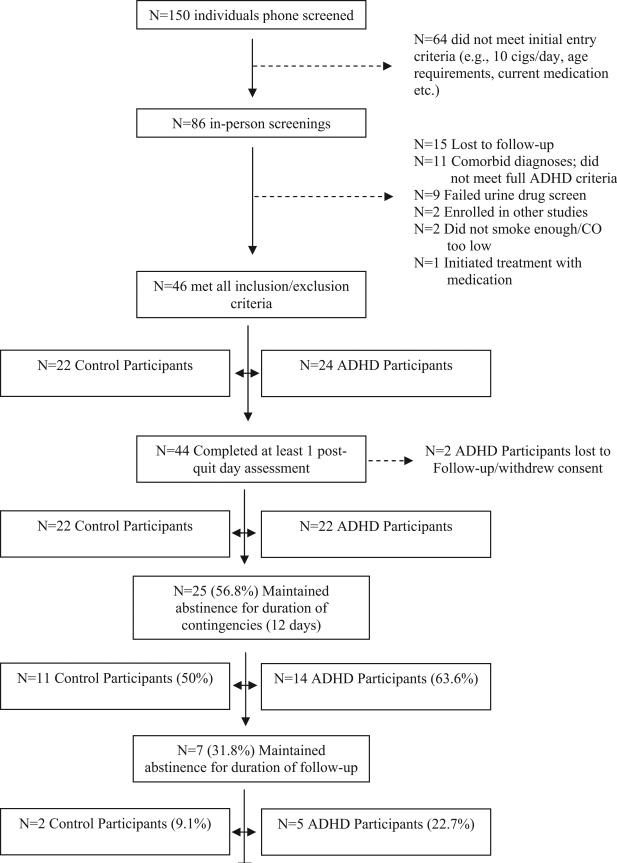
There were 155 individuals who completed the telephone prescreen and 86 were scheduled for screening visits. Of the 40 participants who were excluded after the in-person screening visit, 15 had repeated scheduling difficulties and/or were lost to follow-up; 11 either had exclusionary comorbid diagnoses or did not meet full criteria for ADHD; nine failed the urine drug screen; two were enrolled in other studies; two either did not smoke enough cigarettes per day or had carbon monoxide (CO) levels below the inclusion criterion; and one initiated treatment with St. John's Wort. Figure 1 illustrates the disposition of all participants throughout the study.
Figure 1.
Disposition of participants throughout study. cigs = cigarettes; ADHD = attention deficit hyperactivity disorder; CO = carbon monoxide.
Participants were 46 male and female adult smokers between 18 and 50 years old. Twenty-four of the participants were diagnosed with ADHD and 22 participants had no psychiatric diagnosis (except for nicotine dependence). Two participants in the ADHD group were lost to follow-up prior to the beginning of the monetary incentive phase. There were no significant group differences for any demographic variables at screening for those participants who eventually entered the monetary incentive phase (see Table 1).
Table 1.
Demographic and Baseline Smoking Related Variables for Participants With and Without ADHD
| Demographics | ADHD | non- ADHD | p value |
|---|---|---|---|
|
n (%) |
n (%) |
||
| Men | 13 (59.1) | 9 (40.9) | ns |
| Women | 9 (40.9) | 13 (59.1) | ns |
| White | 17 (77.3) | 15 (68.2) | ns |
|
M (SD) |
M (SD) |
||
| Age (years) | 29.5 (8.9) | 33.0 (8.5) | ns |
| IQ estimate | 105.0 (10.1) | 109.5 (9.5) | ns |
| Smoking related variables | |||
| CO (ppm) | 22.9 (10.0) | 20.8 (6.7) | ns |
| FTND total | 4.8 (2.2) | 4.2 (2.0) | ns |
| Cigarettes/day | 17.0 (5.7) | 16.0 (3.7) | ns |
| Years smoked | 12.4(8.9) | 15.1 (10.0) | ns |
| Age started | 16.4 (2.6) | 17.8 (3.6) | ns |
| Quit attempts | 3.1 (4.7) | 3.9 (3.8) | ns |
| ADHD related variables | |||
| DSM–IV Inattentive | 72.5 (13.1) | 46.6 (7.1) | <.001 |
| DSM–IV Hyperactive-Impulsive | 69.9 (10.4) | 44.0 (4.7) | <.001 |
| DSM–IV total | 74.6 (11.0) | 45.1 (5.9) | <.001 |
| Currently taking medication for ADHD (n) | 6 | 0 | |
Note. ADHD = attention deficit hyperactivity disorder; CO = carbon monoxide; FTND = Fagerstrom Test of Nicotine Dependence; DSM–IV = Diagnostic and Statistical Manual of Mental disorders.
