Abstract
Objectives
The incidence of chemotherapy induced peripheral neuropathy (CIPN) is 15–25% with platinum and taxanes. CIPN can be permanent and often requires dose reduction or change in chemotherapy. Acetyl-L-carnitine (ALCAR), an ester of L-carnitine, is used to treat CIPN in humans and in animal models. The goals of this study are: 1) examine the effects of ALCAR on ovarian cancer cells, 2) determine if ALCAR affects the cytotoxicity of standard chemotherapy on ovarian cancer cells.
Methods
OVCAR-3 and SKOV-3 ovarian cancer lines were incubated in ALCAR containing media. Viability, proliferation, and expression of the nerve growth factor receptors (NGFR) Trk-A and p-75 were determined by flow cytometry. Cytotoxicity assays examining ALCAR’s effect on paclitaxel and carboplatin were done by flow cytometry and infrared plate-reader.
Results
Flow cytometry showed no change in percent live (p=0.87) or proliferation (p=0.95) of OVCAR-3 cells when comparing controls with up to 100 μM ALCAR. However, there was a slight but significant decrease in the proliferation of SKOV-3 cells incubated at higher ALCAR concentrations (p=<0.01). Flow cytometry showed no difference in the viability of OVCAR-3 cells when comparing ALCAR: +/− paclitaxel (p=1), +/− carboplatin (p=0.8), or both (p=0.4). Proliferation assays indicated that paclitaxel’s cytotoxicity on OVCAR-3 and SKOV-3 cells was unchanged at higher ALCAR concentrations (p=<0.01–0.4). ALCAR did not affect the expression of NGFR on OVCAR-3 or SKOV-3 cells.
Conclusion
ALCAR does not affect the cytotoxicity of paclitaxel or carboplatin. There was no increase in proliferation, or NGFR of OVCAR-3 or SKOV-3 cells exposed to ALCAR.
Keywords: Acetyl-L-carnitine, ALCAR, Ovarian cancer, Chemotherapy, Nerve growth factor receptor, NGFR
Introduction
Treatment of advanced ovarian cancer consists of a combination of maximal surgical effort and chemotherapy. With aggressive surgery and chemotherapy, certain regimens for this once uniformly fatal disease now show an overall median survival of 66.9 months [1]. The successes in the treatment of advanced and recurrent ovarian cancer have centered on the use of combination chemotherapy regimens including taxanes and platinating agents [2,3]. The international standard of care for both initial and platinum-sensitive recurrent ovarian cancer has evolved towards combination paclitaxel and carboplatin due to its relative tolerability and activity [1,4,5].
The major side effect of this treatment modality includes acute and chronic neuropathies. These sensory and motor chemotherapy induced peripheral neuropathies (CIPN) limit the tolerated cumulative dose. The incidence of CIPN varies between 15 and 54% with more than half of the patients developing grade 2 neuropathies that can sometimes be permanent [4,6,7]. The CIPN greatly affects the quality of life and highly effective chemotherapy regimens are often stopped, altered or dose-reduced [8]. In a landmark Gynecologic Oncology Group trial of intraperitoneal chemotherapy, only 42% of patients were able to complete all 6 cycles of a therapy that showed a clear advantage in overall survival due to toxicities including neuropathy [1].
Over the last decade several studies have shown that Acetyl-L-carnitine (ALCAR), a naturally occurring ester of L-carnitine, can be used to treat painful neuropathies. These studies include neuropathies caused by diabetes [9–11], HIV [12,13], and multi-factorial etiologies [14]. Studies in animal models and small-scale clinical trials have shown a beneficial effect in using ALCAR for the treatment of CIPN [15–18,19–21].
ALCAR exerts its neuroprotective effects by acting as an acyl chain donor and inhibitor of ceramide generation, or by enhancing histone acetylation and attenuating apoptosis [22,23–26]. Additionally, ALCAR facilitates nerve growth by enhancing expression of neurotrophin, nerve growth factor (NGF), nerve growth factor receptor (NGFR) [27–30].
Advanced stage ovarian tumors also express the NGFR p75 and Trk-A [31,32]. In one study, expression of phosphorylated Trk-A NGFR was observed in 21 (18%) of 119 borderline, 12 (21%) of 57 stage I, and 47 (84%) of 56 stage III–IV ovarian carcinomas [32]. The significance of these observations is that stimulation of NGFR expressing ovarian cancer cell lines with NGF induces the cells to produce VEGF [33]. Thus the ovarian tumor cells may utilize the NGF-NGFR pathway to produce angiogenic factors that may promote their growth and metastasis.
Extensive literature supports the anti-apoptotic and growth promoting functions of ALCAR. However, it is pertinent to ask if these same functions of ALCAR may also promote ovarian cancer growth. These preceding observations led us to investigate the potential effect of ALCAR on ovarian cancer cells: growth and proliferation, expression of NGFR p75 and Trk-A, and alteration in the cytotoxic potential of paclitaxel and carboplatin when used in a combinatorial therapeutic regimen.
