Abstract
BACKGROUND
Neoplasms of the pancreas during pregnancy are rare, with less than 25 cases of benign and malignant tumors reported in the literature.
METHODS
We present three unique cases of pancreatic tumors occurring during pregnancy—one mucinous cystic neoplasm and two adenocarcinomas. We review the literature regarding pancreatic neoplasms during pregnancy and discuss the diagnosis, complications, and management of these tumors.
RESULTS
MRI and ultrasound are the imaging modalities of choice in pregnancy. In patients with benign or premalignant tumors, surgical resection may be postponed until the second trimester. In symptomatic patients, or if there is a concern for intrauterine growth restriction (IUGR), urgent surgical intervention should be performed. With malignant tumors, the benefit of delaying surgery must be balanced with the risk of maternal disease progression. Termination of the pregnancy should be discussed when a malignant tumor is diagnosed during the first trimester. Pancreatic tumors diagnosed during the third trimester may be resected after delivery. If malignant, early delivery of the fetus and subsequent maternal operation can be considered at appropriate fetal maturity.
CONCLUSION
When these tumors occur during pregnancy, they present a diagnostic and treatment dilemma, with variation in treatment based on gestational age and patient preference.
Keywords: Pancreas, neoplasm, pregnancy
INTRODUCTION
Pancreatic neoplasms, both benign and malignant, are uncommon during pregnancy. There have been only eight reported cases of pancreatic adenocarcinoma,[1–8] thirteen cases of cystic pancreatic lesions diagnosed during pregnancy,[9–21] and three reported cases of pancreatic neuroendocrine tumors.[22, 23] Their occurrence during pregnancy leads to dilemmas in diagnosis, management, and timing of surgical treatment. In all cases the goal is to minimize both maternal and fetal risk. The timing of surgical resection for pancreatic neoplasms during pregnancy must take into account the risk of maternal disease progression and safety of the developing fetus. In addition, given the poor overall survival for patients with pancreatic adenocarcinoma, consideration and discussion of termination of the pregnancy are also important depending on the stage at diagnosis, gestational age at diagnosis, and maternal wishes/beliefs.
There have been several individual case reports on patients with pancreatic neoplasms in pregnancy,[1–23] the most common of which are mucinous cystic neoplasms (MCNs) and adenocarcinomas. MCNs of the pancreas have been described almost exclusively in women,[24–27] and their hormone-responsiveness and large size may contribute to their recognition during pregnancy.[26, 27] The natural progression of these tumors from adenoma to invasive carcinoma has led to the current recommendation being complete surgical resection for all MCNs of the pancreas.[28]
Adenocarcinomas of the pancreas during pregnancy present a unique treatment challenge. In the case of obvious malignancy (pancreatic adenocarcinoma, IPMN with invasive cancer, preoperatively diagnosed mucinous cystadenocarcinoma) there can be significant maternal consequences if definitive surgery or other therapy is delayed for fetal maturation. In a situation with inconclusive evidence of invasive malignancy, the surgeon and obstetrician must take into account the probability of malignancy in the pancreatic lesion, the gestational age of the fetus, and the wishes of the mother and family.
With large pancreatic tumors (benign, pre-malignant, or malignant), most common in patients with MCNs, tumor size can cause complications during pregnancy; this includes intrauterine growth restriction (IUGR), compression of surrounding structures, pancreatitis, and tumor rupture.
We report three cases of pancreatic neoplasms in pregnancy. The first is a case of a simultaneous mucinous cystic neoplasm of the pancreas and a mature cystic ovarian teratoma diagnosed during the second trimester of pregnancy. The second involves metastatic adenocarcinoma of the pancreas developing during pregnancy. The final case describes a resectable adenocarcinoma diagnosed in the second trimester of pregnancy. We describe the clinical findings, decision-making, and outcomes of all three cases. In addition, we review the literature and discuss the complex decision-making process and management for patients diagnosed with pancreatic cancer during pregnancy.
CASE 1
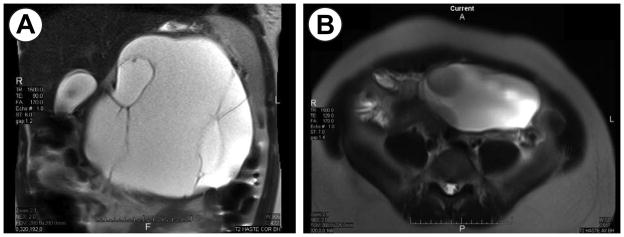
A 21-year-old gravida 2, para 1 woman presented at 10 weeks 6 days gestation with abdominal distention and fullness. Signs and symptoms of an upper abdominal mass including abdominal fullness and a palpable mass were present for nine months without any associated nausea, vomiting, or weight loss. A CT scan predating her pregnancy demonstrated a large upper abdominal mass and a separate pelvic mass. The patient refused treatment at that time. An initial pelvic and abdominal ultrasound demonstrated a normally-developing fetus, a left ovarian 13 cm mass, and a 19 cm mixed echogenicity mass in the upper abdomen. The left ovarian mass was consistent with a teratoma. An MRI revealed a 16.9 × 17.2 × 13.2 cm multiloculated cystic mass arising from the body/tail of the pancreas (Figure 1A), and the pelvic mass (Figure 1B). All laboratory results were within normal limits.
