Abstract
Invasion and metastasis increase after inhibition of vascular endothelial growth factor (VEGF) signaling in some preclinical tumor models. The present study asked whether selective VEGF inhibition is sufficient to increase invasion and metastasis and whether selective c-Met inhibition is sufficient to block this effect. Treatment of pancreatic neuroendocrine tumors in RIP-Tag2 mice with a neutralizing anti-VEGF antibody reduced tumor burden but increased tumor hypoxia, HIF-1α, and c-Met activation, and also increased invasion and metastasis. However, invasion and metastasis were reduced by concurrent inhibition of c-Met by PF-04217903 or PF-02341066 (crizotinib). Similar benefit was found in orthotopic Panc-1 pancreatic carcinomas treated with sunitinib plus PF-04217903 and in RIP-Tag2 tumors treated with XL184 (cabozantinib), which simultaneously blocks VEGF and c-Met signaling. These findings document that invasion and metastasis are promoted by selective inhibition of VEGF signaling and can be reduced by concurrent inhibition of c-Met.
Keywords: Evasive resistance, pancreatic cancer, angiogenesis inhibitors
Introduction
Most angiogenesis inhibitors used in the treatment of cancer block the actions of VEGF, a cytokine that promotes blood vessel growth and survival. Treatment with the anti-VEGF monoclonal antibody bevacizumab, usually administered with chemotherapy, delays progression and prolongs survival of some patients (1, 2).
Inhibition of VEGF signaling is accompanied by increased invasiveness and metastasis in some preclinical models (3-6). The mechanism of the exaggerated aggressiveness is unknown, but contributing factors could be vessel pruning, hypoxia, and increased expression of c-Met, the tyrosine kinase receptor for hepatocyte growth factor (HGF) (7, 8). The HGF/c-Met pathway promotes tumor cell motility, proliferation, survival, invasion, and metastasis (9, 10). c-Met is overexpressed, activated, amplified, or mutated in a wide variety of solid tumors (10-12), correlates with poor prognosis (13-16), and is thought to contribute to tumor aggressiveness and resistance to therapy (17, 18).
Treatment with the multi-targeted tyrosine kinase inhibitor foretinib (GSK1363089, XL880), which blocks VEGF receptors (VEGFRs), c-Met, and multiple other receptor tyrosine kinases (RTKs) including AXL, Tie2, KIT, FLT3, PDGFR, and RON, has a potent anti-angiogenic action that slows tumor growth in RIP-Tag2 mice (19). XL880 also decreases invasion and the appearance of tumor cells in the liver (19). Although the mechanism of the anti-invasive and anti-metastatic actions was not addressed, they are presumed to reflect direct effects on tumor cells. It is unclear whether simultaneous inhibition of VEGFR and c-Met was necessary and sufficient for these actions.
In the present study, we examined the involvement of VEGF and c-Met signaling in invasion and metastasis through the use of selective agonists and inhibitors. We first verified that selective inhibition of VEGF by a function-blocking antibody increases tumor invasion and metastasis, as expected from previous reports (6), and then asked whether concurrent inhibition of c-Met is sufficient to block this effect. We also determined the relationship of vascular pruning, tumor hypoxia, HIF-1α, and c-Met activation to invasion and metastasis. Our approach was to determine whether tumor invasion and metastasis were promoted by inhibition of VEGF with a function-blocking anti-VEGF antibody (20) and whether they were blocked by a selective inhibitor of c-Met (PF-04217903) (21) used alone or together with the anti-VEGF antibody.
We addressed these issues in two complementary tumor models, transgenic RIP-Tag2 mice, which are known to develop more invasive and metastatic tumors after inhibition of VEGF signaling (4, 6), and orthotopically implanted Panc-1 human pancreatic adenocarcinomas, which are known to express both VEGF and c-Met (22, 23). Tumors in RIP-Tag2 mice naturally become more invasive and metastatic with age, but generally do so near the time the mice succumb (24, 25). The distinctive stages of tumor progression in these mice provided the opportunity to determine whether aggressiveness could be advanced, delayed, or reversed by treatment. Luciferase expression in Panc-1 tumor cells made it possible to use in vivo imaging to follow treatment effects on tumor growth (26).
Tumor-bearing mice were treated with anti-mouse VEGF antibody or, for comparison, with sunitinib, a multi-targeted inhibitor of VEGFRs, PDGFRs, KIT, and related RTKs (27). Effect on tumor aggressiveness was assessed for the agents administered alone or together with PF-04217903 or a second c-Met inhibitor, PF-02341066 (crizotinib) (28, 29). To determine whether a single agent that inhibits both targets could mimic the effects of separate inhibitors given together, RIP-Tag2 mice were treated with the multi-targeted RTK inhibitor XL184 (cabozantinib), which blocks VEGFRs and c-Met, among other kinases (19, 30-33).
Overall, the experiments revealed that, as expected from previous work (6), inhibition of VEGF by function-blocking antibody slowed tumor growth but increased invasion and metastasis. The findings fit with a mechanism involving vascular pruning, intratumoral hypoxia, HIF-1α accumulation, and activation of c-Met in tumor cells. Importantly, invasion and metastasis were blocked by selective inhibition of c-Met. Inhibition of both signaling pathways by XL184 also reduced tumor growth, invasion, and metastasis and prolonged survival.
Results
Contrasting effects of VEGF inhibition on growth and invasion of RIP-Tag2 tumors
RIP-Tag2 mice treated with neutralizing anti-mouse VEGF antibody or sunitinib from age 14 to 17 weeks had significantly smaller tumors (Figure 1, A-C). The sectional area of tumors was 75% less after the antibody and 78% less after sunitinib, compared to age-matched controls treated with vehicle (Figure 1D).
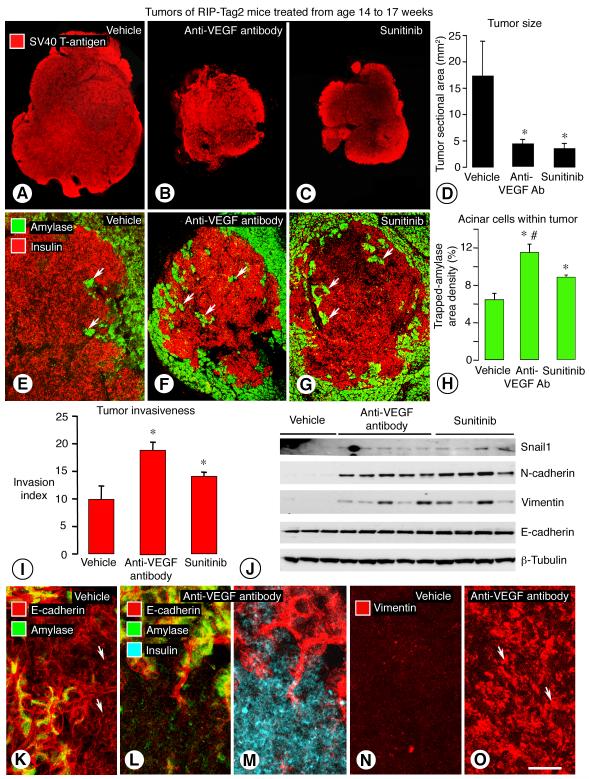
Figure 1. Inhibition of VEGF signaling: Effect on aggressiveness of RIP-Tag2 tumors.
Tumors of RIP-Tag2 mice treated with vehicle, anti-VEGF antibody, or sunitinib from age 14 to 17 weeks (A-C). Composite images of tumor sections immunostained for SV40 T-antigen (red) (A-C). Differences in sectional area after anti-VEGF antibody or sunitinib (4-5 mice/group) (D). Confocal images showing tumor cells (insulin, red) and acinar cells (amylase, green) after vehicle (E), anti-VEGF antibody (F), or sunitinib (G); arrows mark islands of acinar cells surrounded by tumor cells. Area density of trapped amylase-positive cells (H) and Invasion index (I) after vehicle, anti-VEGF antibody, or sunitinib. Western blots showing differences in Snail1, N-cadherin, and vimentin protein in tumors of mice treated from age 14 to 15 weeks (J). Strong E-cadherin immunoreactivity (red) near the border (arrows) of a control tumor (K). Little E-cadherin staining in tumor cells after anti-VEGF antibody for 3 weeks (L), with confirmation of tumor-cell identity by insulin staining (M, cyan). Compared to E-cadherin, vimentin staining shows the opposite treatment-related changes (red, arrows) (N, O). *P < 0.05 vs. vehicle. #P < 0.05 vs. sunitinib. Scale bar in O applies to all images: 800 μm for A-C; 150 μm for E-G; and 30 μm for K-O.
Despite their smaller size, tumors treated with anti-VEGF antibody or sunitinib appeared to be more invasive, as judged by the irregularity of the tumor border and the abundance of clusters of amylase-positive acinar cells of the exocrine pancreas trapped inside tumors (Figure 1, E-G). Quantitative measures of the tortuosity of the tumor border (Invasion index, see Methods) and the number of trapped acinar cells were significantly greater (Figure 1, H and I). The relevance of amylase-positive cells within tumors, as an indicator of invasion, was assessed by comparing amylase staining to the basement membrane protein type IV collagen and to type I collagen, a known constituent of the capsule of RIP-Tag2 tumors (4). The three approaches gave complementary results (Supplemental Figure 1). Tumors with abundant amylase cells inside had strong staining for type IV collagen around the trapped exocrine cells, as in normal pancreatic acini, but the border had little or no type IV collagen or type I collagen (Supplemental Figure 1, A-C, G-I). Tumors that had few or no amylase-stained cells inside had type IV collagen around blood vessels, and the border had a layer of type IV collagen and a capsule of type I collagen (Supplemental Figure 1, D-F, J-L).