Materials and methods
Cell culture
OVCAR-3 and SKOV-3 ovarian cancer cells (ATCC, Rockville, MD) were incubated in a base culture media of RPMI 1640. Cells were incubated at 37 °C, in 5% CO2 (Thermo Electron, Marietta, OH).
Flow cytometry
For flow cytometric analysis, cells (5×105 cells/tube) were washed two times in phosphate buffered saline containing 1% bovine serum albumin (PBS-BSA) and incubated sequentially with anti-Trk-A NGFR and anti-p75 NGFR (Santa Cruz Biotechnology, Santa Cruz, CA) and FITC-labeled goat anti-mouse (GAM) or goat-anti rabbit (GAR) secondary antibodies (1:100 dilution) for 30 min on ice. To examine ALCAR’s effect on NGFR expression the OVCAR-3 and SKOV-3 cells were incubated for 72 h with 0, 1, 10, or 100 μM ALCAR prior to incubation with the antibodies. After labeling the cells were washed with PBS-BSA. Cells were analyzed on an LSRII (Becton Dickinson) flow cytometer using FACSDiva software. DAPI was used as a live/dead indicator. To compare the values of samples acquired on different days, SPHERO™ Rainbow Fluorescent Particles (BD Biosciences) were used to standardize instrument voltages. FlowJo software (v. 8.4, TreeStar) was used for analysis of the raw flow cytometry data.
Proliferation assay
CellTracker Green (Invitrogen, Carlsbad, CA) labeled OVCAR-3 cells (2×106 cells/25 cm2 flask) were grown in quadruplicate in media containing 0,1,10, or 100 μM ALCAR (Spectrum Chemical Manufacturing, Gardena, CA). After 72 hour culture, the cell proliferation was determined by flow cytometry. Propidium iodide was used as the live/dead indicator and ModFit LT (v3.1) was used for data analysis.
CA-125 assay
OVCAR-3 cells (1×105/well) were plated in a 6 well plate and cultured in media supplemented with 0, 1, 10, or 100 μM ALCAR. The experiment was done in duplicate. After culturing for 5 days, the supernatant was harvested and the amount of CA125 was determined by using the standard clinical radioimmunoassay (Abbott Axsym, Abbott Park, IL).
Cytotoxicity assays by flow cytometry
For the combination chemotherapy assays, OVCAR-3 cells (2×106 cells) were cultured in media containing 0 or 10 μM ALCAR. Paclitaxel (Bedford Laboratory, Bedford, OH) (1.4 nM), or carboplatin (Bedford Laboratory, Bedford, OH) (22.2 μM), or combination of paclitaxel and carboplatin at an individual drug concentrations shown to inhibit 50% growth (IC 50) [34] was added to the ALCAR treated and untreated OVCAR-3 cells. After culturing for 72 h, the cells were analyzed by flow cytometry as described above.
Cytotoxicity assays by fluorescent plate reader
Paclitaxel cytotoxicity assays were performed in 96 well black wall assay plates (Corning Inc., Corning, NY). OVCAR-3 or SKOV-3 cells (1×104 cells/well) were incubated for 4 h and paclitaxel and ALCAR (0,1, 10, 100 μM) were added concomitantly or sequentially (by adding the first drug at the start of the assay, culturing for 24 h, followed by addition of the second drug). One-half of the wells received 10 μM (10 times the concentration shown to be lethal to 50% of ovarian cancer cells {LC50}) paclitaxel and the other half did not. Each treatment was repeated in quadruplicate. After the final treatment had been added cells were incubated for 72 h. Cell-Titer Glo (Promega, Inc) was added to determine cell viability, and the plates were then read by a Safire II plate reader (Tecan US, Durham, NC).
Statistics
The GraphPad Prism (v. 4, GraphPad Software, Inc.) program was used to perform statistical analysis. Samples were compared using a Chi square test, when a paired observation on 2 variables was used. One-way analysis of variance (ANOVA) was used for the comparison of multiple groups with multiple variables within each group. Dunnett’s multiple comparison test was performed if ANOVA was significant. A student t-test with two-sided p-values, was used to compare unpaired groups. A p<0.05 was used to indicate significance.