Figure 1.
Figure 1a. T2-weighted coronal MRI of a large pancreatic mucinous cystic neoplasm with adjacent liver, gallbladder, and compressed spleen.
Figure 1b. T2-weighted axial MRI of a mature ovarian teratoma.
Surgical excision of persistent adnexal masses in the second trimester is a common practice, with the intent to prevent subsequent emergent intervention for torsion or rupture. A multidisciplinary conference concluded that surgical excision of the pancreatic mass would be warranted as well, given the high risk of IUGR due to its size. Laparotomy was performed at 20 weeks gestation (Figure 2A). No tocolytics were used intraoperatively. A large pancreatic body cystic mass was resected with a spleen preserving distal pancreatectomy (Figure 2B) with a simultaneous left oophorectomy (Figure 2C). Her postoperative course was uneventful. Labor was induced at 39 weeks gestation with normal spontaneous vaginal delivery. She has no evidence of recurrent disease one year after resection.
Figure 2.
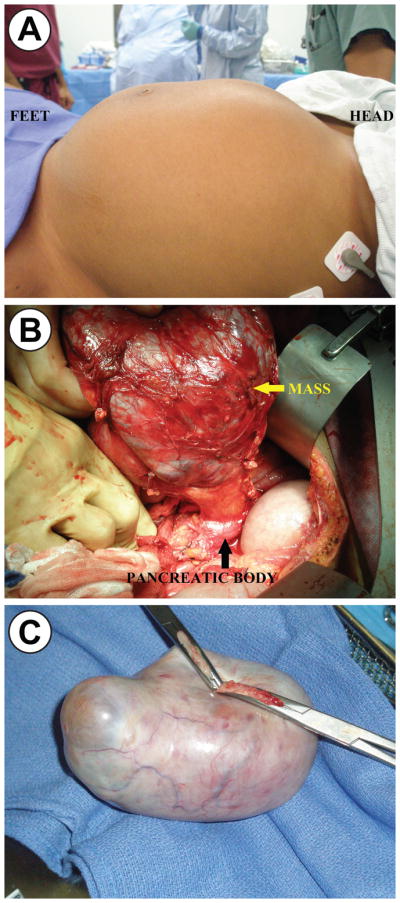
Figure 2a. Preoperative photograph of a 21-year-old patient at 20 weeks gestation with a large pancreatic mucinous cystic neoplasm.
Figure 2b. A large mucinous cystic neoplasm was noted to originate from the body of the pancreas.
Figure 2c. A mature ovarian cystic teratoma was simultaneously resected.
Final pathological examination demonstrated a mucinous cystic neoplasm of the pancreas with moderate dysplasia, measuring 17 cm in greatest dimension. Surgical margins were negative and 2 lymph nodes were negative for malignancy. The left ovarian mass was consistent with a mature cystic teratoma, measuring 14 cm in greatest dimension.
CASE 2
A 29-year-old gravida 1, para 0 Hispanic female presented at 37 weeks gestation with a one week history of emesis and several months of moderately severe epigastric pain that was progressing in severity. She had failed to continue to gain weight as projected. She had no significant past medical or family history, and denied any drug, alcohol, or tobacco use. Radiologic interrogation was deferred by her primary provider due to perceived risks during pregnancy. The patient subsequently was induced at 37 weeks gestation with an uneventful delivery due to preeclampsia.