Participants were recruited from the community via advertisements, word of mouth, and referrals from local clinics. Advertisements specifically sought “regular smokers with no known health problems.” A telephone prescreen was conducted and potential participants were invited to the clinic for a screening visit. Participants who were screened were referred by the following sources: print and web advertisements (ADHD group: 47.5%, control group: 61%), word of mouth (25%, 29%), radio advertisements (10%, 0%), posted flyers (7.5%, 2%), clinic referrals (7.5%, 2%), other/unknown (2.5%, 5%). Because this was not a controlled intervention trial, during prescreening potential participants who explicitly expressed interest in smoking cessation treatment were excluded and referred elsewhere.
During the screening visit, all participants provided informed consent and completed a detailed medical and smoking history. Smoking behavior was assessed, and expired CO concentration was measured to confirm that participants were sufficiently heavy smokers to meet inclusion criteria. To be included in the study, participants were required to report smoking at least 10 cigarettes per day and to provide an afternoon CO concentration of at least 10 ppm. No significant differences were found for expired CO concentration, total score on Fagerström Test of Nicotine Dependence (FTND), cigarettes smoked per day, age started smoking, years smoked, or number of quit attempts (see Table 1).
At screening participants were administered the following measures by a trained data technician: the Conners's Adult ADHD Rating Scale (CAARS)–Self-Report Version (Conners, Erhardt, Sparrow, & Staff, 1998) and the computerized Structured Clinical Interview for the DSM–IV (SCID; First, Gibbon, Williams, & Spitzer, 1997). A PhD-level clinical psychologist reviewed these results, and conducted a follow-up clinical interview that included the Conners's Adult Diagnostic Interview for DSM–IV (CAADID; Epstein, Johnson, & Conners, 2000). An ADHD diagnosis was made if the participant met full DSM–IV diagnostic criteria for ADHD Inattentive, Hyperactive/Impulsive, or Combined Type. Individuals in both groups were excluded if they met diagnostic criteria for any DSM–IV Axis I or II disorder other than ADHD or nicotine dependence. Participants were also excluded for significant neurological disorders, history of brain trauma, current pregnancy, or if they produced a positive urine drug screen for illicit substances. Participants in the ADHD group taking medication for their ADHD were allowed to participate, provided they had been on a stable dose and product for at least 3 weeks prior to baseline and agreed not to alter their medication regimen during the study. Six of the participants in the ADHD group were on stimulant medication during the study (immediate release methylphenidate/Ritalin LA, n = 2; Adderall/Adderall XR, n = 3; Dexedrine, n = 1). There were no significant differences between the participants with ADHD who were medicated or unmedicated with respect to initial CO readings, cigarettes smoked/day, age started smoking, years of regular smoking, or number of clinician rated ADHD symptoms (all ps > .10). Control participants who were taking psychoactive medications were excluded. Comprehensive feedback was provided to participants who were diagnosed with ADHD, and treatment referrals were provided when necessary.
Procedures and Visit Schedule
Following screening, participants completed two baseline sessions where a range of smoking and trait-based personality measures were completed. At the second baseline session, a quit date (QD) was established after which participants were instructed to try to abstain from smoking for up to 12 days. QDs were scheduled for Sunday evenings, so that the first laboratory visit was the following day (Monday). The scheduled duration of abstinence lasted 12 days, starting the day after the QD. During this time, participants visited the laboratory at a set time each day Monday to Friday. No visits were scheduled on Saturday and Sunday. For those participants who remained abstinent throughout the 12-day period, a single follow-up visit was scheduled 7 to 10 days following the last laboratory visit. No contingencies were in place during this follow-up period.
During each visit, participants provided an expired air CO sample and completed a range of smoking-related questionnaires. Participants also kept a daily smoking diary, which was reviewed at each visit, including the follow-up visit.