Tumors of 14-week old RIP-Tag2 mice treated with normal goat IgG for 1 or 3 weeks resembled those of mice treated with vehicle (data not shown).
Tumor cell changes in RIP-Tag2 tumors after VEGF inhibition
Proliferating cells marked by phosphohistone H3 immunoreactivity were abundant throughout vehicle-treated tumors (Supplemental Figure 2A). After treatment with anti-VEGF antibody for 3 days, proliferating cells were still abundant at the tumor border (area density: 14.7% vs. 14.3% for vehicle) but were half the control value at the tumor center (6.8% vs. 13.3% for vehicle, P < 0.05) (Supplemental Figure 2B). Abundant phosphohistone H3-positive cells in finger-like projections of tumor contrasted with rare dividing cells in the surrounding exocrine pancreas (Supplemental Figure 2C).
Apoptotic cells identified by activated caspase-3 immunoreactivity were more abundant after anti-VEGF antibody for 3 days, but were less numerous than proliferating cells under all conditions (Supplemental Figure 2, D-F). Apoptotic cells were no more frequent in finger-like projections than elsewhere in tumors.
Snail1, N-cadherin, and vimentin as markers of mesenchymal phenotype had stronger bands in western blots of tumors after treatment with anti-VEGF antibody or sunitinib than in corresponding mice treated with vehicle from age 14 to 15 weeks (Figure 1J). Densitometry values for Snail1, N-cadherin, and vimentin were 3, 5, and 10 times greater, respectively, after anti-VEGF antibody (P < 0.05) and 3, 10, and 5 times greater after sunitinib (P < 0.05).
E-cadherin, as a marker of epithelial phenotype, was weaker in tumors of RIP-Tag2 mice at age 17 weeks (Figure 1K) than at age 10 weeks (data not shown), but was even less in tumors treated with anti-VEGF antibody (age 14 to 17 weeks), where tumor cell identity was verified by insulin staining (Figure 1, L and M). E-cadherin staining was inversely related to staining for vimentin (Figure 1, K-O) and c-Met (Supplemental Figure 2, G-H). E-cadherin was stronger in vehicle treated mice, and vimentin and c-Met were stronger after anti-VEGF antibody (Supplemental Figure 2, G-J).
Hypoxia and c-Met in RIP-Tag2 tumors after VEGF inhibition
Tumors in RIP-Tag2 mice treated with anti-VEGF antibody or sunitinib from age 14 to 17 weeks had fewer blood vessels than in corresponding vehicle-treated tumors (Figure 2, A-C), as found previously after inhibition of VEGF signaling (19, 34). The reduced vascularity was accompanied by greater hypoxia, reflected by staining for pimonidazole, carbonic anhydrase IX (CA-IX), or glucose transporter 1 (Glut1) (Figure 2, A-C, Supplemental Figure 3, A-B, D-E). The staining patterns for the three markers was similar: staining was patchy in control tumors and was widespread and strongest in regions of vascular pruning in VEGF inhibitor-treated tumors. Measurements confirmed an inverse relationship between tumor vascularity and amount of pimonidazole staining (Figure 2D). The amount of HIF-1α protein, assessed by western blot, was also greater after anti-VEGF antibody or sunitinib (Figure 2E).
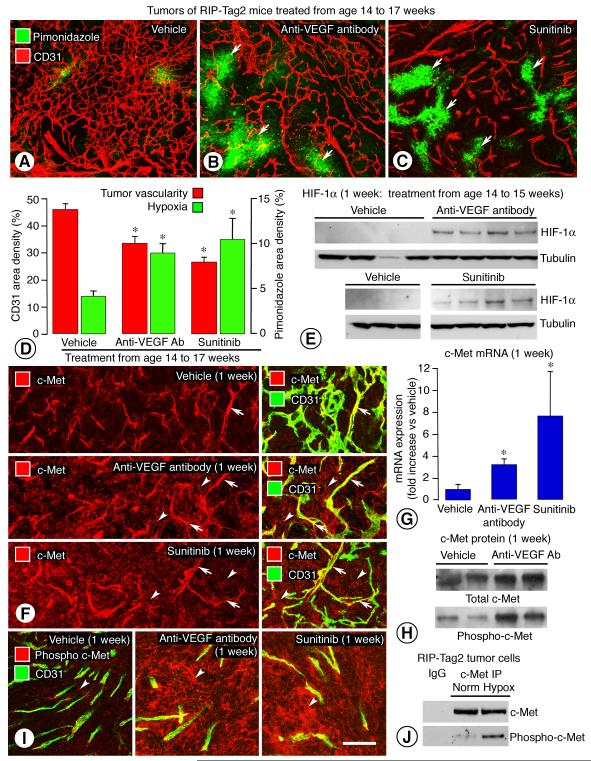
Figure 2. Inhibition of VEGF signaling: Effect on tumor vascularity, hypoxia, and c-Met.
Comparison of blood vessels (CD31, red) and hypoxia (pimonidazole, green, arrows) in tumors of RIP-Tag2 mice treated with vehicle, anti-VEGF antibody, or sunitinib from age 14 to 17 weeks (A-C). Fractional areas of CD31 and pimonidazole after treatment (D). Western blots show little or no HIF-1α in tumors of control mice and strong bands after anti-VEGF antibody or sunitinib from age 14 to 15 weeks (E). c-Met immunoreactivity (red) in scattered tumor cells and many vessels (CD31, green, arrows) after vehicle, but in many tumor cells (arrowheads) after anti-VEGF antibody or sunitinib from age 14 to 15 weeks (F). Greater c-Met mRNA in tumors treated with anti-VEGF antibody or sunitinib from age 14 to 15 weeks (5-7 mice/group) (G). Greater total c-Met and phospho-c-Met in RIP-Tag2 tumors after anti-VEGF antibody from age 14 to 15 weeks, assessed by immunoprecipitation (H). Phospho-c-Met immunoreactivity (red) in scattered tumor cells (arrowheads) in control RIP-Tag2 tumors but in many tumor cells after anti-VEGF antibody or sunitinib from age 14 to 15 weeks (CD31, green) (I). Greater signal for phospho-c-Met in tumor cells isolated from 14-week old RIP-Tag2 mice, exposed in vitro for 4 hours to normal or low oxygen (Norm = 21% oxygen; Hypox = 1% oxygen), and assessed by immunoprecipitation (J). * P < 0.05 vs. vehicle. Scale bar in I applies to all images: 50 μm for A-C; 80 μm for F; 60 μm for I.
Comparison of c-Met immunoreactivity in RIP-Tag2 tumors treated with vehicle, anti-VEGF antibody, or sunitinib confirmed the known relationship between hypoxia and c-Metexpression (8). Staining for c-Met was weak or absent in tumor cells but was strong in tumor vessels of control 15-week old mice (Figure 2F, upper). After anti-VEGF antibody or sunitinib for one week, c-Met staining was strong and widespread in tumor cells (Figure 2F, middle and lower). Tumor vessel staining was present but less conspicuous because of the vascular pruning.
Expression of c-Met mRNA was 3-fold the control value after anti-VEGF antibody and 6-fold the control value after sunitinib (Figure 2G). The amount of c-Met mRNA in RIP-Tag2 tumors treated with normal goat IgG was similar to that of vehicle-treated controls. The amount and distribution of phospho-c-Met was strongly influenced by treatment. Total c-Met and phospho-c-Met assessed by immunoprecipitation were 3- and 5-fold greater, respectively, in RIP-Tag2 tumors after anti-VEGF antibody for one week (Figure 2H). Phospho-c-Met immunoreactivity was mainly in blood vessels in vehicle-treated tumors, but was strong and widespread in tumor cells after anti-VEGF antibody or sunitinib for one week (Figure 2I). The two inhibitors of VEGF signaling had similar effects on phospho-c-Met staining (Figure 2I).
Neither HGF mRNA expression nor HGF immunoreactivity changed detectably in tumors of RIP-Tag2 mice treated with anti-VEGF antibody for 1 week (Supplemental Figure 3, I-K).
To determine whether hypoxia had a direct effect on c-Met activation in tumor cells, we isolated tumor cells from 14-week old RIP-Tag2 mice and exposed them to 21% oxygen (normoxia) or 1% oxygen (hypoxia) for 4 hours in vitro. Phospho-c-Met assessed by immunoprecipitation was greater in tumor cells exposed to hypoxia (Figure 2J).
We addressed the question of whether tumor cells, like blood vessels, are direct targets of inhibitors of VEGF signaling in RIP-Tag2 tumors. Blood vessels in all tumors had strong staining for VEGFR-2 and VEGFR-3 (Supplemental Figure 4), as reported previously (34). In addition, 5-10% of tumor cells in RIP-Tag2 mice at age 17 weeks had moderate VEGFR-2 immunoreactivity (Supplemental Figure 4, A-C). This was not changed by inhibition of VEGF signaling. VEGFR-3 immunoreactivity was not detected in tumor cells under any of the treatment conditions (Supplemental Figure 4, D-F).
Treatment of 14-week old RIP-Tag2 mice with anti-VEGF antibody for 1 week did not result in significant changes in expression of angiopoietin-1 (ANG1), angiopoietin-2 (ANG2), basic fibroblast growth factor (FGF2), insulin-like growth factor 1 receptor (IGF-1R), macrophage stimulating 1 receptor (RON/MST1R), RET, SKY, or MER in tumors, but increases were found in AXL (2.8 times) and TGF-β (15 times) (Supplemental Figure 5A).