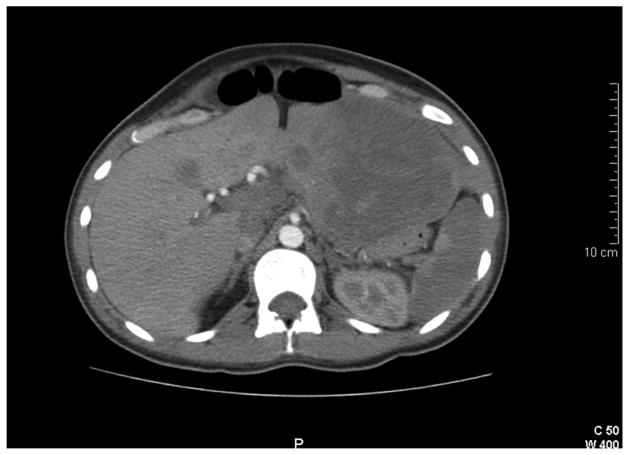
Soon after delivery she underwent imaging with ultrasound and CT scan for continued abdominal pain. The scan demonstrated multiple masses in the liver with compression of the stomach (Figure 3). The masses were primarily located in the left lobe of the liver and were thought to represent symptomatic hepatic adenomas. Initial laboratory exams were normal. Alpha-fetoprotein was 74 ng/ml. A multidisciplinary conference recommended a left hepatic lobectomy due to symptomatology. An exploratory laparotomy on postpartum day five discovered a primary pancreatic mass arising from the tail of the gland. The mass had solid and cystic components with multiple other liver masses consistent with metastatic disease. Intraoperative frozen sections and final pathology were consistent with pancreatic adenocarcinoma with mucinous features. Vimentin and progesterone stains were negative. Subsequently, the CA 19-9 was 1,666,959 U/ml. The patient was discharged home on postoperative day 5 with plans to start chemotherapy as an outpatient. The patient was readmitted 2 weeks later due to uncontrolled abdominal pain, then developed hypoxia and syncope that evening. A CT angiogram demonstrated multiple pulmonary emboli and extensive thrombosis of the iliac and femoral venous systems. An IVC filter was placed. The patient subsequently developed disseminated intravascular coagulation, multiple organ system failure and expired shortly thereafter.
Figure 3.
Postpartum CT scan showing several large liver masses, later found to be metastatic adenocarcinoma of the pancreas.
CASE 3
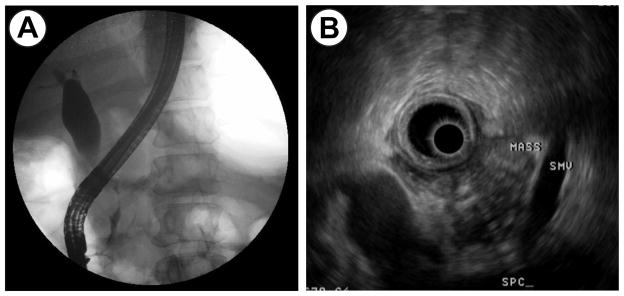
A 37-year old gravida 3, para 1 woman at 17 weeks gestation presented with a two week history of right upper back pain, nausea and vomiting, acholic stools, and dark urine. She had no significant past medical history. The patient’s total bilirubin was 2.9 mg/dl, alkaline phosphatase was 245 IU/L, and lipase was 504 U/L. An abdominal ultrasound demonstrated multiple gallstones and a common bile duct diameter of 16 mm. Endoscopic retrograde cholangiopancreatography (ERCP) was performed for suspected choledocholithiasis, which revealed a common bile duct stricture with marked proximal ductal dilatation (Figure 4A). A stent was placed. An endoscopic ultrasound (EUS) demonstrated a 3.5 × 4.2 cm heterogeneous, hypoechoic mass without vascular or lymphatic involvement (Figure 4B). Fine needle aspiration (FNA) showed poorly differentiated adenocarcinoma. An MRI showed no evidence of metastatic disease.
Figure 4.
Figure 4a. ERCP demonstrating a common bile duct stricture with proximal ductal dilatation.
Figure 4b. Endoscopic ultrasound revealing a 3.5 × 4.2 cm heterogeneous, hypoechoic mass.
After multidisciplinary consultation, the patient was offered the options of resection or termination of the pregnancy with subsequent resection, with the patient consenting to resection alone. A pancreaticoduodenectomy was performed at 19 weeks gestation. Final pathology revealed poorly differentiated ductal adenocarcinoma of the pancreas with negative margins. Eighteen of 33 lymph nodes were positive for carcinoma.
At 24 weeks gestation, the patient was given two 4-week cycles of gemcitabine, which has a low risk profile during pregnancy. At 34 weeks, labor was induced and the patient delivered a 4 lb 9 oz healthy male. Two weeks postpartum, 4 months postoperatively, her first CT scan demonstrated multiple liver lesions consistent with metastases. Palliative chemotherapy was pursued with the patient expiring one year after surgical resection.
