Abstract
Objective. Recent advances in molecular techniques have revealed that there is bi-directional transfer of cells between mother and child during pregnancy, and the presence of a mother's cells in her child has been termed maternal microchimerism (MMc). There is the potential for maternal cells to provoke inappropriate immune responses in the child, which could be a factor in autoimmunity including JDM. The aim of this study was to determine whether maternal (female) cells could be detected in frozen muscle sections from seven males (age range 3–13 years) with JDM participating in the Juvenile Dermatomyositis National (UK and Ireland) Cohort Biomarker Study and Repository for Idiopathic Inflammatory Myopathies and sections of muscle controls (age range 2–12 years).
Methods. At least 1000 cells from each section underwent FISH and confocal imaging through each nucleus. Concomitant IF for CD45 was used to determine whether MMc in muscle were lymphocytes. A non-parametric Mann–Whitney U-test was used to detect statistical differences.
Results. The frequency of MMc was higher in JDM muscle (0.42–1.14%) than in controls (0.08–0.42%) P = 0.01. No CD45+ MMc were observed.
Conclusion. These data confirm an increased frequency of MMc in JDM. More detailed characterization of MMc is required, particularly using phenotypic markers, to explain the role of these cells in JDM.
Keywords: maternal microchimersim, juvenile dermatomyositis, autoimmunity
Introduction
It is now well established that cells cross the feto–maternal interface in both directions with relative efficiency; maternal cells engraft in fetal tissue and vice versa [1, 2]. Persistence of the mother's cells in her offspring is termed maternal microchimerism (MMc), whereas persistence of fetal cells in the mother is termed fetal microchimerism (FMc). Both microchimeric states have persisted for decades [3, 4], but it is unclear what role these cells might have in health and disease. From an immunological perspective, it is intriguing that the engrafting fetal or maternal cells are tolerated despite sharing only 50% genetic identity with the surrounding cells. There are some clinical observations to suggest that these cells may play a role in susceptibility to autoimmune disease [5, 6], supporting the theories that (i) the initial trigger in autoimmunity may be alloimmune rather than autoimmune [7] or (ii) autoimmunity is associated with a breakdown in tolerance to microchimerism.
Some autoimmune diseases such as JDM and scleroderma share features with chronic graft vs host disease (cGvHD), a complication of bone marrow transplantation. Susceptibility to cGvHD is associated with incompatibility between the HLA genes expressed by the invading population from the donor and those of the recipient [8]. Given that most autoimmune diseases like JDM occur in subgroups of the population carrying certain HLA genes, it is reasonable to argue that HLA-incompatible alleles from the invading microchimeric population may cause a similar reaction in these genetically susceptible individuals. Indeed, there are now increasingly robust data supporting a role for microchimerism in JDM [9–11].
JDM is a multisystem autoimmune disease that results from inflammation of the small vessels of the muscle, skin, gastrointestinal tract and other organs. The incidence of JDM has been reported to be between 2 and 3/1 000 000 per year in children under 16 years of age [12], with an excess in females. Although significant progress has been made in the treatment of children with JDM, particularly using steroids, secondary effects can result in growth delay and osteopenia [13]. The underlying disease mechanisms are still poorly understood, but better understanding of the aetiology of the disease could lead to more targeted therapeutic strategies than broad-spectrum steroids. Involvement of the immune system in JDM has been demonstrated by the presence of lymphocytic and phagocytic infiltrates in muscle biopsies from children with JDM [14] and association with the HLA Class II allele DQA1*0501 [15]. Studies over the last decade investigated MMc in JDM: maternally derived chimeric cells were shown to be present more often in muscle biopsies from children with JDM compared with controls [9, 10]. Analysis of whole-blood DNA by quantitative polymerase chain reaction demonstrated increased MMc in children with JDM compared with their healthy siblings [10, 11, 16]. Further, in unrelated healthy offspring, the presence of chimerism was shown to be associated with HLA genotype of the mother, in particular HLA DQA1*0501 [16]. In the same study, experiments on peripheral blood lymphocytes showed that maternally derived microchimeric cells are activated when exposed to the JDM child's lymphocytes, indicating that MMc is immunologically active and may play a direct role in the disease process [16]. The aim of this project was to use state-of-the-art confocal imaging to determine whether MMc could be detected in muscle biopsies from children participating in the Juvenile Dermatomyositis Cohort Biomarker Study and Repository (UK and Ireland) (JDCBS).
