Abstract
The national investment that was made in oncology research with the passage of the National Cancer Act in 1971 is coming to fruition now. Nowhere is this more apparent than with the exciting prospects for genetically informed precision medicine as applied to the treatment of children with cancer. The wealth of information gleaned from intensive genetic analyses and NexGen sequencing studies has identified a number of viable targets in leukemias and solid tumors. The rapid and evolving understanding of the enzymatic controls which regulate chromatin dynamics during normal differentiation of stem cells and their mutation or dysregulation in tumor cells has led to a new library of therapeutically tractable tumor targets. Recent identification of germline variants associated with toxicity and/or response to therapy has further enhanced our ability to deliver individualized treatments for pediatric cancer patients. How best to utilize genomic data and integrate it into evolving clinical protocols to provide more efficacious therapies and a better quality of life for children with cancer is our challenge today.
Introduction
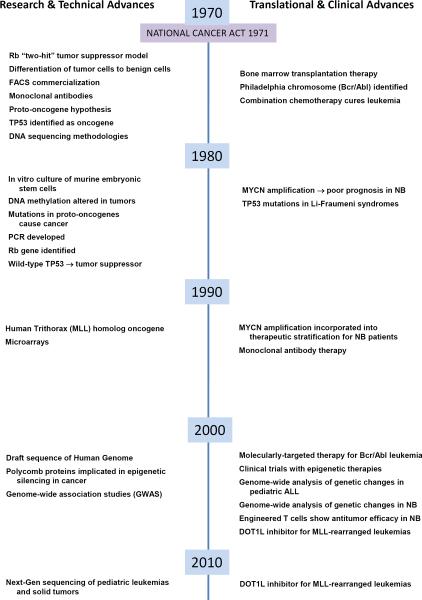
Significant advances in our ability to successfully prevent, detect, and treat malignant tumors have been made since President Nixon signed that National Cancer Act into law on December 23, 1971. As we are now some 40 years out from the historic challenge, a true appreciation of our progress and the challenges that await us necessitates a reflection on our treatments for children and the state of our knowledge about “the cancer cell”, the immune system and cancer therapy at the time the Cancer Act was initiated (Fig. 1).
Figure. 1.
1971–2011 Timeline Marking Research and Clinical Advances since the National Cancer Act of 1971. This timeline represents selected research and technical advances as well as translational and clinical advances that have occurred in the last 40 years that have had a significant impact on our view of cancer biology and clinical practice with specific emphasis on pediatric oncology. Many of these findings relate to topics discussed in the articles that accompany this CCR Focus on pediatric oncology.
In 1971, scientists could not readily clone or sequence genes and a prevailing view was that viruses caused cancers. While today it is known that only 15% of cancers have a viral etiology (EBV, HPV), the role of the host immune or inflammatory responses to viral infection as tumor promoting causations remains to be resolved. From the study of viruses in cancer in 1969 came the hypothesis that retroviruses isolated from animal tumors contained “oncogenes” which encoded proteins capable of transforming cells (1) Their subsequent identification, cellular localization and function would lead to the realization in 1978 that there were normal cellular homologues of viral oncogenes, called “proto-oncogenes” (2) and mutations in these genes caused tumors (3). Based on an epidemiological analysis of the inheritance of retinoblastoma tumors in children, Knudson's landmark study in 1971 postulated the existence of a “tumor suppressor gene” and led to the “2-hit model” of tumorigenesis.(4) Fifteen years later the Rb tumor suppressor gene was isolated and cloned. (5)
In 1971 the plasticity of a cancer cell was noted in studies showing that tumor cells could be differentiated into benign cells (6). With hindsight, this was indicative of a cancer stem cell but it wasn't until 1997 that they would be described in leukemia (7) and much later in 2003 in solid tumors.(8) In 1971 the field of epigenetics was still restricted to model organisms although modifications to histones and their effects on gene expression were known. Cancer epigenetics didn't being in earnest until a decade later with the identification of altered gene methylation in cancer (9). It has only been in the last decade that key enzymes regulating histone modifications that control stem cell lineage specification have been identified. How mutations in theses enzymes contribute to tumorigenesis is an area of intense investigation.