Reduction in invasion of RIP-Tag2 tumors after inhibition of c-Met
To determine whether tumor invasiveness promoted by inhibition of VEGF signaling was dependent on c-Met activity, we treated RIP-Tag2 mice with PF-04217903, a selective inhibitor of c-Met signaling (21), together with anti-VEGF antibody or sunitinib from age 14 to 17 weeks. Tumors in mice treated with the drug combination had a smoother contour, fewer projections into the exocrine pancreas, and fewer intratumoral acinar cells than after anti-VEGF antibody or sunitinib alone (Figure 3, A-D). Measurements of intratumoral acinar cells (Figure 3E) and Invasion index (Supplemental Figure 5B) showed significant reductions after treatment with PF-04317903 alone or in combination with anti-VEGF antibody or sunitinib. Tumors treated with PF-04317903 in combination with sunitinib had 38% fewer amylase-positive cells than the 14-week old onset controls (3.0 ± 0.4% vs. 4.8 ± 0.5%, P < 0.05), indicative of reversal of this measure of invasion.
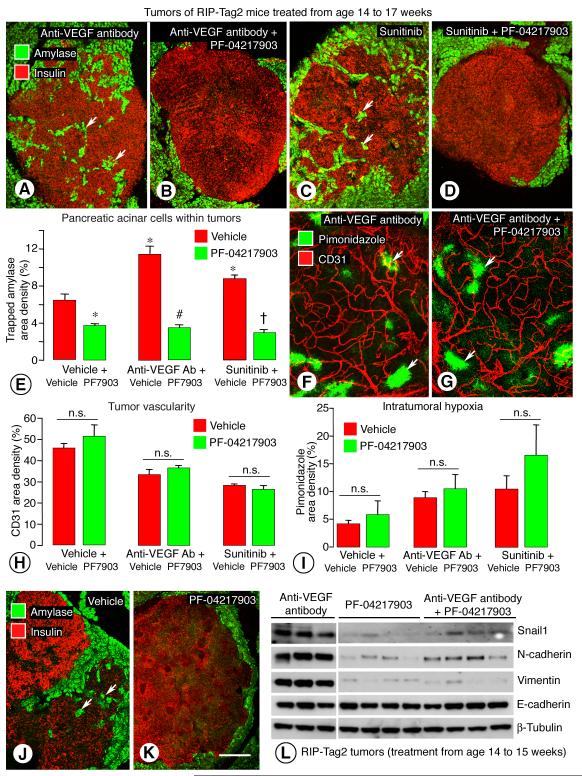
Figure 3. Inhibition of VEGF and/or c-Met: Effect on tumor invasion, vascularity, hypoxia, and cell markers.
Confocal images of RIP-Tag2 tumors treated from age 14 to 17 weeks and stained for insulin (tumor cells, red) and amylase (acinar cells, green) (A-F). Trapped acinar cells marked by amylase (arrows) within tumors are numerous after anti-VEGF antibody (A) or sunitinib (C) alone but not after co-administration of PF-04217903 (B, D). Measurements of amylase staining within tumors show significant treatment-related differences (E). Similarity of vascularity (CD31, red) and hypoxic regions (pimonidazole, green, arrows) in tumors of 17-week old RIP-Tag2 mice after treatment with anti-VEGF antibody (F) or anti-VEGF antibody plus PF-04217903 (G) for 3 weeks. Bar graph showing that addition of PF-04217903 to anti-VEGF therapy did not significantly change vascularity (H) or hypoxic regions (I) in tumors. Differences in number of trapped amylase cells (arrows) after vehicle (J) or PF-04217903 alone (K). Western blot showing differences in mesenchymal markers after anti-VEGF antibody, PF-04217903, or the combination from age 14 to 15 weeks (L). * P < 0.05 vs. vehicle. # P < 0.05 vs. anti-VEGF antibody alone. = P < 0.05 vs. sunitinib alone. Scale bar in K applies to all images: 150 μm for A-D, J-K; 50 μm for F-G.
The addition of PF-04217903 to anti-VEGF antibody or sunitinib was not accompanied by greater vascular pruning (CD31 immunoreactivity, Figure 3, F-H), reduction in tumor size (data not shown), or intratumoral hypoxia (pimonidazole staining, Figure 3, F, G, I) than was found with the single agents. In the assessment of intratumoral hypoxia under these conditions, CA-IX and Glut1 had patterns similar to pimonidazole staining (Supplemental Figure 3, A-F, G). Glut1 had a stronger signal than CA-IX and was more intense in tumors treated with PF-04217903 in combination with anti-VEGF antibody or sunitinib (Supplemental Figure 3, G and H). HIF-1α was not increased in western blots from baseline after treatment with PF-04217903 alone. HIF-1α was increased by anti-VEGF antibody or sunitinib alone (Figure 2E) or by either one combined with PF-04217903 (data not shown).
PF-04217903 given in combination with anti-VEGF antibody or with sunitinib prevented the increase in c-Met and phospho-c-Met immunoreactivities produced by anti-VEGF antibody or sunitinib given alone (Supplemental Figure 5, D and E).
Tumors of RIP-Tag2 mice treated with PF-04217903 alone had a smoother border and fewer trapped acinar cells than after vehicle (Figure 3, J and K). However, tumors treated with PF-04217903 alone for 3 weeks were similar in size to age-matched, vehicle-treated controls (data not shown). PF-04217903-treated tumors had no reduction in overall vascularity (Figure 3H), vascular patency assessed by injection of Lycopersicon esculentum lectin (34), or intratumoral hypoxia (Figure 3I), and had no apparent change in vessel branching or in pericyte coverage assessed by NG2 and α-SMA immunoreactivities (data not shown).
To test whether the anti-invasive effects of PF-04217903 could be reproduced by another c-Met inhibitor, we performed similar experiments using the c-Met inhibitor, PF-02341066 (21, 35), in RIP-Tag2 mice treated from age 14 to 17 weeks. As with PF-04217903, treatment with PF-02341066 reduced invasiveness, as reflected by trapped acinar cells and Invasion index, but did not change tumor vascularity or hypoxia (Supplemental Figure 6, A-B, E-F, G-H, K-L). Importantly, treatment with PF-02341066 in combination with sunitinib resulted in changes similar to those found after PF-04217903 plus sunitinib: tumors were less invasive than after vehicle or sunitinib alone (Supplemental Figure 6, C-F), and tumor vascularity and hypoxia were about the same as after sunitinib alone (Supplemental Figure 6, I-L).
To determine whether inhibition of c-Met suppressed the increase in mesenchymal markers after inhibition of VEGF, we compared Snail1, N-cadherin, and vimentin in western blots prepared from tumors of RIP-Tag2 mice treated with anti-VEGF antibody, PF-04217903, or the combination from age 14 to 15 weeks. The bands for these markers were strong after anti-VEGF antibody but were weaker after the antibody plus PF-04217903 or after PF-04217903 alone (Figure 3L). Similarly, Snail1 was less in tumors treated with sunitinib plus PF-04217903 than with sunitinib alone, but N-cadherin and vimentin showed less difference (Supplemental Figure 5C). The reduction in mesenchymal markers after PF-04217903 fits with the reversal of the mesenchymal phenotype by c-Met blockade.
Reduction in liver metastasis after inhibition of c-Met in RIP-Tag2 mice
The liver is a common site of metastasis in RIP-Tag2 mice. Metastases were rarely visible grossly in the liver at autopsy of untreated 14-week old RIP-Tag2 mice (onset controls), but micrometastases, consisting of single or small groups of cells, were found by microscopy after staining for SV40 T-antigen (0.05 ± 0.01 per mm2 sectional area). Liver metastases were 14 times more numerous in control mice at age 17 weeks (0.7 ± 0.2 per mm2), and were much more abundant and larger after anti-VEGF antibody or sunitinib from age 14 to 17 weeks (Figure 4, A-D). Measurements showed 5 times as many liver metastases after anti-VEGF antibody and twice as many after sunitinib, compared to the 17-week old vehicle group (Figure 4E). Compared to the 90 ± 9 μm mean diameter of metastases in the vehicle group, metastases were nearly twice as large after anti-VEGF antibody (mean diameter, 172 ± 29 μm) and 4 times as large after sunitinib (mean diameter, 369 ± 267 μm). The incidence of liver metastases in RIP-Tag2 mice treated with normal goat IgG from age 14 to 17 weeks was similar to mice treated with vehicle (data not shown).
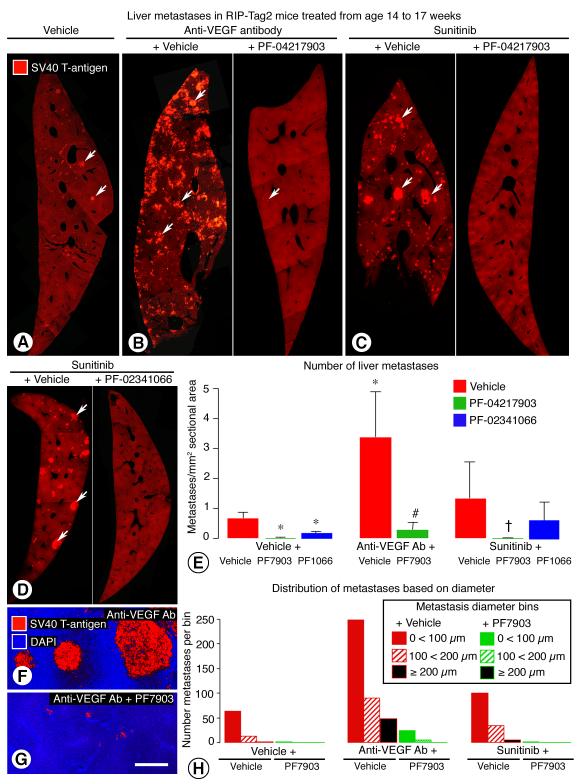
Figure 4. Inhibition of VEGF and/or c-Met: Effect on number and size of liver metastases.
