Abstract
Human impact on biodiversity usually is measured by reduction in species abundance or richness. Just as important, but much more difficult to discern, is the anthropogenic elimination of ecological interactions. Here we report on the persistence of a long ecological interaction chain linking diverse food webs and habitats in the near-pristine portions of a remote Pacific atoll. Using biogeochemical assays, animal tracking, and field surveys we show that seabirds roosting on native trees fertilize soils, increasing coastal nutrients and the abundance of plankton, thus attracting manta rays to native forest coastlines. Partnered observations conducted in regions of this atoll where native trees have been replaced by human propagated palms reveal that this complex interaction chain linking trees to mantas readily breaks down. Taken together these findings provide a compelling example of how anthropogenic disturbance may be contributing to widespread reductions in ecological interaction chain length, thereby isolating and simplifying ecosystems.
From sea to land, from land to sea; And heave round earth, a living chain of interwoven agency. Goethe's Faust1
The chains of interactions that weave together the constituents of ecosystems are critical to their functioning. Such interaction chains include both trophic (e.g. consumption of prey by predators), informational (e.g. behavioral interactions), and abiotic (e.g. physical transport of nutrients across ecosystem boundaries) linkages that assemble both vertically (e.g. from top to bottom of food webs) and horizontally (e.g. across the boundaries of multiple food webs and habitats).
Alteration or elimination of any of the links in an ecological interaction chain can have deleterious and destabilizing effects on community and ecosystem functioning2,3,4,5. Some of these effects are direct, while others reach indirectly across ecosystems (e.g. change at one node in a chain influences another node via intermediary transmitter nodes6,7). There are many means by which human disturbance can negatively impact the integrity of ecological interaction chains. Species removals, species introductions, habitat conversions, pollution, and climate change can all change or abolish species interactions4,8,9,10,11. Given the sensitivity of these ecological links to anthropogenic change, we hypothesized that ecosystems more insulated from human influence may retain longer chains of ecological interactions that host linkages that bind these ecosystems closer together.
We conducted a focused empirical examination of this hypothesis by investigating the effects of anthropogenic disturbance on ecological interaction chain length at Palmyra Atoll, an especially remote collection of coral islets in the tropical central Pacific. The ecosystems of Palmyra have been much less impacted anthropogenically than many inhabited or heavily used coastal and island regions, but are by no means completely “pristine”12. Palmyra's forests in particular have been altered by intermittent human activities. Historically large and contiguous patches of native forest, comprised principally of the native trees Pisonia grandis and Tournefortia argentea, have been threatened by the human-facilitated expansion of the coconut palm (Cocos nucifera)13,14. This type of anthropogenic disturbance is especially common in the tropics where palms of various species are aggressively cultivated for oil production at the expense of native forests15,16. Today, Palmyra's forests are fractured into discrete patches of well conserved native forest surrounded by dense stands of coconut palm17 (Supplementary Fig. S1 and S2 online). This mosaic of recently disturbed habitats intermingled with generally more intact habitats provides a rare opportunity to test (by contrasting patterns in native and palm forests, Figure 1A) if and how human disturbance impacts interaction chain length.
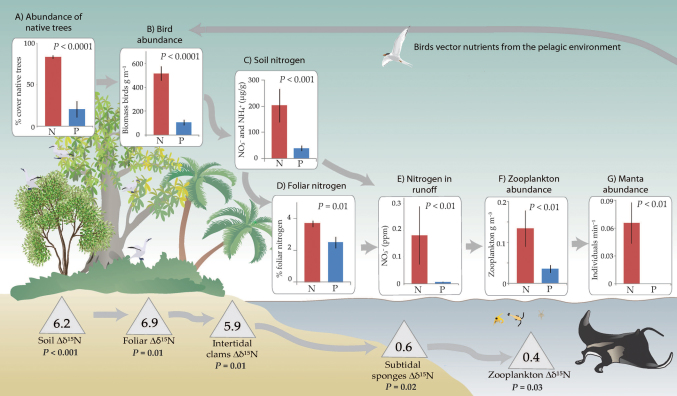
Figure 1. Description of a long interaction chain linking forests to manta rays.