DISCUSSION
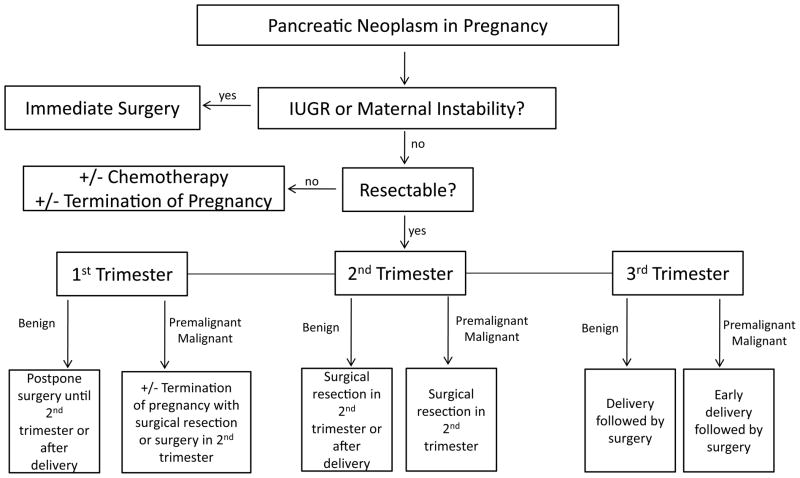
Pancreatic neoplasms during pregnancy are rare, with only 24 cases of all histologic types reported in the literature.[1–23] Table 1 summarizes the existing case reports of pancreatic neoplasms during pregnancy. These tumors present a diagnostic and therapeutic challenge when diagnosed antepartum. For both the mother and the fetus, the benefits and risks of aggressive treatment versus observation must be addressed. In addition, given the poor prognosis of most malignant pancreatic neoplasms, long-term prognosis must be considered in all decision-making and decisions in this regard will be strongly influenced by maternal/family preference. Figure 5 represents a proposed treatment algorithm for patients diagnosed with a pancreatic mass during pregnancy. Knowledge of pancreatic tumors during pregnancy is limited to small studies and case reports; therefore, this figure represents guidelines regarding issues to consider in the treatment of these patients rather than evidence-based recommendations.
Table 1.
Existing case reports of pancreatic neoplasms during pregnancy
| Author | Pathology | Gestational Age at Presentation | Gestational Age at Surgery | Complications |
|---|---|---|---|---|
| Al-Adnani et al.[1] | Adenocarcinoma | 30 weeks | N/A | IUGR, placental metastases |
| Blackbourne et al.[2] | Adenocarcinoma | 14 weeks | 17 weeks | Jaundice |
| Kakoza et al.[4] | Adenocarcinoma | 24 weeks | Postpartum | Pancreatitis, outlet obstruction |
| Marinoni et al.[5] | Adenocarcinoma | 27 weeks | Postpartum | Pancreatitis, jaundice, liver/lung metastases |
| Onuma et al.[6] | Adenocarcinoma | 30 weeks | 34 weeks | Gastric perforation |
| Porcel et al.[7] | Adenocarcinoma | 28 weeks | 30 weeks | Hypercoagulability |
| Simchuk et al.[8] | Adenocarcinoma | 16 weeks | 16 weeks | Biliary obstruction, metastases |
| Hakamada et al.[3] | Anaplastic | 1st trimester, 1st pregnancy | 2nd trimester, 2nd pregnancy | Intractable nausea, hematemesis |
| Asciutti et al.[9] | MCN | 23 weeks | Postpartum | Pancreatitis |
| Baiocchi et al.[10] | MCN | 3rd trimester | Postpartum | None reported |
| Ganepola et al.[11] | MCN | 4 weeks | 23 weeks | None reported |
| Herring et al.[12] | MCN | 3 weeks | 17 weeks | None reported |
| Ikuta et al.[13] | MCN | 10 weeks | After D&C | Missed abortion |
| Ishikawa et al.[14] | MCN | 17 weeks | Postpartum | None reported |
| Kato et al.[15] | MCN | 15 weeks | 23 weeks | IUGR |
| Lopez-Tomassetti et al.[16] | MCN | 20 weeks | 20 weeks | None reported |
| Naganuma et al.[17] | MCN | 33 weeks | 34 weeks | Tumor rupture |
| Ozden et al.[19] | MCN | 36 weeks | 36 weeks | Tumor rupture |
| Wiseman et al.[21] | MCN | 15 weeks | 16 weeks | Intractable nausea |
| Smithers et al.[20] | MCN | 7 weeks | 8 weeks | Tumor leak, necrosis |
| Olsen et al.[18] | MCN | 5.5 weeks | 18 weeks | None reported |
| Kamphues et al.[22] | Neuroendocrine | 19 weeks | 19 weeks | Splenic vein thrombosis, renal artery compression |
| Kamphues et al.[22] | Neuroendocrine | 16 weeks | 18 weeks | Intractable nausea |
| Sciscione et al.[23] | Neuroendocrine | 19 weeks | 20 weeks | Fetal death |
Figure 5.
Decision-making flowchart for a pancreatic mass diagnosed in pregnancy. Ultimate choices will be based on maternal and family preferences and should be discussed extensively with the patient.