Contrasting number and size of metastases (arrows) in sections of liver (SV40 T-antigen, red) from RIP-Tag2 mice treated with vehicle (A), anti-VEGF antibody alone or combined with PF-04217903 (B), sunitinib alone or combined with PF-04217903 (C), or sunitinib alone or combined with PF-02341066 (D) from age 14 to 17 weeks. Treatment-related differences in number of liver metastases per square millimeter of section, with and without co-administration of PF-04217903 or PF-02341066 (E) (4-7 mice/group). * P < 0.05 vs. vehicle alone. # P < 0.05 vs. anti-VEGF antibody alone. = P < 0.05 vs. sunitinib alone. Confocal micrographs comparing the size of liver metastases in mice treated with anti-VEGF antibody alone (F) or combined with PF-04217903 (G). Fewer and smaller metastases in mice treated with PF-04217903 alone or in combination with anti-VEGF antibody or sunitinib shown by sorting all metastases in each treatment group into the three size bins shown in legend (H). Scale bar in G applies to all images: 350 μm for A-C, 450 μm for D, 100 μm for F, G.
Liver metastases assessed by SV40 T-antigen staining were significantly less numerous in mice treated with PF-04217903 or PF-02341066 (Figure 4, right panels of B, C, and D). The baseline number of liver metastases in 17-week old RIP-Tag2 mice (vehicle treatment) was reduced 98% by administration of PF-04217903 and reduced 73% by PF-02341066 (Figure 4E). Metastases in mice treated with sunitinib were reduced 99% by PF-04217903 and reduced 53% by PF-02341066 (Figure 4E). Metastases in mice treated with anti-VEGF antibody were reduced 92% by co-administration of PF-04217903 (Figure 4E). Metastases were visible microscopically in the liver of all 17-week old mice treated with vehicle, anti-VEGF antibody, or sunitinib alone, but were found in only 1 of 7 mice given vehicle plus PF-04217903, 3 of 6 mice given anti-VEGF antibody plus PF-04217903, and 1 of 5 mice given sunitinib plus PF-04217903.
Liver metastases were smaller in all groups that received PF-04217903 (Figure 4, F-H). The mean diameter of metastases was reduced 40% when PF-04217903 was combined with vehicle, 50% when combined with anti-VEGF antibody, and 81% when combined with sunitinib (Figure 4H). These reductions reflect average decreases in volume (metastatic tumor burden) of 78%, 88%, and 99%. Metastases with diameters larger than 100 μm were 3 times more numerous after anti-VEGF antibody or sunitinib than after vehicle, but were rare in the groups receiving PF-04217903 (Figure 4H).
Survival of RIP-Tag2 mice during the 3-week treatment from age 14 to 17 weeks was strongly influenced by the treatment: 42% survival with vehicle, 50% with anti-VEGF antibody, 70% with anti-VEGF antibody plus PF-04217903, and 80% with sunitinib alone or in combination with PF-04217903.
Reduction in invasion of Panc-1 tumors after inhibition of c-Met
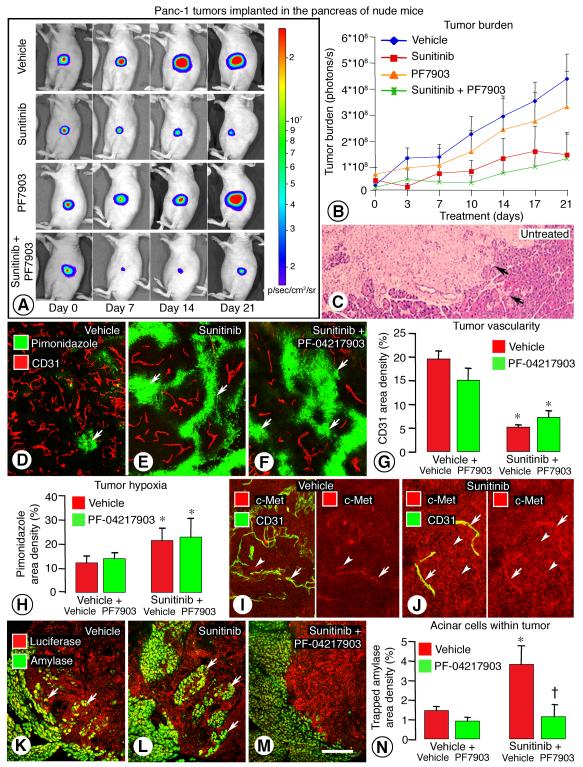
To compare the effects of inhibition of VEGF signaling alone and with concurrent blockade of c-Met signaling in a second tumor model, we performed similar experiments on implanted Panc-1 pancreatic adenocarcinomas (26), which invade locally and can metastasize (36, 37). Growth of luciferase expressing Panc-1 tumors in the pancreas was monitored by bioluminescence imaging (Figure 5A). Tumors in mice treated with vehicle grew steadily and invaded the exocrine pancreas during 3-week treatment (Figure 5, B and C). Tumors treated with sunitinib grew slower, and those treated with sunitinib plus PF-04217903 grew even less (Figure 5, A-C).
Figure 5. Inhibition of VEGF and/or c-Met: Effect on Panc-1 tumor growth, c-Met, and invasion.
Changes in luciferin bioluminescence during the course of 3-week treatment (A). Measurements of bioluminescence showed slower growth of tumors in mice treated with sunitinib alone or in combination with PF-04217903 (B). Irregular edges (arrows) of an untreated Panc-1 tumor section stained with hematoxylin and eosin (H&E) (C). Blood vessels (CD31, red) and hypoxic regions (pimonidazole, green, arrows) in Panc-1 tumors treated with vehicle (D), sunitinib (E) or sunitinib plus PF-04217903 (F) for 3 weeks. Bar graphs showing treatment-related differences in tumor vascularity (CD31) (G) and intratumoral hypoxia (pimonidazole) (H). c-Met immunoreactivity (red) was faint in tumor cells (arrowhead) and blood vessels (arrow) (CD31, green) in vehicle-treated Panc-1 tumors (I) but was stronger in tumor cells (arrowheads) and vessels (arrows) after sunitinib for 3 weeks (J). Trapped acinar cells marked by amylase immunoreactivity (green, arrows) in Panc-1 tumors (luciferase, red) after vehicle (K), sunitinib alone (L), or sunitinib plus PF-04217903 (M). Measurements of intratumoral acinar cells (N). * P < 0.05 vs. vehicle. = P < 0.05 vs. sunitinib plus vehicle. Scale bar in M applies to all images: 30 μm for C; 50 μm for D-F; 70 μm for I-J; 150 μm for K-M.
Treatment with sunitinib was accompanied by 75% reduction in tumor vascularity and doubling of intratumoral hypoxia, assessed by pimonidazole staining (Figure 5, D, E, G, H). Vascular pruning and hypoxia were similar after treatment with sunitinib plus PF-04217903 (Figure 5, F-H). Treatment with PF-04217903 alone had little effect on vascular pruning or intratumoral hypoxia (Figure 5, G and H).
c-Met immunoreactivity was moderate in some blood vessels and faint in others and was faint in most tumor cells of vehicle-treated Panc-1 tumors. However, c-Met was stronger in both locations after treatment with sunitinib (Figure 5I-J). Invasiveness of Panc-1 tumors, reflected by intratumoral acinar cells stained for amylase, was exaggerated by sunitinib (Figure 5, K-L) and was suppressed by co-administration of PF-04217903 (Figure 5, M-N).
Panc-1 tumors metastasize to the liver when the tumors become aggressive (36, 37). At the early stage we studied, micrometastases were found in the liver of 4 of 6 mice treated with sunitinib but in none of 6 mice treated with sunitinib plus PF-04217903 (data not shown).
Reduction in invasion of RIP-Tag2 tumors after treatment with XL184
The effects of agents that block c-Met or VEGF signaling, administered alone or in combination, were compared to those of XL184, which blocks both receptors simultaneously (19, 32). XL184 has potent inhibitory activity against c-Met, VEGFR-2, and multiple other RTKs tested in biochemical and cell-based assays (Supplemental Figure 7A). The IC50 for c-Met (1.3 nM) and VEGFR-2 (0.035 nM) were both in the low nanomolar or sub-nanomolar range and were the lowest of the kinases assayed.
Comparison of tumor size in RIP-Tag2 mice treated from age 14 to 17 weeks revealed that XL184-treated tumors were much smaller (mean sectional area 0.7 ± 0.1 mm2) than those treated with vehicle (17.3 ± 6.5 mm2), anti-VEGF antibody (4.3 ± 0.8 mm2), or sunitinib (3.7 ± 0.8 mm2). XL184-treated tumors of 17-week old mice were also smaller than tumors in 14-week old onset controls (2.8 ± 0.5 mm2). Treatment with XL184 for 3 weeks resulted in widespread vascular pruning and intratumoral hypoxia (Supplemental Figure 8): the 79% reduction in tumor vascularity was greater than found after any of the other agents studied.