Methods
JDM tissue samples
A total of 220 children with JDM were recruited prospectively while 87 diagnosed children were recruited retrospectively to the JDCBS. Recruitment and structure of the study were as described [17]. As well as detailed clinical data, DNA, peripheral blood lymphocytes and muscle biopsies are stored for analysis. As JDM predominantly affects females, muscle biopsy sections were available from seven males with JDM to allow identification of maternal cells using X chromosome-specific FISH.
Control tissue samples
Four control muscle sections were obtained: one from Professor Seth Love, Pathologist North Bristol Trust, from a 12-year-old boy with normal muscle pathology. The remaining three control sections were obtained from the JDCBS control cohort [18]. All three had been reported as normal by two independent expert pathologists. None of the four controls had any known muscle disease.
Ethical approval
Full written consent according to the Declaration of Helsinki was obtained for all samples (MREC/1/3/22) and appropriate local ethical approval was obtained for all tissues (National Research Ethics Service 04/Q2002/35).
Strategy
Our approach was first to stain the X and Y chromosome using FISH and then to carry out concomitant IF to identify the antigen(s) of interest. MMc cells were identified, quantified and characterized using confocal microscopy.
FISH with concomitant IF
Fresh frozen tissue sections of 7 μm (stored at −80°C) were fixed in acetone and air dried prior to a dehydration series. The FISH probes (Vysis CEP X Spectrum Orange/Y SpectrumGreen Direct Labelled Fluorescent DNA Probe, Abbott Molecular Inc., Des Plaines, IL, USA) were prepared according to the manufacturer's recommendation, and then applied on the tissue sections. DNA was denatured at 73°C for 10 min and then renatured with FISH probes by incubating overnight at 42°C. The following day, after post-hybridization washes, IF was started. Non-specific binding was blocked by incubating the slides with normal blocking serum for 60 min. Subsequently, slides were incubated with primary antibody (CD45-Mouse monoclonal; Dako UK Ltd, Ely, UK) for 4 h at 37°C, washed with 1× PBS/0.05% Tween-20, and then incubated with fluorescent secondary antibodies for 2 h at 37°C. For negative control slides, primary antibody was replaced by normal blocking serum. Sequential staining was performed for labelling double antigens. The sections were then dehydrated through an ethanol series, air dried and mounted with 4′,6-diamidino-2-phenylindole (DAPI) counterstain and mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA).
Analysis
All sections were analysed under a Leica SP5-AOBS confocal laser scanning microscope attached to a Leica DM I6000 inverted epifluorescence microscope. Images were taken by using a 63× magnification (oil) lens and 2× digital zoom. Cells were analysed by assigning them to one of four categories: X, Y, XY or XX. Video images are available. The FISH success rate (frequency of nuclei with both the X and Y chromosome observable) was determined in each sample. A Mann–Whitney non-parametric U-test was used to determine statistical differences in MMC frequency between the JDM and control groups.
Results
MMc identification in JDM and control tissue
MMc were identified by three colour fluorescence confocal imaging through individual nuclei as shown in Fig. 1. More than 1000 cells were counted in each sample. The results obtained are shown in Table 1.
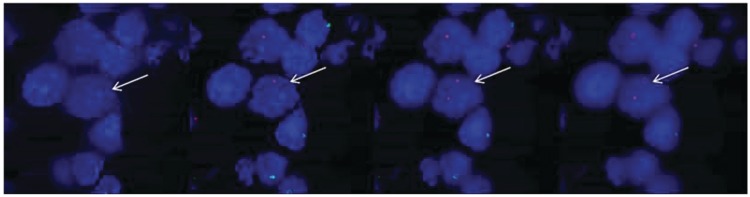
Fig. 1.
Confocal imaging through a maternal cell (highlighted by arrow). The X chromosome is indicated by a red dot and the Y chromosome by a green dot. The nucleus is stained blue with DAPI. Imaging through this maternal cell, 2 X chromosomes (and no Y chromosomes) are observed.