Results
ALCAR does not affect proliferation of expression of CA-125 by ovarian cancer cells
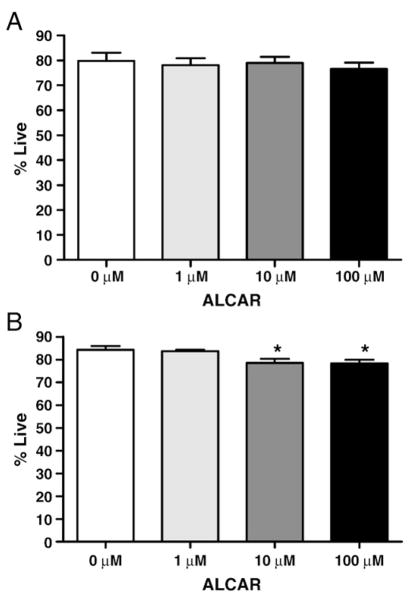
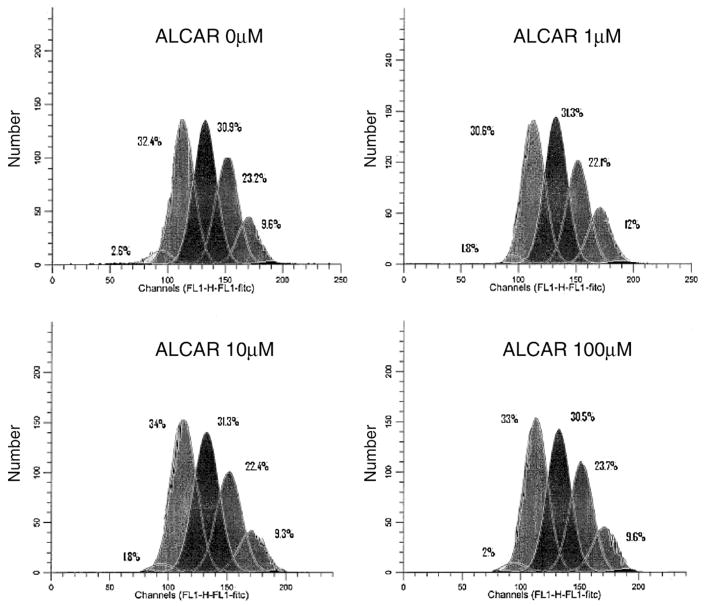
Flow cytometry results showed that a 3 day treatment with ALCAR at up to 100 times the physiologic concentration was not toxic to the OVCAR-3 cells (p=0.87) (Fig. 1A). However, among SKOV-3 cells there was a small, but statistically significant, decrease in the percentage of live cells when comparing the 4 ALCAR groups by ANOVA (p=<0.01). By Dunnett’s multiple comparison, the cells grown in 10 μM and 100 μM ALCAR (both p=<0.05) were significantly decreased when compared to the control (Fig. 1B). No effect (p =0.95) was observed on the proliferation of the OVCAR-3 cells following ALCAR treatment (Fig. 2).
Fig. 1.
ALCAR does not induce proliferation of ovarian cancer cells. (A) OVCAR-3 and SKOV-3 (B) cells were treated with the designated concentrations of ALCAR and proliferation was determined by flow cytometry. Each experiment was repeated in triplicate (SKOV-3) or quadruplicate (OVCAR-3). * Indicates significant from control group (ALCAR 0 μM) by Dunnett’s multiple Comparison (both p=<0.05).
Fig. 2.
OVCAR-3 proliferation profile in response to ALCAR. Shown is the proliferation graph of OVCAR-3 cells grown in varying ALCAR concentrations. Each peak represents a generation, with the higher generation (6th generation) on the left, and down to the 2nd generation on the far right. The addition of ALCAR did not make a significant difference in the rate of proliferation.
OVCAR-3 cells release the tumor antigen CA-125. We therefore measured the concentration of CA125 in the spent media following ALCAR treatment as a surrogate for proliferation of the OVCAR-3 cells. The mean amount (±standard deviation) of CA125 detected following treatment with 0, 1, 10, and 100 mM of ALCAR were 2680±1146 U/ml, 2055±49.50 U/ml, 2395±756.6 U/ml, and 2345±63.64 U/ml respectively. There was no statistical difference in the amount of CA-125 expression among the varying groups (p=0.84).
Cytotoxicity assay by flow cytometry showed no effect on paclitaxel or carboplatin by ALCAR
In the flow cytometry based assay, when comparing paclitaxel vs. paclitaxel+ALCAR, 13.1% and 13.6% of the cell population was dead, respectively (p=1.0). In the carboplatin vs. carboplatin+ALCAR group there were 10.7% and 13% dead cells, respectively (p=0.83). Finally, in the paclitaxel+carboplatin without and with ALCAR there were 12.1% and 17.3% dead cells (p=0.42).
Concurrent and sequential cytotoxicity assays by fluorescent reader showed no effect on cytotoxic potential of paclitaxel by ALCAR
In the concurrent and sequential cytotoxicity assays the LC50 dose of paclitaxel was used to show a larger difference between the control and treatment groups. The cytotoxic potential of paclitaxel was not affected by varying amounts of concomitant ALCAR for OVCAR-3 cells (p=0.19), or SKOV-3 cells (p=0.40) (Table 1). Additionally, by ANOVA there was a small, but statistically significant, difference in plate luminescence between the wells with varying amounts of ALCAR in both OVCAR-3 (p=<0.01) and SKOV-3 (p=<0.01). Dunnett’s multiple comparison showed in the OVCAR-3 cells there was a significant, but minor, difference between the control group and the 1 μM (decrease) and 100 μM ALCAR (increase) group (both p=<0.05). For the SKOV-3 cells Dunnett’s multiple comparison showed a significant, but minor, increase between the control group and the 100 μM ALCAR group (p=<0.01).