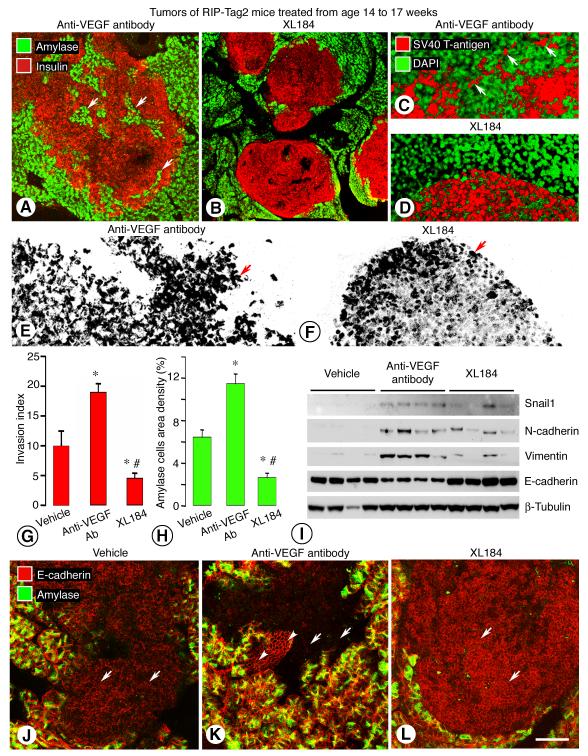
Tumors in RIP-Tag2 mice treated with XL184 had well-defined borders, smooth contours, few projections into the acinar pancreas, and few or no islands of trapped acinar cells, and were distinctly unlike tumors treated with anti-VEGF antibody (Figure 6, A and B). SV40 T-antigen-positive cells were scattered outside the tumor border in mice treated with anti-VEGF antibody (Figure 6C) but not after XL184 (Figure 6D). Many tumor cells in mice treated with vehicle or anti-VEGF antibody were irregular in shape and loosely associated with one another (Figure 6E), but tumor cells treated with XL184 were rounder and tightly clustered (Figure 6F). Consistent with these differences, values for Invasion index and trapped acinar cells were significantly less after XL184 than after vehicle or anti-VEGF antibody (Figure 6, G and H). Area density values for trapped acinar cells in tumors treated from age 14 to 17 weeks with XL184 (2.6 ± 0.4%) were 46% lower than for untreated mice at 14 weeks of age (4.8 ± 0.5%). This significant difference indicates that XL184 partially reversed the invasion present at the beginning of treatment.
Figure 6. XL184 effects on RIP-Tag2 tumor invasion and epithelial/mesenchymal markers.
Tumors from RIP-Tag2 mice treated for 3 weeks, from age 14 to 17 weeks, with anti-VEGF antibody (A) or XL184 (B). Confocal microscopic images showing tumor cells (insulin, red) and acinar cells (amylase, green) after anti-VEGF antibody (A) or XL184 (B); arrows mark islands of acinar cells surrounded by tumor cells (A). Scattered tumor cells infiltrate the exocrine pancreas after anti-VEGF antibody (C) but not XL184 (D). Irregular tumor-cell shape after anti-VEGF antibody compared to rounded shape after XL184 (E, F, arrows). Contrasting effects of vehicle, anti-VEGF antibody, and XL184 on Invasion index (G) and trapped-amylase cells after treatment from age 14 to 17 weeks (H). Western blot showing mesenchymal markers in tumors after the three treatments from age 14 to 15 weeks (I). Contrasting levels of E-cadherin immunoreactivity (red, arrows) in tumors after the three treatments from age 14 to 17 weeks (J-L). * P < 0.05 vs. vehicle; # P < 0.05 vs. anti-VEGF antibody; 4-5 mice/group. Scale bar in L applies to all images: 150 μm for A-B; 25 μm for C-D; 60 μm for E-F; 45 μm for J-L.
RIP-Tag2 tumors treated with XL184 had fainter c-Met immunoreactivity in tumor cells and blood vessels and less phospho-c-Met assessed by immunoprecipitation than corresponding vehicle-treated controls (Supplemental Figure 7B-F). By comparison, c-Met immunoreactivity of the tumors treated with PF-04217903 together with anti-VEGF antibody or sunitinib was still strong in tumor cells and blood vessels (data not shown). Western blots of XL184-treated tumors also showed more E-cadherin protein and less Snail1, N-cadherin, and vimentin than tumors treated with anti-VEGF antibody (Figure 6I). Similarly, tumors treated with XL184 had stronger E-cadherin immunoreactivity than tumors treated with vehicle or anti-VEGF antibody (Figure 6, J-L). Taken together, these results indicate that tumor cells treated with XL184 had a more epithelial phenotype.
Reduction in metastasis and prolonged survival after treatment with XL184
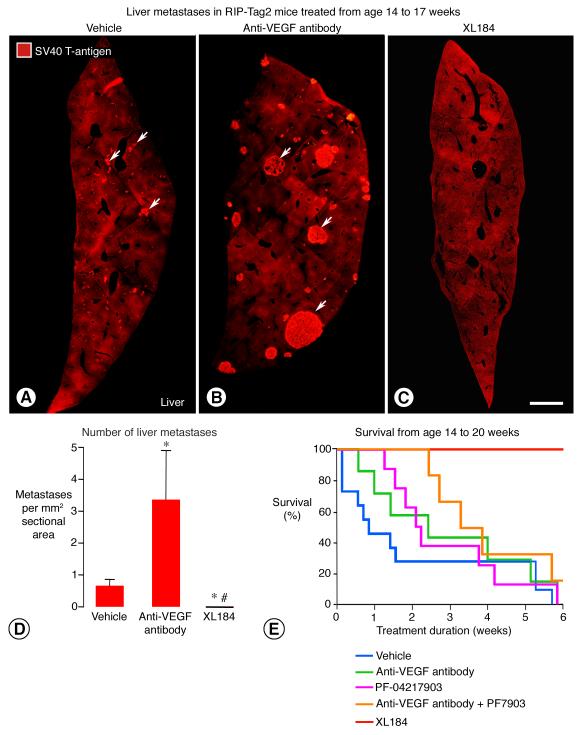
Microscopic liver metastases were consistently found in 17-week old RIP-Tag2 mice after treatment for 3 weeks with vehicle, were more numerous and larger after anti-VEGF antibody, but were not found after treatment with XL184 (Figure 7, A-D).
Figure 7. XL184 effects on liver metastasis and overall survival of older RIP-Tag2 mice.
Metastases (arrows) in liver sections (SV40 T-antigen, red) from RIP-Tag2 mice treated with vehicle (A), anti-VEGF antibody (B), or XL184 (C) from age 14 to 17 weeks. The number of metastases after anti-VEGF antibody was 5-fold the number after vehicle, but none were detected after XL184 (D). Survival of mice after treatment beginning at age 14 weeks with vehicle (blue line, median survival = 14.7 weeks, n = 12), PF-04217903 (pink line, median survival = 16.1 weeks, n = 8), anti-VEGF antibody (green line, median survival = 16.4 weeks, n = 7), anti-VEGF antibody plus PF-04217903 (orange line, median survival = 17.3 weeks, n = 6), or XL184 (red line, 100% survival at 20 weeks, n = 6, P < 0.05 vs. vehicle and anti-VEGF antibody) (E). (One of 7 mice in the XL184 group that died from a gavage injury at 3.5 weeks was excluded.) * P < 0.05 vs. vehicle; # P < 0.05 vs. anti-VEGF antibody. Scale bar in C applies to all images: 700 μm for A-C.
Overall survival of RIP-Tag2 mice treated from 14 weeks of age for up to 6 weeks was strongly influenced by the treatment. Median survival of vehicle-treated mice was 14.7 weeks (Figure 7E). Survival was prolonged to 16.1 weeks in the PF-04217903 group, 16.4 weeks in the anti-VEGF antibody group, 17.3 weeks in the group receiving anti-VEGF antibody plus PF-04217903, and more than 20 weeks in the XL184 group (Figure 7E). Strikingly, all mice treated with XL184 survived until the experiment ended at age 20 weeks. One of 6 mice treated with the combination of anti-VEGF antibody and PF-04217903 survived this long, but none of the mice in the other groups reached 20 weeks of age (Figure 7E).
The 20-week old RIP-Tag2 mice treated with XL184 for 6 weeks had small, round, compact tumors. The mean Invasion index of these tumors (5.8), compared to tumors in mice treated from age 14 to 17 weeks, was lower than for vehicle (12.7, P < 0.05) or anti-VEGF antibody (18.9, P < 0.05) and about the same as for XL184 (4.5) (Figure 6G).
None of 5 mice treated with XL184 from age 14 to 17 weeks had detectable liver metastases. Small liver metastases were present in 5 of 6 mice treated with XL184 from age 14 to 20 weeks (0.3 ± 0.2 metastases/mm2), but these were much less numerous than in 17-week old mice treated with anti-VEGF antibody (3.4 ± 1.5 metastases/mm2) or sunitinib (1.3 ± 1.2 metastases/mm2) for 3 weeks (Figures 4E).
To determine whether XL184 had similar effects on tumors in younger RIP-Tag2 mice, we treated mice from age 10 to 14 weeks. As in the older mice, tumors in younger mice treated with XL184 were rounder and less invasive (Supplemental Figure 9, A-E). Grossly visible liver metastases were not found in any of the mice at age 14 weeks, but micrometastases were visible microscopically after staining for SV40 T-antigen (Supplemental Figure 9, F-H). Four times as many micrometastases were found in mice treated with anti-VEGF antibody as with vehicle, but none was found in 5 mice treated with XL184 (Supplemental Figure 9I). Mice treated with XL184 had better overall survival than age-matched mice treated with vehicle or anti-VEGF antibody (Supplemental Figure 9J).