Monetary incentive procedures
Participants were instructed that starting on the first day following the QD they could earn money contingent on provision of CO samples that met the abstinence criterion of ≤4 ppm. This criterion is generally lower than other studies that have used monetary incentives to reduce smoking, but because we were interested in smoking withdrawal symptoms rather than abstinence per se, we were willing to accept potential false negatives to ensure that participants had not smoked. To allow for potential carryover effects, participants who failed to meet the criterion on the first day, but reported no smoking since the previous day and had a CO level at least half of the previous baseline session, were allowed to continue and were paid according to the contingency management (CM) schedule. An escalating payment schedule was used in which participants earned $4 for the first day and an additional $4 each day thereafter. In addition, an escalating bonus payment was made every 2 days that participants remained abstinent such that on the second day postquit, participants earned $10 for meeting the abstinence requirement, on the fourth day postquit, participants earned $20, and so on. Participants could therefore earn up to a total of $370 for maintaining abstinence across the 10 sessions (Monday to Friday of 2 consecutive weeks). Participants were instructed that if they provided CO samples of >4 ppm at any visit, failed to attend a scheduled laboratory session, or self-reported smoking, that they would be discontinued from the study. Following the end of the monetary incentive condition, participants were asked to keep a smoking diary until their scheduled follow-up visit 7 to 10 days later. Lapses occurring during this period were therefore based only on self-report of smoking.
Data analysis
The proportion of smokers with ADHD and without ADHD who remained abstinent throughout the monetary incentive and follow-up phases were the primary outcomes for the study. Confidence intervals (95%) for each proportion were estimated using the Wald method (Agresti & Coull, 1998). Rates of abstinence for the ADHD and non-ADHD groups during the monetary incentive phase were compared using chi-square analysis. The proportions of participants who maintained abstinence during follow-up were compared across and within the groups using chi-square analysis.
Results
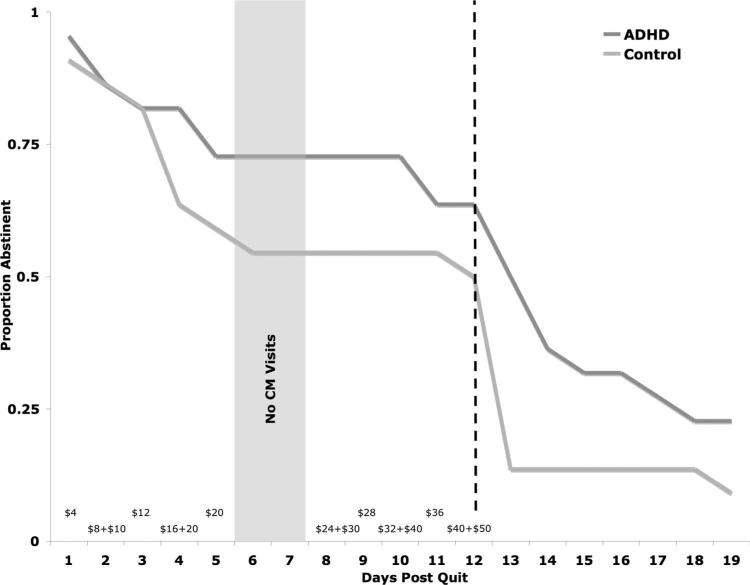
Two participants in the ADHD group discontinued prior to the QD due to scheduling difficulties. Thus, data are based on 22 participants/group. Figure 2 illustrates the proportion of participants in each group who were abstinent across the course of the study. Nearly 64% (14/22; 95% CI [43%, 80%]) of smokers in the ADHD group maintained complete abstinence for the 12 days during which the monetary incentives were in place. Fifty percent (11/22; 95% CI [31%, 69%]) of the smokers without ADHD maintained abstinence during this period. This difference was not statistically significant (Fisher exact p value = .54). At follow-up 22.7% of the smokers with ADHD (5/22; 95% CI [10%, 44%]) remained abstinent, versus 9.1% (2/22; 95% CI [1%, 29%]) of the smokers without ADHD. This difference between groups was not statistically significant (Fisher exact p value = .41). The difference in the proportion of participants who were abstinent during the monetary incentive phase versus the follow-up phase was significant for both groups: ADHD group, χ2 = 2.30, p = .02; control group, χ2 = 2.95, p = .004.
Figure 2.
