Abstract
Objective. The autopsy and clinical information on children dying with anti-SSA/Ro-associated cardiac manifestations of neonatal lupus (cardiac NL) were examined to identify patterns of disease, gain insight into pathogenesis and enhance the search for biomarkers and preventive therapies.
Methods. A retrospective analysis evaluating reports from 18 autopsies of cardiac NL cases and clinical data from the Research Registry for Neonatal Lupus was performed.
Results. Of the 18 cases with autopsies, 15 had advanced heart block, including 3 who died in the second trimester, 9 in the third trimester and 3 post-natally. Three others died of cardiomyopathy without advanced block, including two dying pre-natally and one after birth. Pathological findings included fibrosis/calcification of the atrioventricular (AV) node, sinoatrial (SA) node and bundle of His, endocardial fibroelastosis (EFE), papillary muscle fibrosis, valvular disease, calcification of the atrial septum and mononuclear pancarditis. There was no association of pathology with the timing of death except that in the third-trimester deaths more valvular disease and/or extensive conduction system abnormalities were observed. Clinical rhythm did not always correlate with pathology of the conduction system, and the pre-mortem echocardiograms did not consistently detect the extent of pathology.
Conclusion. Fibrosis of the AV node/distal conduction system is the most characteristic histopathological finding. Fibrosis of the SA node and bundle of His, EFE and valve damage are also part of the anti-Ro spectrum of injury. Discordance between echocardiograms and pathology findings should prompt the search for more sensitive methods to accurately study the phenotype of antibody damage.
Keywords: neonatal lupus, anti-SSA/Ro antibodies, autoimmune congenital heart block
Introduction
Neonatal lupus (NL) is a pathological read-out of maternal anti-SSA/Ro-SSB/La autoantibodies (Abs) affecting 2% of offspring exposed in utero [1, 2]. The target organs of NL comprise the heart, skin, liver and haematological system. The cardiac manifestations associated with NL (cardiac NL) are the most serious, 17.5% being fatal and >60% requiring lifelong pacemakers [3–5]. Moreover, the risk of cardiac NL recurrence in a subsequent pregnancy has been reported to be 17.4% [6]. To date, preventive strategies such as maternal steroids, low-dose IVIG and plasmapheresis have not decreased the incidence [7, 8]. Although recent data from a case–control study suggests that maternal exposure to HCQ may decrease the risk of cardiac NL, prospective controlled studies are warranted to confirm these results [9]. Therapeutic approaches using maternal dexamethasone have not revealed sustained reversibility of third-degree block and have had inconsistent effects on the reversal of incomplete heart blocks [8, 9]. Attempts at early detection via markers such as fetal Doppler mechanical PR interval have been disappointing [2]. Maternal antibodies to amino acids 200–239 (p200) of the Ro52 protein hold promise as an indicator for an increased risk of having a child with cardiac NL; however, its value as a biomarker has not yet been established due to variations in assays and different cohorts studied [10, 11].
Cardiac NL has been classically confined to the atrioventricular (AV) node, and the fibrosis of this structure has been considered as the histological hallmark. This finding has driven research efforts to link the putative maternal autoantibody to cardiac scarring. It has been reported that in an in vitro culturing system, maternal anti-SSA/Ro-SSB/La antibodies bind apoptotic cardiocytes, blocking the normal physiological removal of these cells [12, 13]. Co-culture of macrophages with opsonized apoptotic cardiocytes results in the release of pro-inflammatory and pro-fibrosing cytokines, which transdifferentiate cardiac fibroblasts to a scarring phenotype [12, 14]. This model supports inflammation as a key initiating event in the pathogenesis of cardiac NL and, if correct, would predict that fibrosis might extend beyond the conduction tissue per se. In recent years, fatal cardiomyopathy, including endocardial fibroelastosis (EFE), with or without conduction abnormalities has also been acknowledged as part of the clinical spectrum of cardiac NL [15, 16]. Interestingly, sinoatrial (SA) and infra-Hisian conduction system disease in fetuses exposed to anti-SSA/Ro-SSB/La antibodies have each been described as well [17]. Atrial flutter and valve aberrations were reported among the cardiac manifestations found in these patients [17]. Furthermore, it has been shown that maternal sera containing antibodies against SSA/Ro and SSB/La RNPs inhibit L-type Ca-channel currents in isolated cardiac myocytes and induce sinus bradycardia in a murine model of congenital heart block (CHB), implying that the SA node could also be affected [18]. However, low atrial rates are only rarely documented in the affected fetuses, an observation best explained by the presence of subsidiary atrial pacemakers [19]. Most recently, Cuneo et al. [20] reported two cases of AV valve insufficiency due to chordal rupture from the papillary muscles in a 34-week gestation fetus and a 6-month-old infant born to mothers that tested positive for anti-Ro 52 antibodies.