Discussion
The goal of this project was to learn why inhibition of VEGF signaling increases tumor invasiveness and metastasis in some preclinical models (3-6) and whether this form of evasive resistance can be prevented or reversed by inhibition of c-Met. Our experiments in RIP-Tag2 mice confirmed that inhibition of VEGF signaling by a ligand-specific blocking antibody or by the multi-targeted RTK inhibitor sunitinib slowed tumor growth but promoted invasion of tumor cells into the surrounding exocrine pancreas and increased the number and size of liver metastases. These effects accompanied intratumoral vascular pruning, hypoxia, HIF-1α accumulation, c-Met activation, and change to a more mesenchymal tumor cell phenotype. The heightened tumor aggressiveness did not occur when either of two c-Met inhibitors, PF-04217903 or PF-02341066, was co-administered with the anti-VEGF antibody or with sunitinib. XL184, a multi-targeted RTK inhibitor that blocks both c-Met and VEGF signaling, also reduced tumor invasion and metastasis.
We used the RIP-Tag2 multi-stage tumor model, where invasion and metastasis usually occur late in the disease (24, 25), to build on published evidence that inhibition of VEGF signaling by sunitinib, anti-VEGFR-2 antibody (DC101), or genetic deletion of VEGF advances the onset of invasion and metastasis in this model (6). Our findings confirmed this effect of sunitinib and added the selective anti-VEGF antibody #AF-493-NA to the list of agents that promote invasion and metastasis in RIP-Tag2 mice. These findings were corroborated by parallel experiments on orthotopic Panc-1 pancreatic adenocarcinomas (26): c-Met expression and invasiveness of Panc-1 tumors were increased by treatment with sunitinib, and the increase was blocked by concurrent inhibition of c-Met.
The present findings extend an earlier report of reduction in tumor invasion and liver micrometastases in 10-week old RIP-Tag2 mice treated with XL880 for 1 or 2 weeks (19). That report left unresolved whether XL880 has similar efficacy on more aggressive, later stage tumors. Also, because XL880 inhibits multiple RTKs, it was not possible to ascertain whether concurrent inhibition of VEGFR and c-Met was necessary and sufficient to reproduce all of the robust effects on RIP-Tag2 tumors. Nor was it possible to distinguish actions on blood vessels from direct actions on tumor cells.
These open issues led us to use more selective inhibitors of VEGF and c-Met signaling, to study RIP-Tag2 mice at age 14 to 17 weeks when the tumors are more aggressive, and to examine Panc-1 tumors as a second model. The two models cannot reflect all types of cancer, but their similar responses indicate that the anti-invasive action of c-Met blockade was not unique to one model, and highlight the role of c-Met in evasive resistance associated with anti-VEGF therapy. In both models, inhibition of c-Met and VEGF signaling resulted in smaller, ball-like tumors and less invasiveness.
Mechanism of tumor invasion after VEGF inhibition: Hypoxia, HIF-1α, and c-Met
The present experiments provide multiple lines of evidence for a mechanism of tumor aggressiveness after VEGF inhibition that involves hypoxia, HIF-1α, c-Met, and conversion to a more mesenchymal tumor-cell phenotype. Data from other studies of tumor aggressiveness after inhibition of VEGF signaling are also consistent with this mechanism (4-6).
Agents that block the actions of VEGF not only inhibit angiogenesis but also cause in vascular pruning (34, 38). Vascular pruning and resulting hypoxia are well-documented changes in RIP-Tag2 tumors after treatment with inhibitors of VEGF signaling (6, 19, 34). Increases in HIF-1α and staining for pimonidazole, CA-IX, and Glut1 are consistent with these changes.
Intratumoral hypoxia can activate c-Met (7, 8). Treatment with anti-VEGF antibody or sunitinib for only a week was accompanied by 3-fold or greater increase in c-Met mRNA expression and corresponding increases in c-Met protein and c-Met phosphorylation.
The distribution of c-Met changed with treatment. In vehicle-treated tumors, c-Met immunoreactivity was greatest in blood vessels, where it varied from strong to absent. c-Met staining was visible in only a small proportion of tumor cells. Staining for c-Met and for phospho-c-Met was much greater after sunitinib or anti-VEGF antibody. Most of the increase was in tumor cells. Tumor vessel staining for c-Met was more uniform after VEGF inhibitors, apparently because vessels having little or no c-Met were pruned.
An immune response to the goat anti-mouse VEGF antibody could have developed over time. This response could have reduced the potency of VEGF blockade in the immunocompetent RIP-Tag2 mice, but it is unlikely to have been responsible for increased tumor invasiveness and metastasis, because neither was found in mice treated normal goat IgG. Tumors in these mice had the same appearance, same level of c-Met mRNA, and same incidence of liver metastasis as in mice treated with vehicle.
The increase in c-Met mRNA after anti-VEGF antibody or sunitinib was accompanied by greater tumor aggressiveness, but the invasiveness and liver metastasis were more severe after the anti-VEGF antibody. The significance of this is unclear, but the amount of phospho-c-Met, which was increased by both treatments, should be a better indicator of c-Met activation than mRNA. Differences in pharmacokinetics and pharmacodynamics of the full-length anti-VEGF antibody and 398.48 molecular weight sunitinib, and differential effects on other factors that promote tumor aggressiveness should be considered.
Intratumoral hypoxia is associated with greater risk of metastasis and less favorable prognosis (39). Hypoxia increases c-Met expression in tumor cells through HIF-1α binding sites on the c-Met promoter (8). Activation of c-Met drives cell proliferation, motility, and invasion (8, 9). This process fits with evidence that overexpression of c-Met can promote tumor invasion and metastasis (10, 16, 40, 41). It also fits with our observations of mouse tumor models and of hypoxia-induced c-Met phosphorylation in freshly isolated RIP-Tag2 tumor cells in vitro.
Hypoxia-induced activation of c-Met in RIP-Tag2 tumor cells in vitro occurred in the absence of HGF in the culture medium. Similarly, HGF was not detected in tumor cells or blood vessels of RIP-Tag2 tumors and HGF mRNA was unchanged by treatment. Ligand-independent phosphorylation of c-Met can be triggered by interaction with other tyrosine kinase receptors or with other cell-surface proteins (17, 18, 42, 43). Activation of c-Met in RIP-Tag2 tumor cells can also be promoted by reactive oxygen species induced by hypoxia (Wei et al., unpublished observations).
Persistent intratumoral hypoxia associated with inhibition of c-Met and VEGF together could favor escape routes for evasive resistance driven by other pathways activated under these conditions (44, 45). Evasive resistance involves the actions of many factors, particularly when c-Met signaling is inactive or blocked (46-48). Metalloproteases and changes in properties of tumor cells and blood vessels could contribute. Although no significant treatment-related changes were found in expression of ANG1, ANG2, FGF2, IGFR, RON, RET, SKY, or MER, increases were found in AXL and TGF-β. TGF-β signaling promotes EMT, invasion, and metastasis (49) and can influence c-Met activity (50, 51). Elucidation of effects of elevated TGF-β expression on invasion and metastasis after treatment with anti-VEGF antibody is a worthwhile goal for future studies.
Mechanism of invasiveness after VEGF inhibition: Changes in tumor cell phenotype
The increase in c-Met expression in tumors after treatment with anti-VEGF antibody or sunitinib was accompanied by reduction in the epithelial marker E-cadherin and increase in the mesenchymal markers Snail1, N-cadherin, and vimentin. c-Met staining was high in tumor cells near tumor borders where vimentin was strong and E-cadherin staining was weak. The reduction in E-cadherin assessed by western blot was not as great as the decrease evident by immunohistochemistry. This lack of congruence probably resulted from trapping of pancreatic acinar cells within tumors during invasion of the exocrine pancreas. The presence inside tumors of E-cadherin-positive acinar cells would mask the loss of tumor-cell E-cadherin assessed by western blot but not by immunohistochemistry.
E-cadherin is reportedly lost in end-stage RIP-Tag2 tumors (52). Staining for E-cadherin was clearly less in tumors of RIP-Tag2 mice at age 17 weeks than at 10 weeks but was still evident in some tumor cells. This discrepancy probably reflects differences in tissue preparative and imaging methods.
Expression of mesenchymal cell markers in RIP-Tag2 tumors was reduced by administration of PF-04217903 together with anti-VEGF antibody. This change and accompanying rounder tumor cell shape are consistent with reversal of epithelial-mesenchymal transition (EMT) (53). EMT is thought to contribute to the acquisition of a motile phenotype that favors invasion and metastasis (54). In tumor cells undergoing EMT, E-cadherin is lost, the transcription factor Snail1 is activated, and the mesenchymal cell markers N-cadherin and vimentin increase (52, 55). EMT can be promoted by HGF/c-Met signaling and is reportedly involved in tumor progression and resistance (12, 53).
A more mesenchymal phenotype has been reported in some glioblastomas treated with anti-VEGF antibody (56), raising the possibility that anti-VEGF therapy can lead to EMT and a more aggressive phenotype in some human tumors. Additional studies are needed to determine whether invasion and metastasis in RIP-Tag2 mice treated with VEGF inhibitors are manifestations or consequences of EMT.
Effects of c-Met inhibitors on tumor invasiveness and metastasis
Inhibition of c-Met signaling by anti-ligand or anti-receptor antibodies, decoy receptors, or small molecule receptor tyrosine kinase inhibitors has given promising results in preclinical models (19, 57-59) and in clinical trials (30, 31, 35).