Proportion of smokers in attention deficit hyperactivity disorder (ADHD; (n = 22) and non-ADHD (n = 22) groups maintaining smoking abstinence throughout the study. First 2 weeks were daily, carbon monoxide- (CO) verified assessment of smoking; and the second 2 weeks were not CO-verified and only assessed once at Day 19. Dollar values along x-axis signify the amount available for compliance with abstinence requirement on each day of the contingency management (CM) intervention. Dotted line indicates the point at which contingencies were removed.
Discussion
This study suggests that monetary incentives can promote smoking abstinence in individuals with ADHD in a similar manner as those smokers without ADHD, even among those who are not explicitly seeking smoking cessation treatment. These results are important because, to date, smokers with ADHD have been shown to have difficulty quitting compared to those without ADHD. In the absence of any other evidence for efficacious smoking cessation approaches specifically for this population, this finding is noteworthy and warrants larger trials that more directly evaluate the efficacy and feasibility of incentive-based approaches for reducing smoking among individuals with ADHD.
Incentive-based approaches to treat drug use, including CM, have been used for several decades to effectively reduce a range of substance use problems, including cigarette smoking. In general, most of these approaches provide some form of consequence (e.g., money, vouchers redeemable for goods and services, affordable housing, etc.) contingent on evidence of drug abstinence, verified by a biological assay such as a urine drug test, or in the case of cigarette smoking, expired breath CO (Stitzer & Petry, 2006). Cigarette smoking specifically has been the target of incentive-based interventions for over 25 years, with more than a dozen published studies demonstrating overall efficacy of the approach for reducing smoking. A recent meta-analysis that included 11 studies reported that the effect size for CM as a means to promote cigarette smoking abstinence was .31 (Prendergast, Podus, Finney, Greenwell, & Roll, 2006). This estimate is likely to be conservative, however, because several more recent studies demonstrating high levels of efficacy for CM to promote smoking abstinence were not included in the effect size estimate (Correia & Benson, 2006; Krishnan-Sarin et al., 2006; Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004; Lamb et al., 2007). Large scale dissemination of CM-based treatments for drug addition and other public health problems is currently underway (Petry & Simcic, 2002; Stitzer & Petry, 2006).
In addition to demonstrating efficacy for reducing smoking in typical adult smokers, CM has been shown to be helpful for a range of unique or difficult-to-treat populations, including individuals with schizophrenia (Roll et al., 1998; Tidey et al., 2002), opiate-dependent individuals undergoing methadone maintenance treatment (Dunn, Sigmon, Thomas, Heil, & Higgins, 2008; Shoptaw et al., 2002), adolescent smokers (Krishnan-Sarin et al., 2006), and pregnant women (Heil et al., 2008; Higgins et al., 2004). Even among smokers who report low motivation to quit, CM has been shown to reduce smoking rates significantly (Lamb, Morral, Galbicka, Kirby, & Iguchi, 2005; Lamb et al., 2007).
ADHD has been conceptualized to be the result of altered reinforcement processes (Johansen et al., 2009; Luman et al., 2005), which may help explain both why it is difficult for smokers with ADHD to quit, and also why the incentive-based approach used in this study demonstrated efficacy for reducing smoking. A wide range of both laboratory and clinical studies has demonstrated that the provision of immediate and salient reinforcement that is contingent on some target response is very effective at modifying problem behavior in individuals with the disorder. In fact, reinforcement-based interventions are the most effective nonpharmacological treatment for ADHD (Fabiano et al., 2009).