Harnessing the immune system to fight cancer was also in its infancy in 1971. Lymphoid cells were categorized as B or T cells and natural killer and dendritic cells had not been described. Commercialization of the fluorescence activated cell scanner in the early 1970s (10) coupled with the development of monoclonal antibodies a few years later (11) facilitated the ever-expanding delineation of subsets of functionally important lymphoid cells.
These new discoveries in genetics and cancer biology combined with advances in medical technology and computational expertise not even imagined in 1971, have led to truly remarkable progress in understanding the pathogenesis of childhood cancer and the development of more effective therapy. Initial improvements in survival were observed with treatment regimens stratified by pathologic classification and tumor stage. However, the inadequacy of this approach has become clear with the recognition of the biologic heterogeneity that exists within pathologically defined tumor types. The clinical significance of genetic tumor markers in pediatric cancers was first established in the 1980's, (12, 13) and it soon became clear that more refined prognostication of risk-of-relapse could be established by combining tumor histology and stage with other clinical features, tumor genetics, and assessment of the response to therapy(14–16). Pediatricians were early adopters of risk-group based treatment strategies in cooperative group acute lymphoblastic leukemia and solid tumors clinical trials. This approach led to further improvements in outcome. In patients with “lower-risk” disease treated with reduced therapy, high survival rates were maintained and less toxicity was observed, while increased survival rates were achieved in “higher-risk” patients following with more intensive multi-modality treatment regimens. However, despite this progress, cancer still remains a leading cause of death in the pediatric population and long-term complications of therapy remains a pressing problem.
This issue of Clinical Cancer Research Focus highlights many of the more recent translational and clinical studies in pediatric leukemia and solid tumors that have led to a better understanding of the key molecular events that drive tumorigenesis; the identification of “druggable” targets; and the development of biologically rationale therapies. Interestingly, despite distinct pathologic origins of disease, in some cases the same molecular target identified in adult cancers is also detected in pediatric tumors, as exemplified by the anaplastic lymphoma kinase (ALK) aberrations that are seen in neuroblastoma, lymphoma, non-small-cell lung cancer, as well as other cancer types.(17) Significant progress in our understanding of the fundamental mechanisms that contribute to disruption of the epigenome in both pediatric and adult cancers is providing additional novel avenues for targeted cancer.(18) Targeted immuotherapies have led to dramatic improvements in the outcome of children with neuroblastoma,(19) and in more recent studies, response to treatment with chimeric antigen receptors has been observed in patients with leukemia and solid tumors.(20–22) It is also recognized that germline genetic variants influence response to therapy and outcome.(23, 24) Thus, to optimally link pediatric oncology patients with effective, genetically informed therapies, a deeper understanding of the host genetic variants that influence response and toxicity to chemotherapy will also be needed.
Advances in Targeting Neuroblastoma
Matthay and colleagues review the biologic rationale and clinical efficacy of three promising targeted therapies for neuroblastoma including: 1) radiotherapy with 131Imetaiodobenzylguanidine (MIBG), 2) immunotherapy with monoclonal antibodies directed against the GD2 ganglioside, and 3) biologic therapy with inhibitors of the ALK tyrosine kinase. (25) MIBG targets the norepinephrine transporter which is expressed in 90% of neuroblastoma tumors, resulting in cell-specific uptake and radiation-induced destruction of the cell.(26) A number of early phase studies and more recent trials in newly diagnosed patients have demonstrated the activity of this agent in neuroblastoma.(27) However, a randomized trial, such as the one planned by the Children's Oncology Group (COG), will be needed to confirm its clinical efficacy in high-risk neuroblastoma. Significant anti-neuroblastoma activity has also been observed with immunotherapy targeting a surface glycolipid molecule, disialoganglioside (GD2), which is uniformly expressed by neuroblastoma. A recently completed seminal randomized COG phase III trial demonstrated superior outcome for patients randomized to immunotherapy with ch14.18 antibody+cytokines and isotretinoin versus isotretinoin alone. In an effort to reduce the significant toxicities associated with ch14.18, second-generation anti-GD2 antibodies are being tested in early phase clinical trials.(19) Additional studies testing the efficacy of combining anti-GD2 antibodies with chemotherapy are under development in the COG. Recently, heritable oncogenic ALK mutations have been shown to be the major cause of familial neuroblastoma, and somatic ALK mutations have been detected in 5–8% of low-, intermediate- and high-risk neuroblastoma tumors,(28–31) Although the presence of the activating ALK alleles is not sufficient to confer clinically aggressive, high-risk disease, ALK has been identified as a valid molecular target. Early phase pediatric clinical trials testing crizotinib, a dual ALK/MET inhibitor that has shown efficacy in adults with ALK-rearranged cancers,(32) have been rapidly developed, offering a truly personalized approach to treatment. Aggressive efforts to develop new ALK inhibitors are under way. In addition, the therapeutic efficacy of anti-ALK antibodies is being investigated (33) as ALK is expressed on the surface of most neuroblastoma tumor cells and is restricted to the brain following development. While developing individualized treatment for children with neuroblastoma remains a significant challenge, the great potential of these targeted approaches to treatment of children with high-risk neuroblastoma is now established.