Table 1.
ALCAR does not affect the cytotoxic potential of paclitaxel
| OVCAR-3 cells | Luminescence | SD | Range
|
p-value | |
|---|---|---|---|---|---|
| Min | Max | ||||
| ALCAR | |||||
| 0 μM | 30,700 | ±2965 | 27,410 | 34,210 | Control |
| 1 μM | 26,320 | ±2238 | 23,550 | 28,430 | <.05 |
| 10 μM | 32,950 | ±1085 | 31,670 | 34,230 | >.05 |
| 100 μM | 36,050 | ±1930 | 33,930 | 38,490 | <.05 |
| ALCAR and Paclitaxel | |||||
| 0 μM | 12,720 | ±352 | 12,380 | 13,210 | Control |
| 1 μM | 13,540 | ±811 | 12,390 | 14,300 | NA |
| 10 μM | 13,950 | ±1330 | 12,320 | 15,190 | NA |
| 100 μM | 12,960 | ±462 | 12,400 | 13,420 | NA |
| SKOV-3 cells
| |||||
| ALCAR | |||||
| 0 μM | 24,070 | ±2631 | 20,590 | 26,220 | Control |
| 1 μM | 23,570 | ±2347 | 21,110 | 25,870 | >.05 |
| 10 μM | 27,400 | ±590.1 | 26,570 | 27,910 | >.05 |
| 100 μM | 30,660 | ±894.3 | 29,910 | 31,920 | <.01 |
| ALCAR and Paclitaxel | |||||
| 0 μM | 15,240 | ±486.1 | 14,640 | 15,700 | Control |
| 1 μM | 15,370 | ±611.9 | 14,800 | 15,910 | NA |
| 10 μM | 15,320 | ±1090 | 13,770 | 16,340 | NA |
| 100 μM | 14,440 | ±1052 | 13,720 | 15,980 | NA |
SD—standard deviation; Min—minimum value; Max—maximum value; NA—not available {ANOVA indicated no significant difference}.
In the first sequential cytotoxicity assay, paclitaxel for 24 h followed by addition of ALCAR for 72 h (Table 2), there was a significant, but slight, decrease in the amount of surviving cancer cells whose media contained chemotherapy and higher amounts of ALCAR for both the OVCAR-3 (p=0.02) and the SKOV-3 cells (p=<0.01). For the OVCAR-3 cells, Dunnett’s multiple comparison showed a significant decrease between the control group and the 1 μM (p=<0.05) and 100 μM group (p=<0.01). Among the SKOV-3 groups Dunnett’s multiple comparison did not show a significant decrease between any groups. There was no significant difference between the control and ALCAR only arms (OVCAR-3 p=0.33; SKOV-3 p=0.62).
Table 2.
Sequential treatment with paclitaxel and then ALCAR does not affect the cytotoxic potential
| OVCAR-3 cells | Luminescence | SD | Range
|
p-value | |
|---|---|---|---|---|---|
| Min | Max | ||||
| ALCAR | |||||
| 0 μM | 245,400 | 27,710 | 218,600 | 269,400 | Control |
| 1 μM | 276,300 | 39,840 | 230,900 | 326,600 | NA |
| 10 μM | 278,900 | 25,060 | 244,100 | 303,400 | NA |
| 100 μM | 278,500 | 20,430 | 256,600 | 302,200 | NA |
| ALCAR and Paclitaxel | |||||
| 0 μM | 119,200 | 6973 | 111,100 | 127,000 | Control |
| 1 μM | 104,500 | 8642 | 99,100 | 117,300 | <.05 |
| 10 μM | 107,300 | 6692 | 102,800 | 117,100 | >.05 |
| 100 μM | 100,600 | 5512 | 94,340 | 107,300 | <.01 |
| SKOV-3 cells
| |||||
| ALCAR | |||||
| 0 μM | 246,300 | 81,670 | 126,800 | 310,000 | Control |
| 1 μM | 267,700 | 12,950 | 253,000 | 282,100 | NA |
| 10 μM | 278,900 | 3986 | 273,900 | 283,600 | NA |
| 100 μM | 282,000 | 4147 | 278,400 | 287,000 | NA |
| ALCAR and Paclitaxel | |||||
| 0 μM | 96,620 | 7553 | 90,340 | 107,600 | Control |
| 1 μM | 103,500 | 6833 | 95,770 | 112,200 | >.05 |
| 10 μM | 106,300 | 7939 | 98,250 | 116,600 | >.05 |
| 100 μM | 84,050 | 7760 | 74,690 | 93,000 | >.05 |
SD—standard deviation; Min—minimum value; Max—maximum value; NA—not available {ANOVA indicated no significant difference}.