Diagnosis
Endoscopic and transabdominal ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are the primary imaging modalities used in diagnosing tumors of the pancreas. The risk to the fetus must be taken into account when imaging these tumors during pregnancy. Since its introduction into the field of medicine, diagnostic ultrasound has not been shown to be a health risk to either the fetus or the mother.[29, 30] Specifically, endoscopic ultrasound (EUS) may be used to gain information about tumor resectability and obtain a tissue diagnosis without an associated increase in radiation exposure.[30] A single diagnostic CT scan is not associated with an increased risk of fetal malformations, although multiphase CT scans or repeat CT scans do increase the radiation exposure to the fetus, and should therefore be used sparingly.[29, 30] In addition, there may be an increased risk of spontaneous abortion associated with CT scanning within the first two weeks after conception (greater than the normal risk of spontaneous abortion during pregnancy of 15%), and also a slightly increased risk of childhood cancers in an exposed fetus (one cancer per 500–1000 fetuses exposed to 0.03 Gy in utero).[29, 30] Several studies have failed to demonstrate an increase in fetal teratogenicity or acoustic nerve damage with the use of MRI or MRCP (magnetic resonance cholangiopancreatography).[29] The lower fetal risk compared to CT and improved quality of imaging (when compared to transabdominal ultrasound) for making decisions regarding resectability make MRI the preferred imaging modality in pregnant patients. However, the use of gadolinium should be avoided in pregnancy, as the contrast is excreted by the fetus and subsequently ingested into the gastrointestinal tract for an unknown period of time.[29, 30]
Endoscopic retrograde cholangiopancreatography (ERCP) is also frequently used for diagnosis and biliary drainage for pancreatic masses causing obstructive jaundice. ERCP has been used during pregnancy. The estimated fetal radiation dose received during ERCP with fluoroscopy has been reported to be 0.0031 Gy, while the fetal radiation dose of a CT scan has been reported to be 0.024-0.03 Gy. Currently, the accepted teratogenic dose is 0.05-0.1 Gy.[29–31] Tham et al. reported the outcomes of 15 patients who underwent ERCP during pregnancy, with no fetal adverse effects in any of the cases.[31] In all cases, patients who undergo ERCP during pregnancy should have a lead shield in place to minimize radiation exposure to the fetus. ERCP should be considered for biliary drainage if surgical intervention is not indicated or needs to be delayed in a jaundiced pregnant patient. ERCP is also indicated for acute cholangitis.
Resectable Tumors During Pregnancy
Pancreatic tumors often appear early in pregnancy, with a reported mean gestational age at diagnosis of 15 weeks.[5] This timing allows the patient and treatment team to decide on the optimum time for surgery. For tumors with a large mass effect potentially leading to intrauterine growth restriction (IUGR), resection should be strongly considered, regardless of gestational age.[11, 15]
First Trimester
Surgical intervention during the first trimester may be associated with spontaneous abortion or poor fetal outcome, including congenital anomalies.[4, 12] However, MCNs or IPMNs with obvious malignancy or high malignant potential (main duct variant IPMNs, larger tumors, the presence of mural nodules, multilocularity on imaging, and symptoms/signs such as jaundice, pain, and weight loss[26, 27]) diagnosed during the first trimester should be treated in a similar manner to malignant adenocarcinomas of the pancreas, with resection at the earliest safe opportunity. The benefit of delaying surgery for fetal maturity must be balanced with the risk of maternal disease progression. This decision must be carefully discussed with the patient.
In our literature review, three cases of benign MCNs, or cystadenomas, were diagnosed in the first trimester,[11, 13, 18] with two of these patients undergoing successful surgical resection in the second trimester.[11, 18] Smithers et al. reported a case of a cystadenocarcinoma diagnosed at 7 weeks gestation with successful immediate surgical resection due to maternal instability.[20] Herring et al. were able to successfully delay surgical resection of a cystadenocarcinoma to 17 weeks gestation after diagnosis in the first trimester.[12] In an interesting case of an MCN diagnosed during the first trimester, the patient refused surgical resection until the second trimester of her next pregnancy; she then developed a recurrence, which the pathology demonstrated to be anaplastic carcinoma.[3]
In the case of a malignant tumor of the pancreas diagnosed during the first trimester, the possibility of termination of the pregnancy in order to pursue further treatment should be discussed with the patient. Even in the setting of resectable disease, the 5-year survival rate of patients with pancreatic cancer is, at best, 15–20%,[32] and pregnant patients need to have a realistic expectation of their prognosis.
Second Trimester
The second trimester is the preferred time for surgical intervention for resectable pancreatic tumors.[12–14, 16] The American College of Obstetricians and Gynecologists’ current recommendations are for operation during the second trimester for any nonurgent abdominal surgical procedures during pregnancy.[33] Many authors of existing case reports agree that the second trimester is favorable for surgical resection, as fetal organogenesis is complete and the size of the fetus may allow for an easier surgical procedure in comparison to third trimester operations. In addition, the risk of spontaneous abortion is lowest during the second trimester.[12–14, 16]
Three cases have been reported regarding adenocarcinoma of the pancreas diagnosed during the second trimester. One reported patient underwent successful surgical resection during the second trimester, while another underwent biliary diversion at 16 weeks.[2, 8] Our third patient is the second reported case of a patient undergoing successful surgical resection of pancreatic adenocarcinoma during the second trimester.