This chain is retained in less disturbed native forest but its integrity is compromised in human-altered palm forest. Bar graphs comparing processes in native (N) and palm (P) forests (mean ± SE) indicate that reductions in native tree abundance (A) reduce seabird abundance (B), which diminish the contribution of seabird derived nutrient subsidies to terrestrial ecosystems (C,D), which severely impair the movement of nutrients to the marine environment (E), reducing zooplankton abundance (F), and ultimately eliminating manta ray (Manta birostris) utilization of native forest coastlines (G). Delta values depict the difference between mean δ15N of native forest and palm forest material (Δδ15N = δ15NN − δ15NP). Positive delta values measured at multiple points along this lengthy interaction chain reveal that taxa in native forest zones are causally linked to one another via dependency upon isotopically elevated seabird derived nutrients.
Results
We initiated this comparison by examining how seabird utilization of forest canopies (e.g. as nesting and roosting habitat) differs in patches classified as either native or palm forest. Surveys of seabirds indicate that they show a strong preference for the complex and stable canopies of native forests, and a strong aversion for the simple and mobile canopies of palm forests. Resultant densities of seabirds in native forests are 4.8 times higher than palm forests (Figure 1B, Table 1). These divergent patterns of bird use have important effects on forest biogeochemistry. Soils in native forests are significantly elevated in plant available nitrogen—the likely limiting nutrient in this ecosystem (5.1 times higher than palm forests; Figure 1C, Table 1). It has been previously established that these changes in soil properties were caused by the importation and concentration of oceanic-derived nutrients in preferred nesting/roosting areas (e.g. guano) by these wide-ranging birds18 and that these changes do not pre-date forest alteration14. Plants resident in native forests capitalize on these elevated nutrient levels, as is reflected by increased levels of foliar nitrogen in these sites (Figure 1D).
Table 1. Comparisons of processes in less disturbed native forests to those in more altered palm forests. Paired comparisons (pooled and analyzed by date) were made for all responses that were sampled repeatedly over time *. Na represents the total number of measurements conducted. Nb (unpaired comparisons only) represents the number of replicates included in all statistical analyses after measurements were pooled at the level of transect for analysis. Nc (paired comparisons only) represents the number of temporal comparisons included in analyses where repeated sampling was conducted over time. Parametric or nonparametric (†) test statistics are reported for each comparison. Data collection was distributed equally between native and palm forests.
| Native forests | Palm forests | ||||||
|---|---|---|---|---|---|---|---|
| Mean (± SE) | Mean (± SE) | t or W† (df) | P | Na | Nb | Nc | |
| % cover native trees | 83.6 (± 2.0) | 20.5 (± 9.6) | 10.6 (8) | <0.0001 | 10 | 10 | - |
| Bird biomass (g m−1 coastline) | 515.7 (± 60.2) | 107.1 (± 22.8) | 9.5 (8) | <0.0001 | 46 | 10 | - |
| Soil nitrogen (NO3− and NH4+; μg/g) | 201.5 (± 63.0) | 39.7 (± 10.5) | 3.6 (23) | <0.001 | 25 | 25 | - |
| % foliar nitrogen | 3.7 (± 0.2) | 2.5 (± 0.4) | 3.0 (12) | 0.01 | 14 | 14 | - |
| Runoff water nitrogen (NO3−; ppm) * | 0.18 (± 0.1) | 0.01 (± 0.001) | 4.1 (7) | <0.01 | 51 | - | 8 |
| % change in Chl a * | 372.5 (± 96.3) | 291.9 (± 102.1) | 5.0 (4) | <0.01 | 47 | - | 5 |
| Zooplankton biomass (g m−3) * | 0.13 (± 0.04) | 0.04 (± 0.01) | 4.0 (7) | <0.01 | 77 | - | 8 |
| Copepod length (mm) * | 1.03 (± 0.03) | 0.97 (± 0.03) | 2.2 (8) | 0.06 | 4,970 | 9 | |
| No. individual mantas (survey min−1) *† | 0.07 (± 0.02) | 0 (± 0) | 105 | <0.01 | 196 | - | 21 |
The influence of birds on forests extends beyond the boundaries of the terrestrial ecosystem. Water running off nutrient rich native forest islets into the marine environment carries with it 26.5 times higher loads of nitrogenous compounds than runoff from palm forests (Figure 1E). Data from moored, in-situ, phytoplankton growth chambers showed that relative changes in chlorophyll a (Chl a) were significantly higher in growth chambers situated along native forest coastlines, suggesting that nutrient additions in these zones may stimulate phytoplankton productivity (Table 1). In these same waters adjacent to native forests zooplankton are greater in biomass (Figure 1F) and certain zooplankton taxa (Copepoda) are larger (Table 1). Differences in the foraging ecology and behavior of a large and conspicuous obligate plankton consumer, the giant manta ray (Manta birostris), were also detected in native forest regions. In extensive visual surveys of mantas we documented that they are significantly more abundant along native forest coastlines than along palm forest coasts with similar bathymetry and morphology (Figure 1G; Supplementary Fig. S3 online). As a supplement to these visual surveys of mantas, we electronically tagged three adult individuals and tracked their movements. Tracking data showed the same pattern as visual surveys: mantas can and do range across the entire lagoon basin—but when they elect to use coastlines, tagged animals exclusively selected areas near native forests. We observed 86.4%, 78.4%, and 43.9% overlap of individual manta core use areas with native forest coastline. No overlap was observed by any of these animals with palm forest coastline.