Accordingly, this study was initiated to provide critical clues toward elucidating not only the spectrum of cardiac pathology, but the potential temporal sequence of injury. This was approached by studying the autopsy reports of hearts from 18 fetuses/infants with cardiac NL enrolled in the Research Registry for Neonatal Lupus (RRNL). Clinical information and pathology reports were abstracted to determine maternal and fetal demographic and health status, with emphasis on the pathological findings and timing of death relative to initial diagnosis of cardiac NL and echocardiographic findings.
Patients and methods
Subjects
As previously described, mothers enrolled in the RRNL satisfy two requirements: (i) antibodies to SSA/Ro or SSB/La RNPs and (ii) a child with any manifestation of NL [4], verified by review of medical records. The RRNL and its informed consent documents were approved by the New York University School of Medicine Institutional Review Board. This covers ethical approval for all studies involving retrospective analysis of autopsy or clinical materials submitted by the families at our request. The enrolment period for this study extended from September 1994 to March 2010; however, a mother could enter the RRNL if the child was born before 1994.
Inclusion criteria for the present study were: (i) enrolment in the RRNL and (ii) available autopsy studies on a fetus/infant with cardiac NL defined herein as the presence of heart block (first-, second- or third-degree) documented by electrocardiogram, echocardiogram, history of pacemaker or statement in the medical record; and/or presence of cardiac injury, which specifically included histological evidence of a mononuclear infiltrate or fibrosis in the endocardium, myocardium and pericardium; and/or EFE or dilated cardiac chambers with evidence of decreased cardiac output on echocardiogram. Maternal health status, ethnicity and medications were based on phone interviews and information obtained from medical records as well as from enrolment and follow-up questionnaires available in the RRNL. For the purposes of this study, the maternal health status at the time of the affected pregnancy is reported.
Detection of antibodies to SSA/Ro and SSB/La protein
Determination of maternal antibodies to SSA/Ro and SSB/La was done by the clinical immunology laboratory at the Hospital for Joint Diseases using a commercial ELISA kit (Diamedix, Miami, FL, USA). In this commercial test, the cut-off for normal has been established at 19 EU for both SSA/Ro and SSB/La. Titres of antibodies to Ro52 were evaluated by ELISA using recombinant Ro52, as previously described [10, 21].
Pathology studies
Eighteen autopsy reports were included in this study. The autopsies were performed between March 1982 and March 2010 in different centres across the USA by the patients’ health-care providers. Fourteen of the deaths occurred before the mother's enrolment in the RRNL and four deaths occurred at the time of or subsequent to enrolment. Three additional autopsy reports were obtained (all from fetuses with third-degree block) but were excluded because the microscopic evaluation of conduction system was not available or was not adequate due to severe autolysis of the heart tissue. Four of the cases included have been previously published [22, 23, 28].