We used three complementary approaches to block c-Met signaling. In the first approach, we used PF-04217903 because of its selectivity for c-Met. PF-04217903 is one of the most selective inhibitors currently available, with an IC50 for c-Met > 1000-fold more than any other target tested in overlapping panels of 208 kinases, which included VEGFR-2, PDGFR-β, TIE2, AXL, SKY, RET, and IGF1R (21). This selectivity made PF-04217903 a reasonable choice to combine with the anti-VEGF antibody or with sunitinib. As a second approach, we used PF-02341066, a tyrosine kinase inhibitor that has high potency against c-Met but also blocks anaplastic lymphoma kinase (ALK) (35). PF-02341066 is less selective than PF-04217903 but has a higher tolerance for molecular variation and is less susceptible to mutations in c-Met that attenuate potency (21). In the third approach, we used XL184 as a single agent that simultaneously blocks c-Met and VEGFR among other RTKs (32).
Treatment with either PF-04217903 or PF-02341066 for 3 weeks significantly reduced invasion and metastasis in RIP-Tag2 mice. Tumors had a distinctive ball-like shape, with a smoother contour and fewer trapped acinar cells, and liver metastases were smaller and much less numerous. Only one of 7 mice treated with PF-04217903 had liver metastasis detected by SV40 T-antigen immunoreactivity, compared to 100% of corresponding vehicle-treated mice, which had an average of 20 liver metastases per mouse. Although 3 of 4 mice treated with PF-02341066 had liver metastases, only 4 liver metastases were found per mouse. This difference could reflect the higher c-Met selectivity of PF-04217903 (21). The anti-invasive and anti-metastatic effect of these agents in otherwise untreated older RIP-Tag2 mice fits with a contribution of c-Met activation to aggressiveness of the late stage tumors.
Synergistic effects of inhibiting c-Met and VEGF signaling together
Synergistic effects of the drug combinations were found in both RIP-Tag2 and Panc-1 tumors. Inhibition of c-Met and VEGF together, by administration of PF-04217903 or PF-02341066 together with anti-VEGF antibody or sunitinib, prevented the invasiveness and metastasis found in RIP-Tag2 mice after inhibition of VEGF signaling. These effects were greater than was found with either c-Met inhibitor used alone. Similarly, PF-04217903 administered in combination with sunitinib caused greater slowing of Panc-1 tumor growth and invasion and had greater survival benefit in RIP-Tag2 mice than when given alone. Co-administration of PF-04217903 also blocked the increase in Snail1, N-cadherin, and vimentin found after inhibition of VEGF signaling. The anti-invasive and anti-metastatic actions of PF-04217903 or PF-02341066 appeared to be independent of effects on tumor vessels or hypoxia, as neither agent caused appreciable vascular pruning, staining for pimonidazole, CA-IX, or Glut1, or increase in HIF-1α.
Because of these favorable effects of the drug combinations, we asked whether XL184, which blocks both c-Met and VEGFR (32), had similarly beneficial actions. We found that XL184 reduced invasion and metastasis in RIP-Tag2 mice as much as the drug combinations, and prolonged survival even more. The similarity of effects of XL184 on tumor invasion and metastasis to those of the combinations of more selective single agents argues for c-Met and VEGFR being relevant targets of XL184, even though it also inhibits multiple other RTKs, including KIT, RET, and AXL, albeit with less potency. AXL has been implicated in tumor invasion and metastasis (60).
Treatment with XL184 had a more robust anti-angiogenic action than the combinations of the selective inhibitors. Similarly robust vascular pruning was found with XL880 (19). This could be due to inhibition of other targeted kinases or to differences in pharmacokinetics or pharmacodynamics and differences in the extent or duration of target inhibition in vivo. These findings are consistent with reported effects of XL184, XL880 and E7050 (32, 61, 62).
To test whether concurrent inhibition of c-Met and VEGF signaling could reverse tumor invasion, we compared tumors in RIP-Tag2 mice treated from age 14 to 17 weeks to those in untreated mice at 14 weeks of age (onset controls). In support of reversal of tumor invasion, we found significantly fewer acinar cells trapped inside tumors after treatment with PF-042107903 plus sunitinib or with XL184 as a single agent. XL184 reduced the metastatic burden in the liver to undetectable, which was even lower than in onset controls.
Concurrent inhibition of c-Met and VEGF signaling, either by the combination of anti-VEGF antibody and PF-04217903 or by XL184, substantially prolonged the survival of RIP-Tag2 mice over vehicle or anti-VEGF antibody alone. The greatest survival benefit was with XL184, where all mice survived until the 6-week experiment ended at 20 weeks of age. The mice had small tumors with little invasion and few or no liver metastases.
The present findings and published reports indicate that treatment with inhibitors of VEGF signaling prolongs the survival of RIP-Tag2 mice (4, 6). Although the survival benefit would seem contradictory to greater invasiveness and metastasis in these mice, overall survival reflects the balance of competing effects of treatment on tumor growth, invasion, and metastasis. In the several-week timeframe of the experiments, the beneficial effects of VEGF blockade on tumor growth outweighed the negative effects of greater invasion and metastasis.
Treatment with sunitinib provided greater survival benefit in RIP-Tag2 mice than was found with the anti-VEGF antibody. In our experiments, 80% of mice treated with sunitinib survived from age 14 to 17 weeks, compared to 50% treated with anti-VEGF antibody. The multi-targeted activity of sunitinib is among the contributing factors. Also, sunitinib treatment began at age 12 weeks in an earlier report (6), but anti-VEGF antibody treatment began in our studies at age 14 weeks, when tumors were more advanced. Another factor is that an immune response to the goat anti-VEGF antibody in mice could have reduced VEGF-blocking activity over time.
The synergistic effects of inhibiting c-Met and VEGF signaling together reflect the complementary features of the two pathways as drug targets. Still to be determined in elucidating the underlying mechanism of synergy is whether activation of c-Met signaling in invasive RIP-Tag2 tumors is ligand-independent, as suggested by our in vitro studies of hypoxic tumor cells. Interactions between c-Met and VEGFR could also involve heterodimer formation, kinase transphosphorylation, cross-talk between signaling pathways, or other mechanisms (17, 18, 42, 63).
The hypothesis that c-Met is necessary and sufficient for promotion of tumor aggressiveness by inhibition of VEGF signaling, which we tested by pharmacological approaches, could also be addressed by genetic overexpression or deletion of c-Met (40, 41, 64). From published evidence, c-Met activation would be expected to promote tumor aggressiveness and deletion to have the opposite effect (13-18).
Overall, our findings are consistent with previous evidence of exaggerated tumor invasiveness and metastasis after inhibition of VEGF signaling. Data from studies using the anti-VEGF antibody indicate that the tumor aggressiveness observed previously (4-6) is not dependent on an off-target action of sunitinib or a peculiarity of the anti-VEGFR-2 antibody DC101. If present in humans, this consequence of selective VEGF blockade could contribute to resistance to angiogenesis inhibitors.
Multiple lines of evidence implicate hypoxia, HIF-1α, and c-Met activation in the tumor aggressiveness. Blockade of c-Met and VEGF signaling together – either by a combination of two selective agents or by a single multi-targeted agent - reduced tumor invasion and metastasis, and prolonged overall survival. Concurrent inhibition of the two signaling pathways had the favorable growth-slowing effects of angiogenesis inhibition and vascular pruning without the undesirable consequences of intratumoral hypoxia. Experience with c-Met inhibitors in preclinical models is now being translated into clinical trials. Promising results in metastatic prostate cancer and other tumor types (28, 30, 31) provide optimism that the approach will be useful in the treatment of human cancer.
Methods
Tumor models and treatments
Tumor-bearing RIP-Tag2 transgenic mice (C57BL/6 background) received one of ten treatments for 3 days or 1, 3, or 6 weeks beginning at 14 weeks of age: (i) sterile saline (vehicle, 5 μL/g) daily by gavage; (ii) normal goat IgG (Jackson ImmunoResearch, 150 μg in 50 μL sterile PBS) injected ip three times per week (MWF); (iii) affinity purified, function-blocking goat anti-mouse VEGF antibody (#AF-493-NA, R&D Systems, 150 μg in 50 μL sterile PBS) injected ip three times per week (MWF); (iv) sunitinib (Pfizer, 40 mg/kg in 5 μL/g sterile saline with 0.5% methylcellulose suspension) daily by gavage; (v) PF-04217903 (Pfizer, 30 mg/kg in 5 μL/g sterile saline with 0.5% methylcellulose suspension) daily by gavage; (vi) PF-02341066 (Pfizer, 50 mg/kg in 5 μL/g sterile saline with 0.5% methylcellulose suspension) daily by gavage; (vii) XL184 (cabozantinib, Exelixis, 40 mg/kg in 5 μL/g sterile saline) daily by gavage; (viii) anti-VEGF antibody plus PF-04217903; (ix) sunitinib plus PF-04217903; or (x) sunitinib plus PF-02341066. Agents used in combination were administered as when used alone. Treatment effects on earlier stage tumors were examined in mice treated from age 10 to 14 weeks with vehicle, anti-VEGF antibody, or XL184. Body weight and survival were recorded during the treatment.
Alternatively, luciferase-expressing human Panc-1 tumor cells were implanted in the pancreas of nude mice (Nu/Nu mice, Charles River) (26). When tumors were detectable 2 or 3 weeks after implantation, mice were treated for 3 weeks with vehicle, sunitinib (40 mg/kg), PF-04217903 (30 mg/kg), or sunitinib plus PF-04217903 daily by gavage (5-6 mice/group). Tumor growth was monitored twice a week by bioluminescence imaging (see Supplemental Methods).
All animal procedures were approved by the Institutional Animal Care and Use Committee of UCSF.