The elevated nitrogen (N) isotope levels of seabird-derived nutrients (which result from the high trophic position of these predators) provide a convenient means for examining whether this complex string of ecological connections are causally connected to one another19,20. Consistent with the hypothesis that manta rays are interactively linked to native forests via changes triggered by alterations in forest and seabird communities, we measured significantly higher δ15N levels (indicative of utilization of seabird derived nutrients) at five key nodes in this long-range interaction chain (Figure 1; Table 2). The strength of this seabird isotope signal attenuates with increasing distance from the origin of this interaction chain (forests), as would be expected as a result of dilution in these aquatic systems, but is still discernable along native forest coastlines all the way out to zooplankton.
Table 2. Comparisons of the δ15N of materials associated with native and palm forests. Differences in zooplankton δ15N were evaluated using paired comparisons (pooled by date; indicated with *) owing to their rapid turnover. All other parameters were compared using unpaired tests. Na represents the total number of measurements conducted. Nb (zooplankton only) denotes the number of temporal comparisons conducted. Sampling was evenly split between native and palm forest sites.
| Native forests Mean (± SE) | Palm forests Mean (± SE) | t (df) | P | Na; Nb | |
|---|---|---|---|---|---|
| Soils | 16.6 (± 0.9) | 10.4 (± 1.1) | 4.4 (16) | <0.001 | 18 |
| Tree leaves (T. argentea) | 15.4 (± 1.3) | 8.3 (± 2.3) | 2.6 (12) | 0.01 | 14 |
| Intertidal clams (Macoma dispar) | 11.0 (± 0.9) | 5.9 (± 0.6) | 4.6 (11) | 0.01 | 13 |
| Subtidal sponges (Spirastrella sp.) | 11.0 (± 0.1) | 10.4 (± 0.2) | 2.4 (31) | 0.02 | 46 |
| Zooplankton* | 11.3 (± 0.2) | 10.9 (± 0.2) | 3.0 (5) | 0.03 | 46; 6 |
Discussion
Ecological interaction chains in the native forests of Palmyra connect processes in these forests to the ecology of manta rays through a diverse series of trophic, non-trophic (behavioral), and physical mechanisms. Native trees provide needed nesting/roosting habitat for seabirds and thus help to maintain high local abundances of seabirds. These seabirds vector large quantities of marine-derived materials into the nutrient impoverished atoll terrestrial communities defining biogeochemical patterns of both plants and soils in native forest areas. Many of the nutrients concentrated in these native forests are returned to the adjacent oligotrophic ocean waters via rain and tidal vectoring. Sampling of the plankton communities directly along these native forest coastlines revealed that phytoplankton are more productive, zooplankton are more abundant, and key zooplankton taxa achieve larger sizes. The most parsimonious explanation for these observed patterns in the plankton is that they are responding to the forest-facilitated, seabird-vectored nutrient additions. The last key observation that we made in these native forest associated habitats was that manta rays, which feed exclusively on plankton, were more abundant and active along these plankton rich native forest coasts. While manta rays are wide ranging animals, this attraction to and persistence along these native forest coasts represents an important and unexpected link between their foraging ecology and forest dynamics. The detectable presence of seabird-derived δ15N materials in terrestrial, intertidal, subtidal, and pelagic organisms situated along this interaction pathway provides compelling support that this is indeed a unified long chain of dependant interactions. Sampling of other potential N sources in this system has revealed no evidence of alternative allochthonous or autochthonous origin materials which could have otherwise created these δ15N patterns14.