Results
Demographics and clinical findings
Seventeen women enrolled in the RRNL and pathology findings on their 18 offspring with cardiac NL were studied. The demographic characteristics of the mothers, including medications, health and antibody status at the time of the affected pregnancy are summarized in Table 1. Maternal ethnicity was as follows: 10 women were Caucasian (59%), 3 (18%) were African-American, 2 Asian (12%), 1 Hispanic (6%) and 1 (6%) Indian. The majority (59%) of the mothers at the time of the studied pregnancy were classified as having a defined rheumatological disease, five (29%) had SLE, three (18%) had SS and two (12%) met criteria for both diseases. The remainder (41%) had either an undifferentiated autoimmune syndrome (UAS) or were completely asymptomatic at the time of pregnancy [24]. The majority of mothers (71%) had antibodies against both SSA/Ro and SSB/La, with 29% having only antibodies reactive with SSA/Ro. Fifteen of the 17 (88%) mothers had antibodies to Ro52.
Table 1.
Maternal demographics and clinical information
| n (%) | |
|---|---|
| Maternal race/ethnicity | |
| Caucasian | 10 (59) |
| African-American | 3 (18) |
| Asian | 2 (12) |
| Hispanic | 1 (6) |
| Indian | 1 (6) |
| Maternal diagnosis | |
| UAS/Asym | 7 (41) |
| SLE | 5 (29) |
| SS | 3 (18) |
| SLE/SS | 2 (12) |
| Maternal antibody status | |
| Anti SSA/Ro-SSB/La | 12 (71) |
| Anti SSA/Ro only | 5 (29) |
| Anti-Ro52 | 15 (88) |
| Maternal medications | |
| Dexamethasone | 8 (47) |
| HCQ | 1 (6) |
The clinical information on the reported cases, including degree of heart block and clinical manifestations, age of detection and age of death, is summarized in Table 2. Overall, 14 (78%) of the 18 deceased cardiac NL children had third-degree block and 1 (6%) had second-degree block. In three cases (17%), two fetuses and one 3-month-old infant, advanced conduction disease was not demonstrated. One woman had two dead offspring; both fetuses had third-degree block and died during the third trimester of gestation.
Table 2.
Summary of clinical information on the reported cases
| Second-degree heart block | Third-degree heart block | Mean time of detection | Mean time of death | AV nodal disease | SA nodal disease | Bundle of His disease | Valvular disease | EFE/carditis | |
|---|---|---|---|---|---|---|---|---|---|
| CHB | |||||||||
| Second-trimester deaths (n = 3) | 0 (0) | 3 (100) | 20 weeks of gestation | 26 weeks of gestation | 2 (67) | 0 (0) | 0 (0) | 1 (33) | 2 (67) |
| Third-trimester deaths (n = 9) | 1 (11) | 8 (89) | 23.3 weeks of gestation | 32.4 weeks of gestation | 7 (78) | 2 (22) | 4 (44) | 4 (44) | 5 (56) |
| Post-natal deaths (n = 3) | 0 (0) | 3 (100) | 19 weeks of gestationa | 13.3 months | 1 (33) | 0 (0) | 1 (33) | 1 (33) | 2 (67) |
| Cardiomyopathy | |||||||||
| Second-trimester deaths (n = 1)b | – | – | 16 weeks of gestation | 17 weeks of gestation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Third-trimester deaths (n = 1) | – | – | 33 weeks of gestation | 34 weeks of gestation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Post-natal deaths (n = 1) | – | – | 3 months | 3 months | 0 (0)c | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Values are provided as n (%). aExcluding one baby who was detected at 3 months of age and is not included in the mean. bCardiomyopathy + first-degree heart block. cMultiple foci of fibrosis and calcification surrounding the area of the AV node while the conduction system itself appeared intact.
Second-trimester deaths with advanced conduction disease
Of the total cases studied, there were three second-trimester deaths associated with advanced conduction disease. The mean time of detection for this group was 20 weeks and the mean time of death was 26 weeks. All three cases had third-degree heart block, with ventricular rates ranging from 44 to 55 beats per minute (b.p.m.) on available echocardiograms performed within 6 days before death. Despite the clinical finding of complete block, the histological evaluation revealed fibrosis with collagen deposition and calcification of the AV node in only two of the cases. In the third case, dilated ventricles, EFE, and fibrosis and calcification of the papillary muscles was described. No abnormalities in the SA were reported in any of these fetuses. Two of the autopsies reported EFE, but in only one of these cases was this mentioned in an earlier echocardiogram. In addition, a mononuclear inflammatory infiltrate with giant cells in the myocardium was also noted in one of the cases studied.