Advances in Targeting Childhood Leukemias
In the comprehensive review high risk B-progenitor acute lymphocytic leukemia (ALL) and juvenile monomyelocytic leukemia (JMLL) by Loh and Mulligan(34), the promise as well as the complexity of intensive genomic analyses and NexGen sequencing is detailed. In studying the mutational spectrum in genes associated with congenital disorders such as NF1, Noonan syndrome or Cbl syndrome that are frequently accompanied by myeloproliferative disorders and JMML, the challenge will be to understand the tumorigenic potential of different mutations. Here robust cell based and animal modeling systems will be needed. It seems that a variety of different mutations in signaling proteins converge on a common pathway RAS/MAPK path providing an opportunity for targeting a common downstream node.
Starting from leukemias that shared the BCR-ABL1 transcriptome without carrying the cytogenetic alteration, detailed genomic analyses revealed that some “PH-like” ALLs are marked by chromosomal rearrangements involving different cytokine receptors. These cytokine signaling receptors predominantly converge on a common signaling pathway - JAK/STAT. With a high frequency of accompanying JAK mutations, this highlights the promise of therapies guided by precision genomic interrogation of patients' tumors. The discovery of activating JAK2 mutations in other myeloproliferative disorders and the hypersensitivity of JMML cells to cytokine stimulation and STAT activation has led to their inclusion in the recent COG clinical trial of Ruxolitinib which also includes ALL with JAK mutations. We shall know in the next few years if the promise of this target is fulfilled.
With the exception of Gleevac®, the accumulating evidence from single agent-targeted clinical trials is that while tumors may be initially responsive, the responses are not durable. This may be a particularly perplexing problem for ALL in which it is already recognized that there are minor leukemic clones with unique but overlapping sets of genetic alterations. Do these clones or new ones appear at relapse? The authors point out that mutations in the acetyltransferase CREBBP emerge as a dominant clone in relapsed B-ALL(20%). Based on this finding, they propose the incorporation of HDAC inhibitors for the treatment of this type of B-ALL.
Targeting the Epigenome in Pediatric Solid Tumors
Lawlor and Thiele review recent advances in chromatin biology in the context of embryonic development, drawing parallels to the aberrant developmental programs in pediatric solid tumors such as the Ewings sarcoma family tumors (EFT), neuroblastoma (NB) and brain tumors.(35) They present emerging data that indicates that genetic alterations characteristic of these tumors lead to fundamental dysregulation of the epigenome. This is exemplified by the studies modeling Ewings in normal neural crest stem cells. Upon transduction with EWS/FLI, the most common chromosomal translocation in EFT increases in BMI-1 and EZH2 key components of polycomb repressor complex proteins 1(PRC1) and 2(PRC2) are found. This leads to increased stemness, and blocks in differentiation similar to those found in EWS tumors.
In NB elevated EZH2 levels have been noted in patients tumors with an undifferentiated histopathology and NB cell models indicate that pharmacologic or genetic inhibition of EZH2 directly or via destabilization of the PRC2 complex with HDAC inhibitors relieves EZH2-mediated repression of genes with tumor suppressor activity.
That many of the enzymes that control chromatin dynamics such as the histone methylase/demethylases and acetylases/deacetylase are druggable has elicited much excitement. The challenge will be knowing how to best use them in clinical trials.