The data presented here need to be considered in light of several important aspects of the monetary incentive manipulation as it pertains to more traditional CM interventions for smoking. First, because the manipulation used in this study was designed only to promote abstinence to study withdrawal effects, individuals who lapsed even a single time were discontinued from further participation. Traditional CM interventions for smoking cessation allow for lapses and have typically used a reset contingency in which lapses result in the amount of the contingencies being reset to the initial value (Heil et al., 2008). As such, the extent to which more conventional CM may help promote smoking cessation in individuals with ADHD is difficult to assess in the present study due to the stringency of our abstinence requirement. Second, the duration of the monetary incentive manipulation was relatively short. Although some studies using true CM interventions have assessed durations as short as 1 week to promote smoking abstinence (Roll et al., 1998; Tidey et al., 2002), most larger clinical trials have used longer durations (Heil et al., 2008; Shoptaw et al., 2002). The durability of the abstinence effect observed in the present study will be important to evaluate across longer durations in future studies. Third, because the present study was not designed as a controlled treatment trial, participants were not explicitly interested in quitting. Although CM has been shown to reduce cigarette smoking even among those uninterested in quitting (Lamb et al., 2005; Lamb et al., 2007), it is not clear how the monetary incentives used in this study would have worked in smokers with ADHD who were interested in or motivated to quit. Fourth, the present study did not have a control group that did not receive monetary incentives for abstinence, nor did it use any other interventions (e.g., nicotine replacement therapy, counseling). Therefore, it is impossible to attribute the observed reductions in smoking solely to the monetary incentive manipulation. Fifth, the use of CO as the sole marker of abstinence is potentially problematic and future studies should use nicotine metabolites, such as cotinine, to verify abstinence. Finally, the comparison of abstinence rates between the ADHD and non-ADHD groups needs to be interpreted carefully because this was not designed as a true equivalence trial. As such, the study was underpowered to adequately assess the efficacy of the intervention across groups. However, concerns about power are mitigated to some extent given the numerical superiority of the ADHD group compared to the non-ADHD group.
Despite these limitations, the data provide promising evidence that monetary incentives can effectively promote smoking abstinence in a clinical sample of individuals who have been reported to fare poorly in smoking cessation trials. Contrary to other studies showing that a diagnosis of ADHD or high levels of symptoms predict poorer treatment outcomes (Covey et al., 2008; Humfleet et al., 2005), the present study found no difference between smokers with ADHD and without ADHD in rates of abstinence facilitated by monetary incentives. The data from the present study suggest that larger randomized clinical trials of more traditional CM in smokers with ADHD would be warranted. Such studies should be designed to be more consistent with other trials that have evaluated the effects of CM on smoking, such as allowing for lapses, having a longer treatment duration, including a non-CM control group, and perhaps combining CM with potentially efficacious pharmacological treatments for smoking. In addition, given the high rates of other substance use problems in individuals with ADHD (Biederman et al., 1997) and the widely demonstrated effects of CM for reducing other kinds of drug use (Prendergast et al., 2006), this type of intervention might also be considered for individuals with ADHD and other drug use problems.
Acknowledgments
This research was supported by Grants R21DA020806 (Scott H. Kollins), K24DA023464 (Scott H. Kollins), and K23DA017261 (F. Joseph McClernon).
Footnotes
E-Mail Notification of Your Latest Issue Online!
Would you like to know when the next issue of your favorite APA journal will be available online? This service is now available to you. Sign up at http://notify.apa.org/ and you will be notified by e-mail when issues of interest to you become available!
Contributor Information
Scott H. Kollins, Duke ADHD Program, Department of Psychiatry, Duke University Medical Center
F. Joseph McClernon, Health Behavior Neuroscience Research Program, Department of Psychiatry, Duke University Medical Center..
Elizabeth E. Van Voorhees, Duke ADHD Program, Department of Psychiatry, Duke University Medical Center
References
- Agresti A, Coull B. Approximate is better than “exact” for interval estimation of binomial proportions. The American Statistician. 1998;52:119–126. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, Soriano J. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Delay aversion in attention deficit/hyperactivity disorder: An empirical investigation of the broader phenotype. Neuropsychologia. 2009;47:446–456. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: The influence of arousal on prefrontal cortical function. Annals of the New York Academy of Sciences. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocki K, Fan J, Fossella J. Placing neuroanatomical models of executive function in a developmental context: Imaging and imaging–genetic strategies. Annals of the New York Academy of Sciences. 2008;1129:246–255. doi: 10.1196/annals.1417.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CL, Mann M, Alexander DK. Effects of reward and response cost on the performance and motivation of children with ADHD. Cognitive Therapy & Research. 2000;24:87–98. [Google Scholar]