Third-trimester deaths with advanced conduction disease
Nine deaths associated with advanced conduction disease occurred in the third trimester. The respective means for time of detection and death were 23.3 and 32.4 weeks of gestation. Seven of nine (78%) autopsies showed fibrosis of the AV node. In two of nine (22%) cases, fibrosis of the SA node was reported; in one of these cases, inflammatory infiltrates were identified in the SA node. However, no decrease in atrial rates was recorded; the lowest atrial rates ranged from 128 to 130 b.p.m. In four (44.4%) fetuses, fibrosis of the conduction system included the bundle of His, broadening the extension of damage. The two cases without reported fibrosis of the AV node had clinical advanced block, with one of them showing an alternating second- to third-degree block. In this fetus with alternating block, the bundle of His was fibrotic and calcified and the AV node and right and left bundle branches appeared unremarkable. This fetal heart also demonstrated evidence of pancarditis in the context of biventricular hypertrophy with dilated atria and ventricles. In the other case, microcalcifications surrounding soft tissue adjacent to the AV node and fatty replacement adjacent to the AV were noted. No inflammatory infiltrate was described on autopsy report.
In five (56%) of the third-trimester deaths, EFE was noted. This finding was only described in one case on the echocardiogram; in the other four cases, EFE was not reported by echocardiographic evaluation. One autopsy study showed a lympho-histiocytic infiltrate with giant cells in the interventricular septum and another showed apoptotic debris between myofibres. Representative findings are shown in Fig. 1.
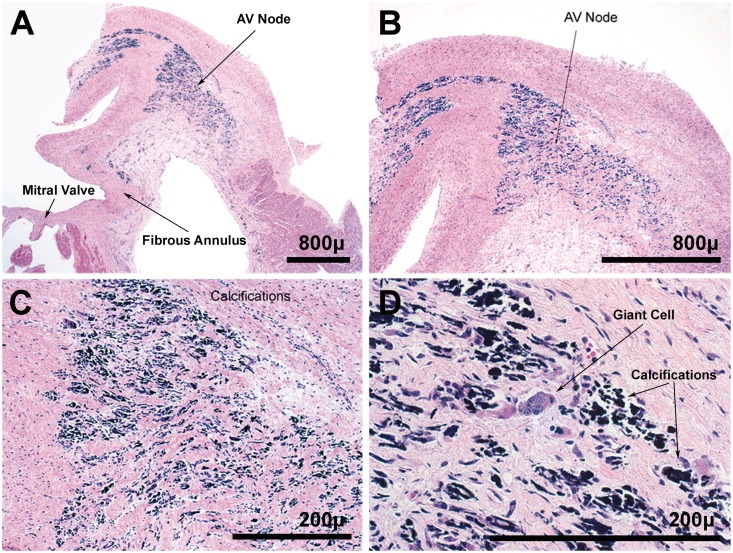
Fig. 1.
Neonatal heart at 29 weeks of gestation. (A) At low power, loss of normal AV nodal architecture is clear. (B and C) At higher power, calcified remnants of cells are noted. (D) Multinucleated giant cells are part of the scattered inflammatory cell infiltrate (haematoxylin–eosin staining).
Four (44%) had pathology findings of the valves/valve apparatus, including the tricuspid, mitral, aortic and pulmonary valves. One of these fetuses had aortic valve insufficiency and stenosis together with severe pulmonary valve stenosis and mild hypoplasia of the mitral and tricuspid valve leaflets. In only one case were these valvular abnormalities reported on the echocardiogram performed before death.