Table 1.
Subject characteristics, FISH success rate and MMc frequency in individuals with maternal microchimerism and controls
| Sample | Age at diagnosis | Age at biopsy | Total cell count | FISH success rate, % | Number of MMc by confocal imaging | MMc frequency |
|---|---|---|---|---|---|---|
| JDM 1 | 13 years 4 months | 13 years 5 months | 1242 | 84 | 10 | 0.81 |
| JDM 2 | 9 years 11 months | 10 years 1 months | 1052 | 78 | 12 | 1.14 |
| JDM 3 | 4 years 7 months | 4 years 9 months | 1113 | 84 | 9 | 0.81 |
| JDM 4 | 5 years 8 months | 6 years 3 months | 2362 | 87 | 10 | 0.42 |
| JDM 5 | 3 years 11 months | 4 years | 1518 | 80 | 16 | 1.05 |
| JDM 6 | 2 years 10 months | 3 years 11 months | 1128 | 76 | 10 | 0.89 |
| JDM 7 | 4 years 2 months | 4 years 3 months | 1357 | 87 | 12 | 0.88 |
| Control 1 | NA | 12 years | 2618 | 80 | 2 | 0.08 |
| Control 2 | NA | 5 years 8 months | 1781 | 79 | 4 | 0.22 |
| Control 3 | NA | 5 years 7 months | 1420 | 74 | 5 | 0.42 |
| Control 4 | NA | 2 years 5 months | 1287 | 72 | 5 | 0.39 |
NA: not applicable.
Analysis of MMc frequency in JDM and control tissue
MMc were identified in all tissues undergoing detailed examination (Fig. 2). More than 1000 cells underwent analysis in each sample. The results obtained are shown in Table 1. Comparison of data between cases and controls showed a significant difference in MMc level (P = 0.01).
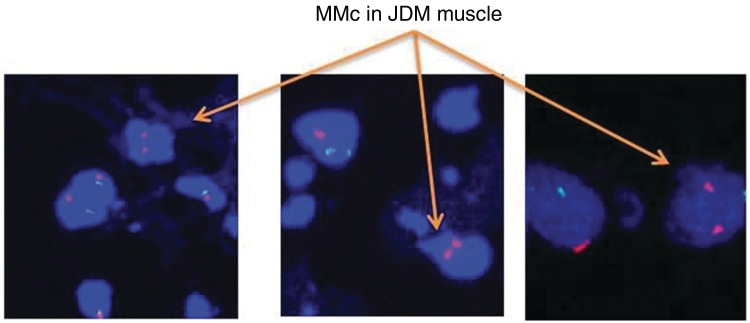
Fig. 2.
Female (presumed maternal) cells identified in male JDM tissue from three JDM cases. The X chromosome is shown by a red dot, the Y chromosome by a green dot and the nucleus with DAPI (blue).
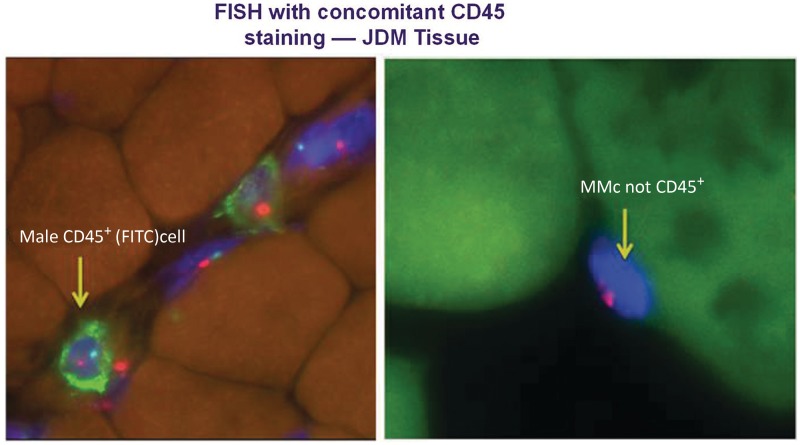
CD45 phenotyping
In order to establish whether maternal cells were of lymphoid origin, CD45 immunoflourescence with concomitant FISH was carried out on JDM sample 6 and control sample 2. As expected, CD45+ cells were observed more frequently in JDM tissue but no CD45+ MMc were identified (Fig. 3).