In the second sequential cytotoxicity assay, ALCAR for 24 h followed by addition of paclitaxel for 72 h (Table 3), there was a significant, but minor, decrease in the amount of surviving cancer cells in the paclitaxel and ALCAR containing wells for SKOV-3 (p=<0.01), but not OVCAR-3 (p=0.584). Among the SKOV-3 groups, Dunnett’s multiple comparison showed a significant decrease between the control group and the 100 μM group (p=<0.01). There was no significant difference between the control arms with varying amounts of ALCAR (OVCAR-3 p=0.82; SKOV-3 p=0.51).
Table 3.
Sequential treatment with ALCAR and then paclitaxel does not affect the cytotoxic potential
| OVCAR-3 cells | Luminescence | SD | Range
|
p-value | |
|---|---|---|---|---|---|
| Min | Max | ||||
| ALCAR | |||||
| 0 μM | 295,300 | 30,330 | 268,900 | 338,100 | Control |
| 1 μM | 295,800 | 20,780 | 268,200 | 315,300 | NA |
| 10 μM | 310,000 | 26,220 | 281,400 | 334,800 | NA |
| 100 μM | 298,900 | 20,650 | 280,300 | 318,100 | NA |
| ALCAR and Paclitaxel | |||||
| 0 μM | 136,900 | 11,340 | 121,900 | 149,400 | Control |
| 1 μM | 146,000 | 3928 | 140,900 | 150,400 | NA |
| 10 μM | 143,200 | 8483 | 136,700 | 155,600 | NA |
| 100 μM | 142,700 | 11,250 | 127,100 | 153,800 | NA |
| SKOV-3 cells
| |||||
| ALCAR | |||||
| 0 μM | 298,000 | 14,360 | 277,600 | 311,300 | Control |
| 1 μM | 306,600 | 24,400 | 277,100 | 336,200 | NA |
| 10 μM | 319,800 | 21,690 | 295,800 | 339,100 | NA |
| 100 μM | 306,100 | 18,270 | 285,200 | 322,300 | NA |
| ALCAR and Paclitaxel | |||||
| 0 μM | 147,600 | 6593 | 140,200 | 154,900 | Control |
| 1 μM | 151,700 | 10,020 | 137,700 | 159,400 | >.05 |
| 10 μM | 142,500 | 7056 | 134,800 | 151,600 | >.05 |
| 100 μM | 119,000 | 4874 | 112,500 | 123,400 | <.01 |
SD—standard deviation; Min—minimum value; Max—maximum value; NA—not available {ANOVA indicated no significant difference}.
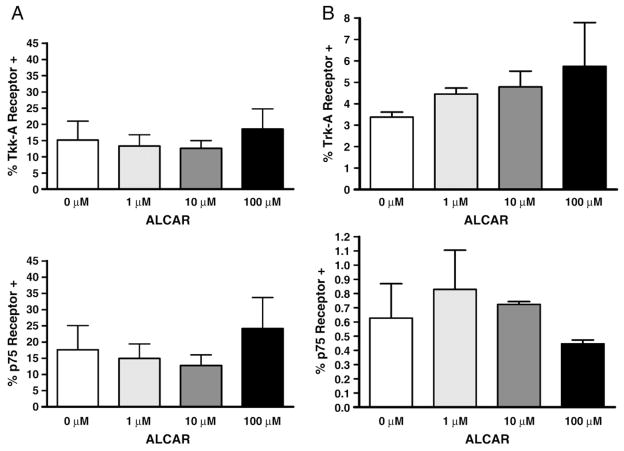
Nerve growth factor receptor expression was not increased by ALCAR
Between 5 and 30% of OVCAR-3 cells express Trk-A NGFR and 4 and 31% of these cells express p75 NGFR (Fig. 3A). There was neither a statistically significant difference in the Trk-A NGFR (p=0.66), nor the p75 NGFR (0.82) between the different ALCAR treatment groups (Fig. 3A). Of the OVCAR-3 cells expressing NGFR, the mean fluorescent intensity (MFI), a measure of the density of the receptors on the cell, showed the MFI for p75 NGFR for the varying amounts of ALCAR (0, 1, 10, 100 μM) to be 521±91, 532±114, 516±108, 537± 124 MFI respectively. The MFI for Trk-A NGFR for the varying amounts of ALCAR (0, 1, 10, 100 μM) are 598±55, 541±124, 545± 126, and 539±98 MFI respectively. There was no statistical difference between the control and ALCAR groups (p75 NGFR p=0.99; Trk-A NGFR p=0.83).
Fig. 3.
ALCAR does not affect the expression of Trk-A and p75. OVCAR-3 (A) and SKOV-3 (B) were treated with ALCAR and expression of Trk-A and p75 was determined by flow cytometry. Each experiment was repeated in triplicate (SKOV-3) or quadruplicate (OVCAR-3). There was no statistically significant difference among the groups.