This series of connections defines one of the longest ecological interaction chains yet observed in nature20,21,22,23. Other work has demonstrated that the majority of species involved in ecological interactions are only two links apart24. The interaction chain linking trees to manta rays in Palmyra's native forests is at least five linkages long. The circuitous architecture of this particular interaction chain is as noteworthy as its length. This interaction presents an interesting route through which oceans affect change on land, and changes on land can feed back to influence ecological processes in the oceans (Figure 1). Reports of unidirectional transboundary ecological connections have garnered much attention25,26 – but this example of a complex bi-directional interaction adds to our understanding of the degree to which ecosystems can be interconnected. This interaction includes an interesting mix of both top-down (i.e. loss of birds affects plant and soil ecology) and bottom-up ecological effects (i.e. increases in bird-derived nutrients appears to increase plankton abundance). Instances of complex top-down and bottom-up interactions may be quite common in nature, but good empirical examples of these dynamics are as yet still emerging27.
We posit that this long interaction chain present in and near the native forests of Palmyra is maintained by the relative lack of human disturbance in the better protected parts of this unusually remote site. Data collected from our altered palm sites support this conclusion by demonstrating that forest alteration severely degrades the efficacy of this series of interactions (Figure 1). The corruption of these interactions very likely has a major negative affect upon the strength of the cross-taxonomic and cross-system connections that they supported. Observations from other systems suggest that many complex ecological interaction chains and associated sources of connectivity may be similarly vulnerable to anthropogenic perturbation. The introduction of non-native predators to Aleutian Islands caused the disintegration of ecologically important sea to land nutrient connections20. Intense increases in nutrient inputs associated with human sewage and agriculture contributed to the collapse of the biologically, structurally, and interactively complex coral reef communities in Kaneohe Bay, Hawaii28. Overfishing of salmon in the Pacific Northwest USA compromised the transfer of nutrients from marine to freshwater ecosystems affecting terrestrial plant and animal communities in a variety of ways26,29.
While numerous other such examples exist, anthropogenic disturbances should not universally be expected to cause contractions in ecological interaction chain length or reductions in system connectivity. The character of the disturbance in question as well as the properties of the recipient system will both determine the final effects that human change has on networks of ecological interactions. However, because many sources of anthropogenic change have the effect of rapidly altering the overall and relative abundance of particular species, directly removing species, introducing species foreign to established ecological interaction networks, eliminating habitat, and changing the physical and chemical properties of local environments – we argue that human-induced constrictions or eliminations of ecological interaction series are likely to have occurred and be occurring much more commonly than is presently appreciated.
Recognizing the effects on anthropogenic activities on ecological interaction chains is more difficult than documenting more tangible disturbance effects (e.g. species extinctions or introductions) because interactions between species and ecosystems do not fossilize and leave little material evidence behind to chronicle their disappearance. However, observations made in more-intact ecosystems, such as those reported herein, help bring these losses in interaction chain length to light and highlight the implications that this type of environmental change may be having upon ecosystem connectedness. Sustained investigation of our remaining uniquely pristine environments will help to extend our understanding of the ubiquity and importance of this intangible, but potentially important type of shifting baseline.
Methods
Study Site
Data collection took place at Palmyra Atoll (5° 52′ N, 162° 04′ W; principally in the eastern lagoon basin) from 2009–2010. Palmyra is located in the Northern Line Islands in the central Pacific. The 12 km long atoll is composed of a series of small islets that are all composed of coral-derived materials. Islets encircle an interior saltwater lagoon system. The lagoons of Palmyra have been characterized as having a well-mixed surface layer that is dynamically influenced by the wind and tides which overlays a relatively static and stable deep water pool30. Palmyra is presently protected as a National Wildlife Refuge by the United States Fish and Wildlife Service.