Pancreatic neuroendocrine tumors and cystadenomas have also been diagnosed during the second trimester of pregnancy.[9, 15, 16, 21–23, 34] Typically, pancreatic neuroendocrine tumors behave more indolently and may be able to wait until after delivery in the asymptomatic patient.[23] These should be resected during pregnancy if they are causing IUGR or other problems due to size.[22]
Third Trimester
Surgical resection of a pancreatic mass during the third trimester may be associated with an increased risk of premature induction of labor.[12, 23] However, since fetal maturity is typically achieved by this time,[4, 5] delivery may be performed early with postpartum surgical resection. Early delivery during the third trimester balances the benefit of survival of the fetus with the risk of maternal disease progression. Masses that are not clinically suspicious for malignancy may be expectantly managed until after full-term delivery.[9, 10, 14]
Immediate threat to the fetus or mother is an indication for early delivery and resection. Several cases have been reported of rupture of pancreatic tumors causing maternal instability during pregnancy.[17, 19] In this situation, emergent surgical intervention should take place, regardless of gestational age.
Unresectable Tumors During Pregnancy
Pancreatic tumors are frequently diagnosed at an advanced stage, and those occurring during pregnancy are no exception. Symptoms of a pancreatic mass, including abdominal discomfort and nausea, may be interpreted as normal symptoms of pregnancy, thus leading to a delayed diagnosis of adenocarcinoma and disease progression.[8] Metastatic adenocarcinoma of the pancreas occurring during pregnancy has been reported five times in the literature. In these cases, the tumor was associated with complications such as pancreatitis, biliary obstruction, lung and liver metastases, hypercoagulability, and gastric outlet obstruction. Four of these patients died shortly after delivery.[4–8] In our second case, the patient was delivered due to suspected preeclampsia. Her postpartum CT scan findings were thought to be consistent with hepatic adenomas, and only upon laparotomy was the patient determined to have unresectable pancreatic cancer.
Metastatic adenocarcinoma of the pancreas has a poor prognosis.[32] When faced with an unresectable tumor, the patient should discuss her treatment options with a multidisciplinary team; termination of the pregnancy may be chosen in order to allow for maternal chemotherapy treatment or due to concern about long-term prognosis. Chemotherapy is not currently recommended during the first trimester of pregnancy, as its use has been shown to increase the risk of spontaneous abortion, fetal death, and major malformations.[35] In a review of 321 published cases of chemotherapy use for cancer during pregnancy, 9 of 11 malformations occurred after chemotherapy had been given during the first trimester.[35] In a second study of 210 pregnant women diagnosed with cancer during pregnancy, the rate of fetal malformations among patients who had received chemotherapy during any trimester of pregnancy was 3.8%, similar to that of the general population. In addition, the rates of IUGR with and without chemotherapy were similar (7.6% vs 7.1%, respectively).[36] One patient in this study received gemcitabine for pancreatic adjuvant gemcitabine during her second trimester, as recommended by her medical oncologist due to the good risk profile of gemcitabine during pregnancy. Currently, chemotherapy is not advised during the first trimester of pregnancy or after 34 weeks gestation, in order to avoid spontaneous delivery during that time.[36]
CONCLUSION
We report three unique cases of pancreatic tumors occurring during pregnancy. The first is a case of a large MCN occurring simultaneously with a large ovarian cystic tumor. The second case involves delayed diagnosis of metastatic carcinoma presenting during the third trimester, while the third case entails a patient with resectable adenocarcinoma diagnosed during the second trimester.
Pancreatic tumors occurring during pregnancy present a unique diagnostic and treatment dilemma. Mucinous cystic neoplasms (MCNs) and adenocarcinomas represent the most commonly reported tumors of the pancreas during pregnancy. In the case of intrauterine growth restriction (IUGR) or maternal instability, urgent surgical intervention should be undertaken regardless of gestational age. Benign tumors diagnosed during the first trimester and without concern for IUGR may be managed expectantly, while the diagnosis of a malignant tumor during the first trimester should prompt a discussion about termination of the pregnancy in order to pursue optimal therapy.
The second trimester of pregnancy remains the most favorable time for surgical intervention for tumors of the pancreas, and resectable tumors diagnosed during this trimester should undergo surgical resection. Tumors of the pancreas diagnosed during the third trimester can be resected after an early delivery. Unresectable tumors have a poor prognosis, and a multidisciplinary approach to these patients can allow for the best outcome for both the mother and the fetus.