Post-natal deaths with advanced conduction disease
Three post-natal deaths associated with advanced conduction disease were included, with a mean time of detection of 19 gestational weeks and a mean time of death of 13.3 months of age. All three had third-degree block as detected by pre-natal echocardiograms. Two (67%) of the post-natal autopsy studies showed fibrosis of the conduction system; one showed fibrosis and calcification of the AV node and the other reported fibrosis of the bundle of His. In the third case, a few foci of microscopic calcification were reported in the atrial septum in proximity to the AV node, but not involving the node. No pathology of the SA node was identified in any of the three cases. In addition, two (67%) autopsy studies showed EFE, both of which were not described in any of the pre-mortem echocardiograms. One study showed hypoplasia of the tricuspid valve papillary muscle. The echocardiogram performed 2 days before death did not describe any abnormalities (functional or anatomical) of the tricuspid valve.
Cardiomyopathy without advanced conduction disease
In addition to the cases with advanced conduction disease, there were three cases of cardiomyopathy without associated advanced conduction abnormalities. One of these fetuses was diagnosed with first-degree block at 19 weeks of gestation with a mechanical PR interval of 150 ms; the echocardiogram done 1 week before death revealed echodensities of both atrial walls and interatrial septum with virtually no motion of the atria, a normal heart rate of 160 b.p.m. and no structural abnormalities. A week later, the fetus developed pleural and pericardial effusions and no heartbeat was detected. The autopsy findings of this fetus included pancarditis and a predominant mononuclear inflammatory infiltrate with few neutrophils. A fetus that died in the third trimester showed pancarditis with a mononuclear infiltrate in the endocardium, pericardium and myocardium with cells that stained for CD68 and CD45 and T/B lymphocytes as well. Patchy calcification of the ventricles and extensive calcification of the atria were noted; a normal heartbeat was recorded at Week 33 by US. Finally, the autopsy on a neonate that died at 3 months old revealed multiple foci of fibrosis and calcification surrounding the area of the AV node, whereas the conduction system itself appeared intact. No anomalies were noted on an echocardiogram at Day 9 of life and EKGs done up to 3 h before death.
Discussion
Damage to the AV node, including calcification and collagen deposition, was the main finding in the autopsy reports studied. Disease also extended to other areas of the conduction system, including the SA node and the bundle of His. In addition, damage to the valves and valve apparatus, which included fibrosis and calcification of the papillary muscles, was noted in a considerable proportion of cases. These valvular abnormalities were most frequently reported in the third-trimester deaths. Signs of cardiomyopathy were seen in a large proportion of the fetal and post-natal autopsies and not invariably associated with conduction disease. Although most of these histological changes were consistent with EFE, which has been previously described as part of the spectrum of cardiac NL [15, 25, 26], a mononuclear inflammatory infiltrate involving endocardium, myocardium and pericardium was seen in several cases, as well as apoptotic debris in one case.
The clinical and serological features of NL syndromes have been extensively described; however, publications detailing the anatomical pathology findings in the heart are limited [15, 20]. The majority of the initially reported cases demonstrated fibrosis and or calcification of the AV node, and in several cases SA nodal scarring was described [17, 23, 27–30]. EFE has been previously demonstrated in pathological studies of anti-SSA/Ro-SSB/La-exposed fetuses with and without the presence of heart block, and it is currently acknowledged as part of the spectrum of cardiac NL [9, 15, 25, 26]. Valvular abnormalities have been only rarely reported. Nield et al. [25] described dystrophic and calcified mitral valve papillary muscles in the post-mortem study of an anti-SSB/La-positive but anti-SSA/Ro-negative female fetus with diffuse EFE, as well as calcification of the SA and AV nodes. Litsey et al. [28] has previously reported one of the cases herein with tricuspid valve dysplasia and Cuneo et al. [20] described chordal avulsion of the mitral valve and tricuspid valve in one case. These findings, taken together with the other valvular abnormalities depicted in our cohort, support evaluation of anti-SSA/Ro-SSB/La antibodies in the mothers of children with more diffuse but unexplained valvular defects.