Experimental procedures and field measurements
We established ten spatially independent, 240 m transects in lagoons of the atoll running parallel to islet coastlines (Supplementary Fig. S4 online). Transects were spaced approximately 500 m apart from one another. These transects defined the areas where all sampling for this project was conducted. The forest type associated with each transect was determined from satellite images by analyzing tree canopy cover (ArcGISv.10; Google Earth Pro) in a 20 m swath of coastline enclosed within a 200 m radius area extending from each transect midpoint. These dimensions were selected so as include sections of forest that might be reasonably assumed to influence lagoon transects (e.g. via runoff). The diagnostic morphology of C. nucifera canopies enables accurate classifications to be made of forest type from satellite imagery (Supplementary Fig. S2 online). Transects were defined as either “native forest” when native tree cover (i.e. P. grandis, T. argentea, and Scaevola sericea; n = 5) was >75%; or “palm forest” when native tree cover was <75% (i.e. predominantly C. nucifera; n = 5); these classifications constructed following natural breaking points observed in frequency distribution histograms of C. nucifera abundance31. While individual native trees were present in transects classified as palm forest, their abundance was (in aggregate) approximately four times lower than in native forest transects (Fig. 1A). The precise distribution of forest composition in all transects is depicted in Supplementary Table S1 online. All transects are located on sites that share a common geologic origin, are composed of largely the same base substrate type, are exposed to the same climatic forces, and are the same elevation14. Measurements of the surface seawater temperature and salinity reveal that differences in both factors between native and palm forests were significant, but extremely small; and that variation was greater within sites than between sites (Supplementary Methods S1 online). There is no known pattern that explains the proliferation of palms at certain sites and it is presumed that their current distribution is the result of anthropogenic forest alternation, dispersal subsequent to these disturbances, and endogenous ecological processes that control tree establishment17.
Biomass of birds m−1 was calculated by counting seabirds on foot and boat along 10 m swaths of transect associated coastline and multiplying bird abundance by species specific mass conversion factors32. We measured biologically relevant nitrogen compounds in soils (plant available NO3− and NH4+, µg g−1), plant leaves (%N), and runoff water (NO3−, ppm) in native and palm forest coastal zones associated with transects. Replicate surface soil (0–15 cm depth) and leaf samples (mature, nonsenescent leaves in full sun from T. argentea) were collected on all transects. Sieved soils were KCl extracted and analyzed using a discrete analyzer (Westco SmartChem 2000). Leaf samples (washed, dried, and milled) were analyzed on an elemental analyzer (Carlo-Erba). Runoff water was collected in acid washed containers at high water line during high tide or after a rain event on transects. All water samples were collected within 30 min of one another. Water was frozen and analyzed on the discrete analyzer (WestCo). Zooplankton communities and manta abundance were sampled in surface waters along all lagoon transects. Zooplankton was collected in timed tows using 350µm mesh nets. We estimated zooplankton biomass (total dry weight; standardized by flow volume) and mean size of the most abundant zooplankton taxa (copepods, species pooled; subsampled at 5% dilution) in each tow. Observers in anchored boats measured manta abundance along all transects using surface point counts (10 m radius; 10 min; total 196 surveys conducted).
We tagged 3 mantas encountered during haphazard lagoon searches with acoustic transmitters (Vemco) and followed each continuously for 48 h. Two of these individuals were tagged outside of established tracking areas along palm forest coastlines and one individual was tagged in a native forest transect. Positional data is obtained using this technology by following animals in a small vessel using a hydrophone and recording their location with GPS every 5 minutes33. This permits high precision determinations of animal location (5–15 m error). These tracking data were used to establish each manta's core use area (defined by convention as their 50% kernel utilization distribution, KUD)34. We then examined overlap between 50% KUD areas and 100 m wide buffers drawn along the contours of all lagoon transects. Animal movement outside of an area of this size was assumed to be unrelated to coastal features.
Relative changes in Chl a concentrations (used as a proxy for primary productivity) were monitored on a subset of transects by entraining known quantities of phytoplankton in transparent acrylic growth chambers fitted with 1μm, 142 mm diameter polycarbonate membranes35. Percent changes in Chl a were fluorometrically determined36 relative to starting concentrations after 3 days. Replicate temporal measurements were made during the duration of the study of runoff water, zooplankton biomass/size, manta visual surveys, and Chl a concentrations (Table 1).