Acknowledgments
Support: NIH K07 Cancer Prevention, Control, and Population Sciences Career Development Award (Grant Number 1K07CA130983-01A1)
Footnotes
Presented at: American Hepato-Pancreato-Biliary Association Annual Meeting, March 2011
References
- 1.Al-Adnani M, Kiho L, Scheimberg I. Maternal pancreatic carcinoma metastatic to the placenta: a case report and literature review. Pediatr Dev Pathol. 2007;10:61–5. doi: 10.2350/06-06-0119.1. [DOI] [PubMed] [Google Scholar]
- 2.Blackbourne LH, Jones RS, Catalano CJ, Iezzoni JC, Bourgeois FJ. Pancreatic adenocarcinoma in the pregnant patient: case report and review of the literature. Cancer. 1997;79:1776–9. doi: 10.1002/(sici)1097-0142(19970501)79:9<1776::aid-cncr20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Hakamada K, Miura T, Kimura A, Nara M, Toyoki Y, Narumi S, Sasak M. Anaplastic carcinoma associated with a mucinous cystic neoplasm of the pancreas during pregnancy: report of a case and a review of the literature. World J Gastroenterol. 2008;14:132–5. doi: 10.3748/wjg.14.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakoza RM, Vollmer CM, Jr, Stuart KE, Takoudes T, Hanto DW. Pancreatic adenocarcinoma in the pregnant patient: a case report and literature review. J Gastrointest Surg. 2009;13:535–41. doi: 10.1007/s11605-008-0697-2. [DOI] [PubMed] [Google Scholar]
- 5.Marinoni E, Di Netta T, Caramanico L, Tomei B, Moscarini M, Di Iorio R. Metastatic pancreatic cancer in late pregnancy: a case report and review of the literature. J Matern Fetal Neonatal Med. 2006;19:247–9. doi: 10.1080/14767050600591407. [DOI] [PubMed] [Google Scholar]
- 6.Onuma T, Yoshida Y, Yamamoto T, Kotsuji F. Diagnosis and management of pancreatic carcinoma during pregnancy. Obstet Gynecol. 2010;116 (Suppl 2):518–20. doi: 10.1097/AOG.0b013e3181de8995. [DOI] [PubMed] [Google Scholar]
- 7.Porcel JM, Ordi J, Castells L, Farran I. Probable pancreatic cancer in a pre-eclamptic patient. Eur J Obstet Gynecol Reprod Biol. 1992;44:80–2. doi: 10.1016/0028-2243(92)90318-s. [DOI] [PubMed] [Google Scholar]
- 8.Simchuk EJ, 3rd, Welch JP, Orlando R., 3rd Antepartum diagnosis of pancreatic carcinoma: a case report. Conn Med. 1995;59:259–62. [PubMed] [Google Scholar]
- 9.Asciutti S, Kanninen TT, Clerici G, Nardi E, Castellani D, GCDIR, Clerici C. Acute pancreatitis with a mucinous cystoadenoma of the pancreas in pregnancy. Anticancer Res. 2010;30:1025–8. [PubMed] [Google Scholar]
- 10.Baiocchi C, Landonio G, Majno M, Minola E, Scanzi F, Ghislandi E. Pancreatic cystadenocarcinoma and pregnancy: a case report. Tumori. 1990;76:294–5. doi: 10.1177/030089169007600319. [DOI] [PubMed] [Google Scholar]
- 11.Ganepola GA, Gritsman AY, Asimakopulos N, Yiengpruksawan A. Are pancreatic tumors hormone dependent?: A case report of unusual, rapidly growing pancreatic tumor during pregnancy, its possible relationship to female sex hormones, and review of the literature. Am Surg. 1999;65:105–11. [PubMed] [Google Scholar]
- 12.Herring AA, Graubard MB, Gan SI, Schwaitzberg SD. Mucinous cystadenocarcinoma of the pancreas during pregnancy. Pancreas. 2007;34:470–3. doi: 10.1097/mpa.0b013e31803799d8. [DOI] [PubMed] [Google Scholar]
- 13.Ikuta S, Aihara T, Yasui C, et al. Large mucinous cystic neoplasm of the pancreas associated with pregnancy. World J Gastroenterol. 2008;14:7252–5. doi: 10.3748/wjg.14.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa K, Hirashita T, Kinoshita H, Kitano M, Matsuo S, Matsumata T, Kitano S. Large mucinous cystadenoma of the pancreas during pregnancy: report of a case. Surg Today. 2007;37:1013–7. doi: 10.1007/s00595-007-3500-1. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Kubota K, Kita J, et al. Huge mucinous cystadenoma of the pancreas developing during pregnancy: a case report. Pancreas. 2005;30:186–8. doi: 10.1097/01.mpa.0000148512.92744.dc. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Tomassetti Fernandez EM, Martin Malagon A, Arteaga Gonzalez I, Muniz Montes JR, Diaz Luis H, Gonzalez Hermoso F, Carrillo Pallares A. Mucinous cystic neoplasm of the pancreas during pregnancy: the importance of proper management. J Hepatobiliary Pancreat Surg. 2005;12:494–7. doi: 10.1007/s00534-005-1005-0. [DOI] [PubMed] [Google Scholar]