Fig. 3.
Maternal cells identified in JDM biopsies do not express CD45. FISH staining as in Fig. 1. CD45+ lymphocytes (stained with FITC–green) were observed in JDM tissue, but no CD45+ MMc were observed.
Discussion
Our analysis identified female (presumed maternal) cells in frozen muscle sections from seven males with well-characterized JDM using FISH. The frequency of MMc was higher in seven JDM muscle sections (0.42–1.14%) than in muscle sections from four controls (0.08–0.42%) (P = 0.01). These data, generated using state-of-the-art confocal imaging, validate and expand previous observations of increased frequency of MMc in male JDM tissue [9, 10]. This increase in frequency suggests a role for MMc in the pathogenetic mechanisms underlying JDM, yet proof of this role has been elusive. One theory suggests that these cells may be effector cells of the immune response, whereas another proposes that a frequency of MMc in tissue above a certain threshold could trigger an immune reaction.
To determine whether MMc in JDM muscle was lymphocytic in origin, the sections were co-stained with an antibody to CD45 that recognizes the human CD45 antigen, a tyrosine phosphatase also known as the leucocyte common antigen (LCA). CD45 is present in all human cells of haematopoietic origin, except erythroid cells, platelets and their precursor cells. While CD45+ host cells were identified and MMc was identified, no MMc co-stained positive for CD45. This supports recent data from Reed et al. [19] who did not identify CD45+ maternal cells. Further detailed characterization of maternal cells in JDM tissue is required to clarify the role of MMc in JDM. Work by the authors on the role of MMc in type 1 diabetes suggests that MMc in type 1 diabetes pancreas cells are tissue-specific insulin-producing β cells [20, 21] rather than cells of the immune system. Stevens et al. [22] showed that maternal cells constituted 0.017–1.9% of parenchymal cells and were found in liver, pancreas, lung, kidney, bladder, skin and spleen from seven male infant studies in detail. It is therefore possible that MMc plays different roles in different autoimmune diseases.
A strength of this study is the well-characterized JDM patient population from whom muscle biopsies were generally obtained shortly after diagnosis, but a weakness is that FISH is only useful to detect maternal cells in male biopsies with JDM. Improved strategies to detect MMc in female tissue are an important area for future development. The JDCBS represents a unique resource in that multiple tissue samples are available from each participant. It will therefore be possible to detect MMc levels in different tissues from the same individuals. One possibility is quantitative measurement of the non-inherited maternal antigen in peripheral blood samples that will require HLA genotyping of maternal DNA samples. This is likely to provide unique insights into the role of MMc in JDM.
Further characterization is required to address the role of MMc in JDM, in particular, phenotyping muscle microchimeric cells using a wide range of immunological, muscle and muscle stem cell markers to determine whether MMc in JDM are immune system or muscle derived. Another future aim is to use state-of-the-art laser capture technology to isolate and genotype these maternal cells and adjacent host cells to prove that the female cells in male tissue are maternal.

Supplementary Material
Acknowledgements
We are grateful to the families participating in the Juvenile Dermatomyositis Cohort Biomarker Study and Repository (UK and Ireland). The UK JDM Cohort and Biomarker Study is supported by grants from the Wellcome Trust UK, Action Medical Research UK and the Henry Smith Charity.