To study the SKOV-3 cell line and NGFR, we performed a flow experiment done in triplicate. The control arm, without ALCAR, showed that of the SKOV-3 cells, approximately 3% express Trk-A NGFR. The NGFR p75 is expressed by 0.3–1% of SKOV-3 cells with a mean of 0.4%. There was neither a statistically significant difference in the Trk-A NGFR (p=0.53), nor the p75 NGFR (0.54) between the different ALCAR groups (Fig. 3B). Of the SKOV-3 cells expressing NGFR, the MFI for p75 NGFR for the varying amounts of ALCAR (0, 1, 10, 100 μM) are 636±25, 636±11, 628±15, 643±28 MFI respectively. The MFI for Trk-A NGFR for the varying amounts of ALCAR (0, 1, 10, 100 μM) are 1068±36, 999±108, 987±94, 915±174 MFI respectively. There was no statistical difference between the control and ALCAR groups (p75 NGFR p=0.85; Trk-A NGFR p=0.48).
Discussion
Presently, there are no proven preventative or treatment measures available for CIPN. ALCAR may be one of the most promising compounds to help allay this side effect that so negatively impacts the quality of life for cancer patients.
ALCAR, an ester of L-carnitine, transports fatty acids in and out of the inner matrix membrane of the mitochondria and thereby supports energy production via β-oxidation [35]. In humans ALCAR comes from dietary absorption, biosynthesis in the liver, brain and kidney (from lysine and methionine conversion), and resorption in the renal collecting system [36]. L-carnitine is reversibly converted to ALCAR by carnitine acetyltransferase [37]. In human plasma, ALCAR has a physiologic range around 1–5 μM [38,39].
ALCAR may exert neuroprotective effects via several separate mechanisms [22]. First, ALCAR enhances the synthesis of acetylcholine which in turn binds to muscarinic acid receptors and produces analgesic effects [40]. ALCAR also increases the expression of metabotropic glutamate receptors 2 and 3 (mGlu2/3) in the dorsal horn and dorsal root ganglion [41], and induces the acetylation of the transcription factor NF-κB resulting in the up-regulation of mGlu2/3 gene expression [42,43]. These mechanisms along with the previously mentioned anti-apoptosis and increased NGF-NGFR expression help explain its neuroprotective effect.
Additional studies have examined the use of ALCAR in CIPN. These trials include both rodent models [15–18] as well as small clinical trials [19–21]. Concurrent administration of ALCAR and paclitaxel, vincristine or cisplatin in rats decreases the incidence of subsequent allodynia, a form of peripheral neuropathy.
In a clinical study, cancer patients with CIPN were treated with IV ALCAR (1 g per day for a median of 14 days) [19]. Using the WHO Toxicity Grading list it was observed that 73% (19/26) patients reported a rapid (usually within 14 days) improvement in their neuropathy by at least one grade. The only adverse effect reported was one case of insomnia.
In another study, 25 patients with either Common Toxicity Criteria (CTC) Grade ≥3 neuropathy undergoing treatment with paclitaxel or cisplatin, or with CTC Grade ≥2 persisting at least 3 months after discontinuation of the same chemotherapy were included [21]. Oral ALCAR administration (1 g three times daily for 8 consecutive weeks) resulted in improvement of sensory neuropathy in 60% (15/25) of patients (2 grades in 6 patients, and 1 grade in 9). Motor neuropathy improved in 92% (11/14) of patients. Improvement was measurable at just 4 weeks, but was more common at the end of the 8 weeks study period. Sensory action potential (p=<0.03) and conduction velocity (p=<0.01) were also significantly improved and clinical symptom relief occurred in all but one patient, and only 2 patients reported mild nausea. After 1 year, symptomatic relief was maintained in 12 of the 13 surviving patients.
However, while there is growing evidence that ALCAR is neuroprotective, it is paramount to confirm its lack of tumor potentiation. We have addressed this important issue by conducting exhaustive in vitro analysis. Our data indicates that ALCAR does not enhance the proliferation of the ovarian cancer cell lines OVCAR-3 and SKOV-3. These data are congruent with the results presented in two previous studies [16,17]. The effects of ALCAR on proliferation observed in some instances, although statistically relevant, were very minor. This point is also mirrored by the fact in one instance the addition of ALCAR caused a minor, but statistically significant decrease. Perhaps more importantly, it was shown that ALCAR had no deleterious effect on the cytotoxicity of paclitaxel when given either concomitantly or sequentially. In some instances there was a statistically significant, albeit minor decrease in surviving cancer cells with the combination of ALCAR and paclitaxel.
Overall the results of this study suggest that ALCAR will likely not have any deleterious in the treatment of ovarian cancer. It is therefore our hope that these experiments will support clinical trials to study the use of ALCAR in the prevention of CIPN in ovarian cancer patients.
Acknowledgments
Statistical analysis was done in consultation with Jens Eickhoff, a statistician at the UWCCC.
Footnotes
Conflict of interest statement
DBE was funded in part by NIH grant T32 CA009614 Physician Scientist Training in Cancer Medicine. PRH received direct or indirect consulting fees from Baxter Health Care, Roche Pharmaceuticals, Nektar, and Millenium Pharmaceuticals. All other authors have no conflicts of interest to declare.