With the exception of valvular disease, overall, the histological findings appear similar across all ages of death. These data do not support the hypothesis that there is an orderly progression over time of anti-SSA/Ro-SSB/La-mediated damage with initial injury manifest as first-degree block, followed by advanced block, cardiomyopathy and culminating in EFE. The absence of AV nodal pathology in one case of first-degree block suggests that this early finding may be functional and transient rather than part of a continuum of the inflammatory/fibrotic cascade. Although the data are limited, it is possible that severe valvular disease is a late third-trimester finding.
In this series there were several notable discordances between the echocardiographic and pathological findings at the SA node, the AV node and endomyocardium. In several cases, abnormal SA nodal tissue was observed, yet abnormally slow atrial rates had not been clinically recorded. This observation raises the question of whether the SA node has an escape mechanism via atrial subsidiary pacemakers, which are used at times of SA node dysfunction [19]. There were two cases of advanced block observed on the echocardiogram but with no reported pathology of the AV node or surrounding tissue. Although speculative, this could be explained by an exit block mechanism or similarly related functional, rather than anatomical, disorder. This may include transient dysfunction of L-type calcium channels [18] or damage to the surrounding tissues. In several instances, EFE that was clearly demonstrated by histological evaluation was not observed on the pre-mortem echocardiograms. Finally, there were several cases in which the valvular abnormalities were only noted on autopsy. Thus the fetal echocardiogram may not be a sensitive enough tool to detect changes that may be of clinical significance.
Although the largest series of autopsies reported to date, several limitations of this study should be noted. Data were retrospectively collected and the autopsies were done at different centres and read by different pathologists. Due to the small size of the conducting system in fetal and neonatal hearts, it may not have been possible to fully assess this system in all autopsies, artificially reducing the incidence of immunohistological support for clinical disease in this population.
In summary, NL is a rare condition with a substantial mortality and morbidity. Considering the few reports available in the literature, information on any autopsies is invaluable since it provides the blueprint to pathological mechanisms and will surely enhance the search for reliable biomarkers and novel preventive therapies (our suggested autopsy protocol can be found as supplementary data available at Rheumatology Online). Currently available techniques, such as echocardiography and magnetocardiography, and the extensive research efforts in recent years have allowed us to better identify the clinical phenotype of anti-SSA/Ro-SSB/La cardiac disease. Increasing evidence suggests that AV node fibrosis and complete heart block are only the tip of the iceberg. There is clinical precedent in the literature for an expanded spectrum of cardiac NL, and although our findings support the concept of AV node fibrosis as the most clinically obvious manifestation, several other disease manifestations in the conductive and non-conductive tissues have been identified. The apparent discordance in several cases between echocardiographic and histological findings suggests that more sensitive methods should be utilized to adequately evaluate these fetuses and anticipate perinatal complications.

Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
We thank all the mothers enrolled in the RRNL.