Targeting Childhood Cancer with Chimeric Antigen Receptors
Tantalized by the sporadic successes of Coley using heat-killed bacteria to stimulate the immune system of patients with sarcomas at the turn of the 20th century (36), proponents of cancer vaccines were stymied up until now. Lee and colleagues discuss the exciting advances in the use of tumor focused chimeric antigen receptors (CAR) to target potent cytolytic T-cells to tumor cells.(37) This technology brings together the genetic engineering expertise garnered over the last 40 years from the study of the genetics of viruses, antibodies and gene therapy with a better appreciation of the complexity of cellular T-cell mediated immune responses and host lymphoid homeostatic mechanisms.
The success in neuroblastoma using anti-GD2 virus specific T-cells in a pilot phase I study stimulated interest in this therapeutic approach.(38) While anti-GD-2 remains a validated target in neuroblastoma, a number of other pediatric tumors such as Ewings sarcoma and osteosarcoma express GD2 suggesting a wider application for anti-GD2 CAR therapy. In pediatrics, most clinical studies are targeting lymphoid tumors using CD19CAR engineered alone (1st generation) or with co-stimulatory modules (2nd generation) transduced into stimulated T-cells. While initially the focus was on transducing more cells into the patients, the toxicity associated cytokine storms has limited numbers. The challenge now is to extend the lifespan of the CAR-T-cells either by genetically or immunologically enhancing their survivial and/or infusing them into lymphopenic patients.
Individualizing Pediatric Cancer Treatments using Germline Genetics
Pinto and co-workers discuss a number of important germline pharmacogenetic and pharmacogenomic studies that demonstrate the potential of using germline genetic biomarkers to personalize therapy and improve the overall care of children with cancer.(39) Although the majority of these pediatric oncology studies have focused on identifying variants associated with toxicity, more recent research has demonstrated that germline variants also contribute to response.(24) The progress in our understanding of how germline genetic variation influences drug toxicity and/or efficacy has largely been made using candidate gene approaches, specifically evaluating variants known to important in metabolic or pharmacokinetic pathways.(40) However, clinically important genomic variants also have been identified using whole genome approaches.(41) Well genotyped human EBV-immortalized lymphoblastoid cell lines (LCLs) derived from healthy individuals in the International HapMap Project have provided additional tools to identify genetic variants associated with chemotherapy resistance,(42) and the potential of this cell-based approach has recently been demonstrated adult cancer patients.(43) Pediatric studies testing the association of chemotherapy-resistant genetic variants identified in the LCL model with outcome in a cohort of neuroblastoma patients is ongoing. To develop more effective, personalized treatment regimens, it will be critical to evaluate heritable genomic variants associated with chemotherapy resistance so that patients at greatest risk for nonresponse to specific chemotherapeutic agents can be identified.
Conclusion
Clinical experience with targeted cancer therapeutics in the adult cancer population has revealed their great potential. Although the experience in pediatrics is more limited, the results of pediatric clinical trials are equally promising. Nevertheless, many challenges exist and significantly more work needs to be done before personalized therapy can become a reality for children with cancer. First, for many pediatric cancers, molecular targets have not been identified, emphasizing the need for more research and additional molecular profiling. However, even when a putative pediatric cancer target is identified, there are significant economic challenges that limit drug development unless there is also biologic rationale for using the agent in more common adult diseases. In addition to molecular profiling of tumors, additional knowledge regarding germline genetic variants associated with nonresponse and/or toxicity will be needed to develop effective, individualized treatment strategies. Finally, until technologies are developed that will yield rapid and reproducible results in a cost-effective manner, the ability to efficiently link pediatric cancer patients with biologically relevant therapeutics in a clinically relevant timeline will remain a significant challenge.