Funding: This project was funded by a grant from North Bristol Trust. The development of the methodology described was funded by grants from Diabetes UK, the Juvenile Diabetes Research Foundation and the European Foundation for the Study of Diabetes.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Hall JM, Lingenfelter P, Adams SL, et al. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–32. [PubMed] [Google Scholar]
- 2.Lo YM, Lo ES, Watson N, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–5. [PubMed] [Google Scholar]
- 3.Maloney S, Smith A, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–7. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi DW, Zickwolf GK, Weil GJ, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in women with scleroderma. Lancet. 1998;351:559–62. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 6.Srivatsa B, Srivatsa S, Johnson KL, et al. Microchimerism of presumed fetal origin in thyroid specimens from women: a case-control study. Lancet. 2001;358:2034–8. doi: 10.1016/S0140-6736(01)07099-4. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JL. Maternal-fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191–4. doi: 10.1002/art.1780390203. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz ME, Sullivan KM. Chronic graft-versus-host disease. Blood Rev. 2006;20:15–27. doi: 10.1016/j.blre.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Artlett CM, Ramos R, Jiminez SA, et al. Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Lancet. 2000;356:2155–6. doi: 10.1016/s0140-6736(00)03499-1. [DOI] [PubMed] [Google Scholar]
- 10.Reed AM, Picornell YJ, Harwood A, et al. Chimerism in children with juvenile dermatomyositis. Lancet. 2000;356:2156–7. doi: 10.1016/S0140-6736(00)03500-5. [DOI] [PubMed] [Google Scholar]
- 11.Artlett CM, Miller FW, Rider LG. Persistent maternally derived peripheral microchimerism is associated with the juvenile idiopathic inflammatory myopathies. Rheumatology. 2001;40:1279–84. doi: 10.1093/rheumatology/40.11.1279. [DOI] [PubMed] [Google Scholar]
- 12.Symmons DP, Sills JA, Davis SM. The incidence of juvenile dermatomyositis: results from a nation-wide study. Br J Rheumatol. 1995;34:732–6. doi: 10.1093/rheumatology/34.8.732. [DOI] [PubMed] [Google Scholar]
- 13.Tymms KE, Webb J. Dermatopolymyositis and other connective tissue diseases: a review of 105 cases. J Rheumatol. 1985;12:1140–8. [PubMed] [Google Scholar]
- 14.Pedrol E, Grau JM, Casademont J, et al. Idiopathic inflammatory myopathies. Immunohistochemical analysis of the major histocompatibility complex antigen expression, inflammatory infiltrate phenotype and activation cell markers. Clin Neuropathol. 1995;14:179–84. [PubMed] [Google Scholar]
- 15.Reed AM, Pachman L, Ober C. Molecular genetic studies of major histocompatibility complex genes in children with juvenile dermatomyositis: increased risk associated with HLA-DQA1 *0501. Hum Immunol. 1991;32:235–40. doi: 10.1016/0198-8859(91)90085-n. [DOI] [PubMed] [Google Scholar]
- 16.Reed AM, McNallan K, Wettstein P, et al. Does HLA-dependent chimerism underlie the pathogenesis of juvenile dermatomyositis? J Immunol. 2004;172:5041–6. doi: 10.4049/jimmunol.172.8.5041. [DOI] [PubMed] [Google Scholar]
- 17.Martin N, Krol P, Smith S, et al. A national registry for juvenile dermatomyositis and other paediatric idiopathic inflammatory myopathies: 10 years’ experience; the Juvenile Dermatomyositis National (UK and Ireland) Cohort Biomarker Study and Repository for Idiopathic Inflammatory Myopathies. Rheumatology. 2011;50:137–45. doi: 10.1093/rheumatology/keq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varsani H, Newton KR, Li CK, et al. Quantification of normal range of inflammatory changes in morphologically normal pediatric muscle. Muscle Nerve. 2008;37:259–61. doi: 10.1002/mus.20898. [DOI] [PubMed] [Google Scholar]
- 19.López De Padilla CM, Vallejo AN, et al. Extranodal lymphoid microstructures in inflamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum. 2009;60:1160–72. doi: 10.1002/art.24411. [DOI] [PubMed] [Google Scholar]
- 20.van Zyl B, Planas R, Ye Y, et al. Why are levels of maternal microchimerism higher in type 1 diabetes pancreas? Chimerism. 2010;1:45–50. doi: 10.4161/chim.1.2.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson JL, Gillespie KM, Lambert NC, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci USA. 2007;104:1637–42. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens AM, Hermes HM, Kiefer MM, et al. Chimeric maternal cells with tissue-specific antigen expression and morphology are common in infant tissues. Pediatr Dev Pathol. 2009;12:337–46. doi: 10.2350/08-07-0499.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.