References
- 1.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006 Jan 5;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 2.Alberts DS, Green S, Hannigan EV, O’Toole R, Stock-Novack D, Anderson P, et al. Improved therapeutic index of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide: final report by the Southwest Oncology Group of a phase III randomized trial in stages III and IV ovarian cancer. J Clin Oncol. 1992 May;10( 5):706–17. doi: 10.1200/JCO.1992.10.5.706. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003 Sep 1;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003 Jun 21;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 5.du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2005 Oct 1;16(Suppl 8):viii7–12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- 6.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004 Nov 17;96(22):1682–91. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 7.Pignata S, De Placido S, Biamonte R, Scambia G, Di Vagno G, Colucci G, et al. Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study. BMC Cancer. 2006;6:5. doi: 10.1186/1471-2407-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007 Feb 1;25(4):437–43. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 9.De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R&D. 2002;3(4):223–31. doi: 10.2165/00126839-200203040-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sima AA. Acetyl-L-carnitine in diabetic polyneuropathy: experimental and clinical data. CNS Drugs. 2007;21(Suppl 1):13–23. doi: 10.2165/00023210-200721001-00003. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 11.Sima AA, Calvani M, Mehra M, Amato A. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005 Jan;28(1):89–94. doi: 10.2337/diacare.28.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Hart AM, Wilson AD, Montovani C, Smith C, Johnson M, Terenghi G, et al. Acetyl-L-carnitine: a pathogenesis based treatment for HIV-associated anti-retroviral toxic neuropathy. AIDS (London, England) 2004 Jul 23;18(11):1549–60. doi: 10.1097/01.aids.0000131354.14408.fb. [DOI] [PubMed] [Google Scholar]
- 13.Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst. 1997;2(3):250–2. [PubMed] [Google Scholar]
- 14.De Grandis D. Tolerability and efficacy of L-acetylcarnitine in patients with peripheral neuropathies. Clin Drug Investig. 1998;15(2):73–9. doi: 10.2165/00044011-199815020-00001. [DOI] [PubMed] [Google Scholar]
- 15.Flatters SJ, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett. 2006 Apr 24;397(3):219–23. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisano C, Pratesi G, Laccabue D, Zunino F, Lo Giudice P, Bellucci A, et al. Paclitaxel and Cisplatin-induced neurotoxicity: a protective role of acetyl-L-carnitine. Clin Cancer Res. 2003 Nov 15;9(15):5756–67. [PubMed] [Google Scholar]
- 17.Ghirardi O, Lo Giudice P, Pisano C, Vertechy M, Bellucci A, Vesci L, et al. Acetyl-L-carnitine prevents and reverts experimental chronic neurotoxicity induced by oxaliplatin, without altering its antitumor properties. Anticancer Res. 2005 Jul-Aug;25(4):2681–7. [PubMed] [Google Scholar]
- 18.Ghirardi O, Vertechy M, Vesci L, Canta A, Nicolini G, Galbiati S, et al. Chemotherapy-induced allodinia: neuroprotective effect of acetyl-L-carnitine. In vivo (Athens, Greece) 2005 May-Jun;19(3):631–7. [PubMed] [Google Scholar]
- 19.Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crino L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005 Mar-Apr;91(2):135–8. doi: 10.1177/030089160509100206. [DOI] [PubMed] [Google Scholar]
- 20.Mancinelli A, D’Iddio S, Bisonni R, Graziano F, Lippe P, Calvani M. Urinary excretion of L-carnitine and its short-chain acetyl-L-carnitine in patients undergoing carboplatin treatment. Cancer Chemother Pharmacol. 2007 Jun;60(1):19–26. doi: 10.1007/s00280-006-0341-3. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi G, Vitali G, Caraceni A, Ravaglia S, Capri G, Cundari S, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005 Aug;41(12):1746–50. doi: 10.1016/j.ejca.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Chiechio S, Copani A, Gereau RWt, Nicoletti F. Acetyl-L-carnitine in neuropathic pain: experimental data. CNS Drugs. 2007;21(Suppl 1):31–8. doi: 10.2165/00023210-200721001-00005. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 23.Andrieu-Abadie N, Jaffrezou JP, Hatem S, Laurent G, Levade T, Mercadier JJ. L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. FASEB J. 1999 Sep;13(12):1501–10. doi: 10.1096/fasebj.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 24.Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Vivoli E, et al. Protective effect of acetyl-L-carnitine on the apoptotic pathway of peripheral neuropathy. Eur J Neurosci. 2007;26(4):820–7. doi: 10.1111/j.1460-9568.2007.05722.x. [DOI] [PubMed] [Google Scholar]