- Carlson CL, Tamm L. Responsiveness of children with attention deficit-hyperactivity disorder to reward and response cost: Differential impact on performance and motivation. Journal of Consulting and Clinical Psychology. 2000;68:73–83. doi: 10.1037/0022-006X.68.1.73. [DOI] [PubMed] [Google Scholar]
- Conners C, Erhardt D, Sparrow E. The Conner's Adult ADHD Rating Scale (CAARS) Multi-Health Systems; Toronto, Canada: 1998. Staff. [Google Scholar]
- Correia CJ, Benson TA. The use of contingency management to reduce cigarette smoking among college students. Experimental and Clinical Psychopharmacology. 2006;14:171–179. doi: 10.1037/1064-1297.14.2.171. [DOI] [PubMed] [Google Scholar]
- Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D. Smoking cessation and inattention or hyperactivity/impulsivity: A post hoc analysis. Nicotine & Tobacco Research. 2008;10:1717–1725. doi: 10.1080/14622200802443536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavior Analysis. 2008;41:527–538. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Johnson D, Conners CK. Conners's Adult ADHD Diagnostic Interview for DSM–IV. Multi-Health Systems; North Tonawanda, NY: 2000. [Google Scholar]
- Fabiano GA, Pelham WE, Jr., Coles EK, Gnagy EM, Chronis-Tuscano A, O'Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clinical Psychology Review. 2009;29:129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Williams JBW, Spitzer RL. SCID Screen Patient Questionnaire–Extended version. Multi-Health Systems; North Tonawanda, NY: 1997. [Google Scholar]
- Gilbert DG, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: Influences of trait depression, personality, and habitual nicotine intake. Experimental and Clinical Psychopharmacology. 1999;7(2):174–181. doi: 10.1037//1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, Gehlbach BA. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: Relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmacology. 1999;7:427–443. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addicition. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, Lynch ME. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103:1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, Badger GJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Munoz R, Reus V, Hall SM. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine & Tobacco Research. 2005;7:453–460. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Killeen PR, Russell VA, Tripp G, Wickens JR, Tannock R, Sagvolden T. Origins of altered reinforcement effects in ADHD. Behavioral and Brain Functions. 2009;5:7. doi: 10.1186/1744-9081-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, Lane SD, Shapiro SK. Experimental analysis of childhood psychopathology: A laboratory matching analysis of the behavior of children diagnosed with Attention-Deficit Hyperactivity Disorder (ADHD). Psychological Record. 1997;47:25–44. [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, Cavallo DA. Contingency management for smoking cessation in adolescent smokers. Experimental and Clinical Psychopharmacology. 2006;14:306–310. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Galbicka G, Kirby KC, Iguchi MY. Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology. 2005;13(2):83–92. doi: 10.1037/1064-1297.13.2.83. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Javors MA, Galbicka G, Iguchi M. Contingencies for change in complacent smokers. Experimental and Clinical Psychopharmacology. 2007;15:245–255. doi: 10.1037/1064-1297.15.3.245. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U, Sonuga-Barke EJ. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: Results of a preliminary study. Psychopharmacology (Berlin) 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- McInerney RJ, Kerns KA. Time reproduction in children with ADHD: Motivation matters. Child Neuropsychology. 2003;9(2):91–108. doi: 10.1076/chin.9.2.91.14506. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Petry NM, Simcic F., Jr. Recent advances in the dissemination of contingency management techniques: Clinical and research perspectives. Journal of Substance Abuse Treat. 2002;23(2):81–86. doi: 10.1016/s0740-5472(02)00251-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. Journal of Substance Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Tucker SB, DuPaul GJ, Merlo M, Stoner G. Hyperactivity and frustration: The influence of control over and size of rewards in delaying gratification. Journal of Abnormal Child Psychology. 1986;14:191–204. doi: 10.1007/BF00915440. [DOI] [PubMed] [Google Scholar]
- Rohde P, Kahler CW, Lewinsohn PM, Brown RA. Psychiatric disorders, familial factors, and cigarette smoking: II. Associations with progression to daily smoking. Nicotine & Tobacco Research. 2004;6:119–132. doi: 10.1080/14622200310001656948. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: A feasibility study. Experimental and Clinical Psychopharmacology. 1998;6:157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response inhibition in children with DSM–IV subtypes of AD/HD and related disruptive disorders: The role of reward. Child Neuropsychology. 2001;7:172–189. doi: 10.1076/chin.7.3.172.8746. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion–I. The effect of delay on choice. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD). Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:691–698. [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Utzinger L, Biederman J. Cigarette smoking associated with attention deficit hyperactivity disorder. Journal of Pediatrics. 2008;153:414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Dorer E. A double-blind, placebo-controlled trial of osmotic release methylpheni-date in initiating and maintaining abstinence in smokers with attention deficit hyperactivity disorder; Paper presented at the College on Problems of Drug Dependence; Reno, NV. Jun, 2009. [Google Scholar]