- 17.Naganuma S, Honda K, Noriki S, et al. Ruptured mucinous cystic neoplasm with an associated invasive carcinoma of pancreatic head in a pregnant woman: report of a case and review of literature. Pathol Int. 2011;61:28–33. doi: 10.1111/j.1440-1827.2010.02609.x. [DOI] [PubMed] [Google Scholar]
- 18.Olsen ME, Greer MS, Feintuch TA. Pancreatic mucinous cystadenoma during pregnancy. The American Journal of Gynecologic Health. 1993;7:27–30. [Google Scholar]
- 19.Ozden S, Haliloglu B, Ilter E, Akin FT, Kebudi A, Peker O. An extremely rare cause of acute abdomen in pregnancy: ruptured pancreatic mucinous cystadenocarcinoma. Pancreas. 2007;34:474–6. doi: 10.1097/mpa.0b013e31803799ee. [DOI] [PubMed] [Google Scholar]
- 20.Smithers BM, Welch C, Goodall P. Cystadenocarcinoma of the pancreas presenting in pregnancy. Br J Surg. 1986;73:591. doi: 10.1002/bjs.1800730727. [DOI] [PubMed] [Google Scholar]
- 21.Wiseman JE, Yamamoto M, Nguyen TD, Bonadio J, Imagawa DK. Cystic pancreatic neoplasm in pregnancy: a case report and review of the literature. Arch Surg. 2008;143:84–6. doi: 10.1001/archsurg.2007.4. [DOI] [PubMed] [Google Scholar]
- 22.Kamphues CH, Rocken C, Neuhaus P, Neumann UP. Non-functioning, malignant pancreatic neuroendocrine tumour (PNET): a rare entity during pregnancy. Langenbecks Arch Surg. 2009;394:387–91. doi: 10.1007/s00423-008-0346-y. [DOI] [PubMed] [Google Scholar]
- 23.Sciscione AC, Villeneuve JB, Pitt HA, Johnson TR. Surgery for pancreatic tumors during pregnancy: a case report and review of the literature. Am J Perinatol. 1996;13:21–5. doi: 10.1055/s-2007-994197. [DOI] [PubMed] [Google Scholar]
- 24.Goh BK, Tan YM, Chung YF, Chow PK, Cheow PC, Wong WK, Ooi LL. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30:2236–45. doi: 10.1007/s00268-006-0126-1. [DOI] [PubMed] [Google Scholar]
- 25.Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152–61. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson LD, Becker RC, Przygodzki RM, Adair CF, Heffess CS. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1–16. doi: 10.1097/00000478-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–22. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 29.Baysinger CL. Imaging during pregnancy. Anesth Analg. 2010;110:863–7. doi: 10.1213/ANE.0b013e3181ca767e. [DOI] [PubMed] [Google Scholar]
- 30.Patel SJ, Reede DL, Katz DS, Subramaniam R, Amorosa JK. Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. Radiographics. 2007;27:1705–22. doi: 10.1148/rg.276075002. [DOI] [PubMed] [Google Scholar]
- 31.Tham TC, Vandervoort J, Wong RC, et al. Safety of ERCP during pregnancy. Am J Gastroenterol. 2003;98:308–11. doi: 10.1111/j.1572-0241.2003.07261.x. [DOI] [PubMed] [Google Scholar]
- 32.Riall TS, Nealon WH, Goodwin JS, Zhang D, Kuo YF, Townsend CM, Jr, Freeman JL. Pancreatic cancer in the general population: Improvements in survival over the last decade. J Gastrointest Surg. 2006;10:1212–23. doi: 10.1016/j.gassur.2006.08.010. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 33.ACOG Committee Opinion No. 474: Nonobstetric surgery during pregnancy. Obstet Gynecol. 117:420–1. doi: 10.1097/AOG.0b013e31820eede9. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa O. Adenosquamous Carcinoma of the Pancreas: A Clinicopathologic Study and Report of Three Cases. Cancer. 1980;46:1192–6. doi: 10.1002/1097-0142(19800901)46:5<1192::aid-cncr2820460519>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 35.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–91. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- 36.Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: results of an international registry. Am J Clin Oncol. 33:221–8. doi: 10.1097/COC.0b013e3181a44ca9. [DOI] [PubMed] [Google Scholar]