Assessment of tumors
At the end of treatment, mice were anesthetized with ketamine (100 mg/kg ip) plus xylazine (10 mg/kg ip), pimonidazole hydrochloride (60 mg/kg, Hypoxyprobe Plus Kit HP2, Chemicon) was injected iv to detect intratumoral hypoxia, and 1 hour later tissues were fixed by vascular perfusion of 1% paraformaldehyde (34). Cryostat sections 80-μm in thickness were stained by immunohistochemistry (34) using combinations of two or three primary antibodies and corresponding secondary antibodies to label endothelial cells (CD31), pericytes (NG2 proteoglycan, ⟨-smooth muscle actin/⟨-SMA), tumor cells (SV40 T-antigen, insulin, luciferase), acinar pancreas (amylase), proliferating cells (phosphohistone H3), apoptotic cells (activated caspase-3), hypoxia (pimonidazole, carbonic anhydrase IX/CA-IX, glucose transporter 1/Glut1), c-Met, phospho-c-Met, HGF, VEGFR-2, VEGFR-3, E-cadherin, vimentin, basement membrane (type IV collagen), and/or type I collagen.
Invasion index was calculated as 1/(4π x area / perimeter2 ) from ImageJ measurements made on digital images of tumors stained for SV40 T-antigen, insulin, or luciferase. The mean value for each treatment group was calculated from the median values for 4-5 mice in the group.
Additional information on methods for staining tumors, for measuring tumor size, vascularity, Invasion index, trapped acinar cells, cell proliferation, hypoxia, metastasis, for isolating tumor cells, and for PCR, immunoblot, and immunoprecipitation are presented in the Supplemental Methods.
Statistical analysis
Experiments done to assess tumor vascularity, hypoxia, and invasion had 5 to 10 mice per group, based on mice that survived until the end of the experiment. Other experiments had 4-8 surviving mice per group. Survival studies began with 5-12 mice per group. Differences among groups were assessed by Student’s t test or ANOVA followed by Fisher’s test for multiple comparisons. Tumor growth was compared by repeated measures ANOVA and survival by the Log-rank (Mantel-Cox) test. P < 0.05 was considered significant. Error bars show means ± SEM.
Supplementary Material
Significance.
This report examines the mechanism of increased tumor aggressiveness after anti-VEGF therapy and presents evidence for roles of vascular pruning, hypoxia, and c-Met activation. The results show that simultaneous inhibition c-Met and VEGF signaling not only slows tumor growth but also reduces invasion and metastasis.
Acknowledgements
We thank Peter Baluk for help with the immunohistochemistry, Katharine Colton, Jeyling Chou, and Debbie Chen for genotyping RIP-Tag2 mice, and Alexis Brumwell, Irene Kwan and Ying Xi for helping with the RIP-Tag2 tumor cell isolation and in vitro determination of effects of hypoxia on c-Met expression.
Grant Support: BS, TI-O, RMN, CWW, SPT, W-KY, DMMcD: NIH grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute, NCI grant CA82923, grants from Exelixis and Pfizer, and funding from AngelWorks Foundation (to DMMcD); YW, HAC: NIH grants HL44712 and CA125564 (to HAC); VB: support of J. Michael Bishop.
Footnotes
Disclosure of Potential Conflicts of Interest: Dana T. Aftab is an employee of Exelixis, Inc. and is holder of company stock or stock options. James G. Christensen is an employee of Pfizer Global Research and Development and is holder of company stock or stock options.
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–14. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature. 2003;423:593–5. doi: 10.1038/423593a. [DOI] [PubMed] [Google Scholar]
- 8.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 9.Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol. 2007;213:316–25. doi: 10.1002/jcp.21183. [DOI] [PubMed] [Google Scholar]
- 10.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 11.Graveel CR, DeGroot JD, Su Y, Koeman J, Dykema K, Leung S, et al. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc Natl Acad Sci U S A. 2009;106:12909–14. doi: 10.1073/pnas.0810403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponzo MG, Lesurf R, Petkiewicz S, O’Malley FP, Pinnaduwage D, Andrulis IL, et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci U S A. 2009;106:12903–8. doi: 10.1073/pnas.0810402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoussoub RA, Dillon DA, D’Aquila T, Rimm EB, Fearon ER, Rimm DL. Expression of c-met is a strong independent prognostic factor in breast carcinoma. Cancer. 1998;82:1513–20. doi: 10.1002/(sici)1097-0142(19980415)82:8<1513::aid-cncr13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Kong DS, Song SY, Kim DH, Joo KM, Yoo JS, Koh JS, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115:140–8. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 15.Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny H, Becker AR, et al. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67:1670–9. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- 16.Lengyel E, Prechtel D, Resau JH, Gauger K, Welk A, Lindemann K, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113:678–82. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 17.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542–51. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Nilsson MB, Saintigny P, Cascone T, Herynk MH, Du Z, et al. Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1alpha in non-small cell lung cancer cells. Oncogene. 2010;29:2616–27. doi: 10.1038/onc.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71:4758–68. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci U S A. 2011;108:5759–64. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timofeevski SL, McTigue MA, Ryan K, Cui J, Zou HY, Zhu JX, et al. Enzymatic characterization of c-Met receptor tyrosine kinase oncogenic mutants and kinetic studies with aminopyridine and triazolopyrazine inhibitors. Biochemistry. 2009;48:5339–49. doi: 10.1021/bi900438w. [DOI] [PubMed] [Google Scholar]
- 22.Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: justification for K-ras-directed therapy. Mol Cancer Res. 2005;3:413–23. doi: 10.1158/1541-7786.MCR-04-0206. [DOI] [PubMed] [Google Scholar]
- 23.Qian LW, Mizumoto K, Inadome N, Nagai E, Sato N, Matsumoto K, et al. Radiation stimulates HGF receptor/c-Met expression that leads to amplifying cellular response to HGF stimulation via upregulated receptor tyrosine phosphorylation and MAP kinase activity in pancreatic cancer cells. Int J Cancer. 2003;104:542–9. doi: 10.1002/ijc.10997. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–22. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 25.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–12. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 26.Beck AW, Luster TA, Miller AF, Holloway SE, Conner CR, Barnett CC, et al. Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer. 2006;118:2639–43. doi: 10.1002/ijc.21684. [DOI] [PubMed] [Google Scholar]
- 27.Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18(Suppl 10):x3–10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- 28.Sampson ER, Martin BA, Morris AE, Xie C, Schwarz EM, O’Keefe RJ, et al. The orally bioavailable met inhibitor PF-2341066 inhibits osteosarcoma growth and osteolysis/matrix production in a xenograft model. J Bone Miner Res. 2011;26:1283–94. doi: 10.1002/jbmr.336. [DOI] [PubMed] [Google Scholar]
- 29.Dulak AM, Gubish CT, Stabile LP, Henry C, Siegfried JM. HGF-independent potentiation of EGFR action by c-Met. Oncogene. 2011 doi: 10.1038/onc.2011.84. doi: 10.1038/onc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–6. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain M, Smith MR, Sweeney C, Corn PG, Elfiky A, Gordon MS, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29(suppl) Abstract 4516. [Google Scholar]
- 32.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 36.Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J Surg Res. 2002;102:39–44. doi: 10.1006/jsre.2001.6318. [DOI] [PubMed] [Google Scholar]
- 37.Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol. 2007;72:152–61. doi: 10.1124/mol.106.029025. [DOI] [PubMed] [Google Scholar]
- 38.Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488–99. [PubMed] [Google Scholar]
- 39.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–34. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Merlino G. Constitutive c-Met signaling through a nonautocrine mechanism promotes metastasis in a transgenic transplantation model. Cancer Res. 2002;62:2951–6. [PubMed] [Google Scholar]
- 42.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–11. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 43.Wang R, Kobayashi R, Bishop JM. Cellular adherence elicits ligand-independent activation of the Met cell-surface receptor. Proc Natl Acad Sci U S A. 1996;93:8425–30. doi: 10.1073/pnas.93.16.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott U, Pusapati RV, Christensen JG, Gray NS, Settleman J. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Res. 2010;70:1625–34. doi: 10.1158/0008-5472.CAN-09-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Janne PA, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. 2011;71:1081–91. doi: 10.1158/0008-5472.CAN-10-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–53. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 47.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chun MG, Mao JH, Chiu CW, Balmain A, Hanahan D. Polymorphic genetic control of tumor invasion in a mouse model of pancreatic neuroendocrine carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:17268–73. doi: 10.1073/pnas.1012705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santibanez JF, Quintanilla M, Bernabeu C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121:233–51. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- 50.Moghul A, Lin L, Beedle A, Kanbour-Shakir A, DeFrances MC, Liu Y, et al. Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-MET transcript. Oncogene. 1994;9:2045–52. [PubMed] [Google Scholar]
- 51.Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Transforming growth factor-beta signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Mol Cancer Res. 2008;6:1521–33. doi: 10.1158/1541-7786.MCR-07-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 53.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 54.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 55.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 56.Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–80. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 57.Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 58.Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006;12:3657–60. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 59.Jin H, Yang R, Zheng Z, Romero M, Ross J, Bou-Reslan H, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–8. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 60.Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107:1124–9. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian F, Engst S, Yamaguchi K, Yu P, Won KA, Mock L, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69:8009–16. doi: 10.1158/0008-5472.CAN-08-4889. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa T, Tohyama O, Yamaguchi A, Matsushima T, Takahashi K, Funasaka S, et al. E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci. 2010;101:210–5. doi: 10.1111/j.1349-7006.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sulpice E, Ding S, Muscatelli-Groux B, Berge M, Han ZC, Plouet J, et al. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101:525–39. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 64.Dai C, Huh CG, Thorgeirsson SS, Liu Y. Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167:429–36. doi: 10.1016/s0002-9440(10)62987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.