Funding: This work was funded by National Institutes of Health, Contract NO1-AR-4-2220 (RRNL) and NIAMS (grant RO1 AR42455-01) (maternal Abs: pathogenesis of NL) to J.P.B.; S.L.E. Foundation NY Inc. grants (to C.L. and P.M.I.); American Heart Association Founders Affiliate Clinical Research Program Award #11CRP7950008 and the 2011-2012/2013 Pfizer Fellowships in Rheumatology/Immunology from Pfizer's Medical and Academic Partnerships programme to A.S.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Brucato A, Frassi M, Franceschini F, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 3.Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5:139–48. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- 4.Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 5.Izmirly PM, Saxena A, Kim MY, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–35. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llanos C, Izmirly PM, Katholi M, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60:3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman DM, Llanos C, Izmirly PM, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62:1138–46. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisoni CN, Brucato A, Ruffatti A, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62:1147–52. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 9.Izmirly PM, Kim MY, Llanos C, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis. 2010;69:1827–30. doi: 10.1136/ard.2009.119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clancy RM, Buyon JP, Ikeda K, et al. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 11.Salomonsson S, Dorner T, Theander E, et al. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum. 2002;46:1233–41. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 12.Clancy RM, Neufing PJ, Zheng P, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–22. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miranda-Carus ME, Askanase AD, Clancy RM, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000;165:5345–51. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 14.Clancy RM, Askanase AD, Kapur RP, et al. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol. 2002;169:2156–63. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 15.Nield LE, Silverman ED, Taylor GP, et al. Maternal anti-Ro and anti-La antibody-associated endocardial fibroelastosis. Circulation. 2002;105:843–8. doi: 10.1161/hc0702.104182. [DOI] [PubMed] [Google Scholar]
- 16.Moak JP, Barron KS, Hougen TJ, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–42. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 17.Cuneo BF, Strasburger JF, Niksch A, et al. An expanded phenotype of maternal SSA/SSB antibody-associated fetal cardiac disease. J Matern Fetal Neonatal Med. 2009;22:233–8. doi: 10.1080/14767050802488220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu Y, Baroudi G, Yue Y, et al. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005;111:3034–41. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- 19.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–82. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 20.Cuneo BF, Fruitman D, Benson DW, et al. Spontaneous rupture of atrioventricular valve tensor apparatus as late manifestation of anti-Ro/SSA antibody-mediated cardiac disease. Am J Cardiol. 2011;107:761–6. doi: 10.1016/j.amjcard.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 21.Llanos C, Chan EK, Li S, et al. Antibody reactivity to alpha-enolase in mothers of children with congenital heart block. J Rheumatol. 2009;36:565–9. doi: 10.3899/jrheum.080860. [DOI] [PubMed] [Google Scholar]
- 22.Clancy RM, Kapur RP, Molad Y, et al. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 23.Meckler KA, Kapur RP. Congenital heart block and associated cardiac pathology in neonatal lupus syndrome. Pediatr Dev Pathol. 1998;1:136–42. doi: 10.1007/s100249900017. [DOI] [PubMed] [Google Scholar]
- 24.Rivera TL, Izmirly PM, Birnbaum BK, et al. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Ann Rheum Dis. 2009;68:828–35. doi: 10.1136/ard.2008.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nield LE, Silverman ED, Smallhorn JF, et al. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. J Am Coll Cardiol. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 26.Guettrot-Imbert G, Cohen L, Fermont L, et al. A new presentation of neonatal lupus: 5 cases of isolated mild endocardial fibroelastosis associated with maternal anti-SSA/Ro and anti-SSB/La antibodies. J Rheumatol. 2010;38:378–86. doi: 10.3899/jrheum.100317. [DOI] [PubMed] [Google Scholar]
- 27.Bharati S, Swerdlow MA, Vitullo D, et al. Neonatal lupus with congenital atrioventricular block and myocarditis. Pacing Clin Electrophysiol. 1987;10:1058–70. doi: 10.1111/j.1540-8159.1987.tb06125.x. [DOI] [PubMed] [Google Scholar]
- 28.Litsey SE, Noonan JA, O'Connor WN, et al. Maternal connective tissue disease and congenital heart block. Demonstration of immunoglobulin in cardiac tissue. N Engl J Med. 1985;312:98–100. doi: 10.1056/NEJM198501103120206. [DOI] [PubMed] [Google Scholar]
- 29.Lee LA, Coulter S, Erner S, et al. Cardiac immunoglobulin deposition in congenital heart block associated with maternal anti-Ro autoantibodies. Am J Med. 1987;83:793–6. doi: 10.1016/0002-9343(87)90918-1. [DOI] [PubMed] [Google Scholar]
- 30.Chameides L, Truex RC, Vetter V, et al. Association of maternal systemic lupus erythematosus with congenital complete heart block. N Engl J Med. 1977;297:1204–7. doi: 10.1056/NEJM197712012972203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.