Isotopic analysis
To examine whether mechanistic ties existed between the components in this proposed interaction chain, we measured the δ15N isotope values of soils, tree leaves (T. argentea), intertidal clams (Macoma dispar), subtidal sponges (Spirastrella sp.), and zooplankton in both native and palm forests transects/coastal areas (Thermo Finnigan Delta-plus IRMS/Carlo Erba elemental analyzer). Comparisons of manta ray δ15N could not be conducted given the rarity of mantas along palm forest coastal transects (Fig. 1G). Sample sizes for each taxa measured were contingent on sampling regime and subject availability (Table 2). Isotopic sampling was distributed evenly across our ten forest transects.
Data analysis
Statistical comparisons between data collected in both forest types were made in Program R37. All samples that were measured repeatedly over time were compared using paired (by date) t-tests as these measures were subject to high temporal variability. Other response variables were compared using Welch t-tests or, when assumptions for parametric tests were not met, Wilcoxon nonparametric tests (manta point count surveys only).
Author Contributions
DJM, PAD, HSY designed and performed research, analyzed data, and wrote the paper. RBD, RD, MMM, and FM designed research, analyzed data, and wrote the paper.
Supplementary Material
Supplementary information
Acknowledgments
For logistical support and research permission we thank the US Fish and Wildlife Service and the Nature Conservancy. Funding was provided by the National Science Foundation, the Woods Institute for the Environment, Stanford University, Sigma Xi, the Explorers Club Exploration Fund, and the National Geographic Society. For helpful advice we thank S. Palumbi, J. Estes, P. Vitousek, and K. Arrigo. Images in Fig. 1 were obtained from IAN image library38.
References
- Goethe J. W. Goethe's Faust, Translated J. Anster, (London, UK, 1887). [Google Scholar]
- Janzen D. H. Deflowering of Central-America. Nat. Hist. 83, 48–53 (1974). [Google Scholar]
- McCann K. Protecting biostructure. Nature 446, 29–29 (2007). [DOI] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J. & Wardle D. A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (2008). [DOI] [PubMed] [Google Scholar]
- Bascompte J. Disentangling the web of life. Science 325, 416–419 (2009). [DOI] [PubMed] [Google Scholar]
- Wooton J. T. The nature and consequences of indirect effects on ecological communities. Ann. Rev. Ecol. Syst. 25, 443–466 (1994). [Google Scholar]
- Abrams P. A., Menge B. A., Mittelbach G. G., Spiller D. A. & Yodzis P. The role of indirect effects in food webs. In:: Food webs: integration of patterns and dynamics (Ed. by Polis G. & Winemiller K.). Pp. 371–395. London, UK: Chapman and Hall, (1996). [Google Scholar]
- Memmott J., Waser N. M. & Price M. V. Tolerance of pollination networks to species extinctions. P. Roy. Soc. B-Biol. Sci. 271, 2605–2611 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis J. M., Tscharntke T. & Lewis O. T. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445, 202–205 (2007). [DOI] [PubMed] [Google Scholar]
- Aizen M. A., Morales C. L. & Morales J. M. Invasive mutualists erode native pollination webs. Plos Biol. 6, 396–403 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovia-Scott J., Spiller D. A. & Schoener T. W. Effects of experimental seaweed deposition on lizard and ant predation in an island food web. Science 331, 461–463 (2011). [DOI] [PubMed] [Google Scholar]
- Collen J. D., Garton D. W. & Gardner J. P. A. Shoreline changes and sediment redistribution at Palmyra Atoll (Equatorial Pacific Ocean): 1874-Present. J. Coastal Res. 25, 711–722 (2009). [Google Scholar]
- Dawson E. Y. Changes in Palmyra atoll and its vegetation through the activities of man 1913–1958. Pac. Nat. 1, 1–51 (1959). [Google Scholar]