Reference List
- 1.Huebner RF, Todaro GJ. Oncogenes of RNA tumor viruses as determinants of cancer. Proc. Natl. Acad. Scie. 1969;64:1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spector D, Varmus H, Bishop JM. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertegrates. Proc Natl Acad of Sci USA. 1978;75(9):4102–6. doi: 10.1073/pnas.75.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabin CJ, Bradley SM, Bargmann CI, Weinberg RA, Papageorge AG, Scolnick EM, Dhar R, Lowy DR, Chang EH. Mechanism of activation of a human oncogene. Nature. 1982;300(5888):143–9. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- 4.Knudson AG., Jr Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68(4):820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA seqment with properties of the gene that predisposes to reinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 6.Pierce GB, Wallace C. Differentiation of malignant to benign cells. Cancer Res. 1971;32(2):127–3. [PubMed] [Google Scholar]
- 7.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 10.Julius MH, Masuda T, Herzenberg LA. Demonstration that antigen-binding cells are precursors of antibody-producing cells after purification with a fluorescence-activated cell sorter. Proc Natl Acad Sci USA. 1972;69(7):1934–8. doi: 10.1073/pnas.69.7.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–49. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 12.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–6. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 13.Look AT, Hayes FA, Nitschke R, McWilliams NB, Green AA. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med. 1984;311:231–5. doi: 10.1056/NEJM198407263110405. [DOI] [PubMed] [Google Scholar]
- 14.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009 Jan 10;27(2):289–97. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh WW, Skapek SX. Childhood rhabdomyosarcoma: new insight on biology and treatment. Curr Oncol Rep. 2010 Nov;12(6):402–10. doi: 10.1007/s11912-010-0130-3. [DOI] [PubMed] [Google Scholar]
- 16.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011 Feb 10;29(5):551–65. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosse YP, Wood A, Maris JM. Inhibition of ALK signaling for cancer therapy. Clin Cancer Res. 2009 Sep 15;15(18):5609–14. doi: 10.1158/1078-0432.CCR-08-2762. [DOI] [PubMed] [Google Scholar]
- 18.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010 Nov 16;19(5):698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010 Sep 30;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011 Dec 1;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011 Aug 25;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008 Nov;14(11):1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitz MR, Wu X, Mills G. Integrative epidemiology: from risk assessment to outcome prediction. J Clin Oncol. 2005 Jan 10;23(2):267–75. doi: 10.1200/JCO.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 24.Yang JJ, Cheng C, Yang W, Pei D, Cao X, Fan Y, et al. Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. JAMA. 2009 Jan 28;301(4):393–403. doi: 10.1001/jama.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuBois SG, Matthay KK. Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nucl Med Biol. 2008 Aug;35(Suppl 1):S35–S48. doi: 10.1016/j.nucmedbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007 Mar 20;25(9):1054–60. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 28.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008 Oct 16;455(7215):930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George RE, Sanda T, Hanna M, Frohling S, Luther W, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008 Oct 16;455(7215):975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008 Oct 16;455(7215):967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008 Oct 16;455(7215):971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 32.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010 Oct 28;363(18):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012 Jan 23; doi: 10.1038/onc.2011.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loh ML, Mullighan CG. Advances in the genetics of high-risk childhood B-progenitor acute lymphoblastic leukemia and juvenile myelomonocytic leukemia—implications for therapy. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1936. [DOI] [PubMed] [Google Scholar]
- 35.Lawlor ER, Thiele CJ. Epigenetic changes in pediatric solid tumors: promising new targets. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiemann B. Starnes CO Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–64. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 37.Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto N, Cohn SL, Dolan ME. Using germline genomics to individualize pediatric cancer treatments. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–39. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- 41.Chen SH, Yang W, Fan Y, Stocco G, Crews KR, Yang JJ, et al. A genome-wide approach identifies that the aspartate metabolism pathway contributes to asparaginase sensitivity. Leukemia. 2011 Jan;25(1):66–74. doi: 10.1038/leu.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang RS, Johnatty SE, Gamazon ER, Im HK, Ziliak D, Duan S, et al. Platinum sensitivity-related germline polymorphism discovered via a cell-based approach and analysis of its association with outcome in ovarian cancer patients. Clin Cancer Res. 2011 Aug 15;17(16):5490–500. doi: 10.1158/1078-0432.CCR-11-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziliak D, O'Donnell PH, Im HK, Gamazon ER, Chen P, Delaney S, et al. Germline polymorphisms discovered via a cell-based, genome-wide approach predict platinum response in head and neck cancers. Transl Res. 2011 May;157(5):265–72. doi: 10.1016/j.trsl.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]