- 25.Galli G, Fratelli M. Activation of apoptosis by serum deprivation in a teratocarcinoma cell line: inhibition by L-acetylcarnitine. Exp Cell Res. 1993;204(1):54–60. doi: 10.1006/excr.1993.1008. [DOI] [PubMed] [Google Scholar]
- 26.Ishii T, Shimpo Y, Matsuoka Y, Kinoshita K. Anti-apoptotic effect of acetyl-L-carnitine and I-carnitine in primary cultured neurons. Jpn J Pharmacol. 2000 Jun;83(2):119–24. doi: 10.1254/jjp.83.119. [DOI] [PubMed] [Google Scholar]
- 27.De Simone R, Ramacci MT, Aloe L. Effect of acetyl-L-carnitine on forebrain cholinergic neurons of developing rats. Int J Dev Neurosci. 1991;9(1):39–46. doi: 10.1016/0736-5748(91)90071-s. [DOI] [PubMed] [Google Scholar]
- 28.Foreman PJ, Perez-Polo JR, Angelucci L, Ramacci MT, Taglialatela G. Effects of acetyl-L-carnitine treatment and stress exposure on the nerve growth factor receptor (p75NGFR) mRNA level in the central nervous system of aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 1995 Jan;19(1):117–33. doi: 10.1016/0278-5846(94)00109-u. [DOI] [PubMed] [Google Scholar]
- 29.Taglialatela G, Angelucci L, Ramacci MT, Werrbach-Perez K, Jackson GR, Perez-Polo JR. Acetyl-L-carnitine enhances the response of PC12 cells to nerve growth factor. Brain Res Dev Brain Res. 1991 Apr 24;59(2):221–30. doi: 10.1016/0165-3806(91)90102-o. [DOI] [PubMed] [Google Scholar]
- 30.Taglialatela G, Angelucci L, Ramacci MT, Werrbach-Perez K, Jackson GR, Perez-Polo JR. Stimulation of nerve growth factor receptors in PC12 by acetyl-L-carnitine. Biochem Pharmacol. 1992 Aug 4;44(3):577–85. doi: 10.1016/0006-2952(92)90452-o. [DOI] [PubMed] [Google Scholar]
- 31.Davidson B, Lazarovici P, Ezersky A, Nesland JM, Berner A, Risberg B, et al. Expression levels of the nerve growth factor receptors TrkA and p75 in effusions and solid tumors of serous ovarian carcinoma patients. Clin Cancer Res. 2001 Nov;7(11):3457–64. [PubMed] [Google Scholar]
- 32.Odegaard E, Staff AC, Abeler VM, Kopolovic J, Onsrud M, Lazarovici P, et al. The activated nerve growth factor receptor p-TrkA is selectively expressed in advanced-stage ovarian carcinoma. Hum Pathol. 2007 Jan;38(1):140–6. doi: 10.1016/j.humpath.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Campos X, Munoz Y, Selman A, Yazigi R, Moyano L, Weinstein-Oppenheimer C, et al. Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol Oncol. 2007 Jan;104(1):168–75. doi: 10.1016/j.ygyno.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Smith JA, Ngo H, Martin MC, Wolf JK. An evaluation of cytotoxicity of the taxane and platinum agents combination treatment in a panel of human ovarian carcinoma cell lines. Gynecol Oncol. 2005;98(1):141–5. doi: 10.1016/j.ygyno.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Bahl JJ, Bressler R. The pharmacology of carnitine. Annu Rev Pharmacol Toxicol. 1987;27:257–77. doi: 10.1146/annurev.pa.27.040187.001353. [DOI] [PubMed] [Google Scholar]
- 36.Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr. 1998;18:39–61. doi: 10.1146/annurev.nutr.18.1.39. [DOI] [PubMed] [Google Scholar]
- 37.Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003;112(1):113–22. doi: 10.1016/s0092-8674(02)01228-x. [DOI] [PubMed] [Google Scholar]
- 38.De Palo E, Gatti R, Crivellaro C, De Palo C, Scandellari C. Plasma carnitine and acetyl-carnitine levels at different times of the day. Clin Physiol Biochem. 1987;5( 2):95–102. [PubMed] [Google Scholar]
- 39.Deufel T. Determination of L-carnitine in biological fluids and tissues. J Clin Chem Clin Biochem. 1990 May;28(5):307–11. [PubMed] [Google Scholar]
- 40.Dolezal V, Tucek S. Utilization of citrate, acetylcarnitine, acetate, pyruvate and glucose for the synthesis of acetylcholine in rat brain slices. J Neurochem. 1981 Apr;36(4):1323–30. doi: 10.1111/j.1471-4159.1981.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 41.Chiechio S, Caricasole A, Barletta E, Storto M, Catania MV, Copani A, et al. L-acetyl-carnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol Pharmacol. 2002 May;61(5):989–96. doi: 10.1124/mol.61.5.989. [DOI] [PubMed] [Google Scholar]
- 42.Chiechio S, Copani A, De Petris L, Morales ME, Nicoletti F, Gereau RWt. Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-kappaB pathway in primary dorsal root ganglia neurons: a possible mechanism for the analgesic effect of L-acetylcarnitine. Mol Pain. 2006;2:20. doi: 10.1186/1744-8069-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Grandis D. Acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: a short review. CNS Drugs. 2007;21(Suppl 1):39–43. doi: 10.2165/00023210-200721001-00006. discussion 5–6. [DOI] [PubMed] [Google Scholar]