- Young H. S., McCauley D. J., Dunbar R. B. & Dirzo R. Plants cause ecosystem nutrient depletion via the interruption of bird-derived spatial subsidies. Proc. Natl. Acad. Sci. USA 107, 2072–2077 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara N. T. & Nair P. K. R. Agroforestry in the South Pacific region – an overview. Agroforest. Syst. 3, 363–379 (1985). [Google Scholar]
- Koh L. P., Miettinen J., Liew S. C. & Ghazoul J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl. Acad. Sci. USA 108, 5127–5132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. S., McCauley D. J., Guevara R. & Dirzo R. Density-dependent seed and seedling predation effects on plant diversity depend on scale. J. Ecol. (In review). [Google Scholar]
- Young H. S. et al. Resource partitioning by species but not sex in sympatric boobies in the central Pacific Ocean. Mar. Ecol. Prog. Ser. 403, 291–301 (2010). [Google Scholar]
- Mizutani H. & Wada E. Nitrogen and carbon isotope ratios in seabird rookeries and their ecological implications. Ecology 69, 340–349 (1988). [Google Scholar]
- Croll D. A., Maron J. L., Estes J. A., Danner E. M. & Byrd G. V. Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961 (2005). [DOI] [PubMed] [Google Scholar]
- Estes J. A., Tinker M. T., Williams T. M. & Doak D. F. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476 (1998). [DOI] [PubMed] [Google Scholar]
- Hebblewhite M. et al. Human activity mediates a trophic cascade caused by wolves. Ecology 86, 2135–2144 (2005). [Google Scholar]
- Knight T. M., McCoy M. W., Chase J. M., McCoy K. A. & Holt R. D. Trophic cascades across ecosystems. Nature 437, 880–883 (2005). [DOI] [PubMed] [Google Scholar]
- Williams R. J., Berlow E. L., Dunne J. A., Barabasi A. L. & Martinez N. D. Two degrees of separation in complex food webs. Proc. Natl. Acad. Sci. USA. 99, 12913–12916 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polis G. A. & Hurd S. D. Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 147, 396–423 (1996). [Google Scholar]
- Hocking M. D. & Reynolds J. D. Impacts of salmon on riparian plant diversity. Science 331, 1609–1612 (2011). [DOI] [PubMed] [Google Scholar]
- McCauley D. J., Keesing F., Young T. P., Allan B. F. & Pringle R. M. Indirect effects of large herbivores on snakes in an African savanna. Ecology 87, 2657–2663 (2006). [DOI] [PubMed] [Google Scholar]
- Hunter C. L. & Evans C. W. Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bull. Mar. Sci. 57, 501–515 (1995). [Google Scholar]
- Schindler D. E. et al. Pacific salmon and the ecology of coastal ecosystems. Front. Ecol. Environ. 1, 31–37 (2003). [Google Scholar]
- Gardner J. P. A., Garton D. W. & Collen J. D. Near-surface mixing and pronounced deep-water stratification in a compartmentalized, human-disturbed atoll lagoon system. Coral Reefs 30, 271–282 (2011). [Google Scholar]
- Young H. S., Raab T. K., McCauley D. J., Briggs A. A. & Dirzo R. The coconut palm, Cocos nucifera, impacts forest composition and soil characteristics at Palmyra Atoll, Central Pacific. J. Veg. Sci. 21, 1058–1068 (2010). [Google Scholar]
- Spear L. B., Ainley D. G. & Walker W. A. Foraging dynamics of seabirds in the eastern tropical Pacific Ocean. J. Avian Biol. 35, 1–99 (2007). [Google Scholar]
- DeSalles P. A. et al. Multiple tools reveal the reliance of mobile species on sensitive habitats: a case study of manta rays (Manta alfredi) and lagoons. (In review).
- Worton B. J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168 (1989). [Google Scholar]
- Furnas M. J. An evaluation of 2 diffusion culture techniques for estimating phytoplankton growth rates in situ. Mar. Biol. 70, 63–72 (1982). [Google Scholar]
- Holm-Hansen O., Lorenzen C. J., Holmes R. W. & Strickland J. D. H. Fluorometric determination of chlorophyll. J. Cons. Int. Explor. Mer. 30, 3–15 (1965). [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.ISBN 3-900051-07-0, URL http://www.R-project.org) (2010)
- Saxby et al. IAN Image Library (ian.umces.edu/imagelibrary/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information