Abstract
Replication-conditional, oncolytic adenoviruses are emerging as powerful tools in the warfare on cancer. The ability to modify cell-specific infectivity or tissue-specific replication machinery, as well as the possibility of modifying viral-cellular protein interactions with cellular checkpoint regulators are emerging as new trends in the design of safer and more effective adenoviruses. The integration of oncolytic adenoviruses with mainstream cancer therapies, such as chemotherapy and radiotherapy, continues to yield significant therapeutic benefits. Adenoviruses can be armed with prodrug-activating enzymes as well as tumor suppressor genes or anti-angiogenic factors, thus providing for enhanced anti-tumor therapy and reduced host toxicity. Thus far, encouraging results have been obtained from extensive preclinical and human clinical studies. However, there is a need to improve adenoviral vectors to overcome unresolved problems facing this promising anti-cancer agent, chief among these issues is the adenovirus-triggered immune response threatening its efficacy. The continued expansion of the knowledge base of adenovirus biology will likely lead to further improvements in the design of the ideal oncolytic adenoviruses for cancer treatment.
Keywords: conditionally-replicating adenoviruses, prodrug activating enzymes, cancer gene therapy, oncolytic viruses
INTRODUCTION
It is a sad fact that most people who develop cancer die from it. The underpinnings of such drama lie in the fact that most cancers become incurable after their metastasis. A factor contributing to this end is the plasticity of tumor cell populations during the course of chemotherapy or radiotherapy, which often leads to tumor resistance. At this juncture the therapeutic index narrows significantly rendering most available treatments ineffective and causing an insufferable stage in the life of a cancer patient, as a result of the high toxicity to normal tissues and to the patient as whole. Except for some remarkable approaches involving the patient’s own immune system [1], there is no silver bullet to address the cancer problem, and the wiser approach where cancer could be treated as a chronic disease using non-toxic novel molecules, such as those in anti-angiogenic therapies, to keep it from progressing is emerging as a protracted war. However, even with their appealing modes of action, anti-angiogenic drugs produce only modest objective responses when administered as single agents [2, 3]. These agents typically are not able to enhance patients survival in clinical trials [4] and may need to be combined with chemotherapy to exert their therapeutic benefit [5, 6]. It was even suggested that an anti-angiogenic resistance mechanism could be developed by reducing tumor response to hypoxia through the loss of p53 function [7] or by switching pro-angiogenic factors [8]. There is thus an urgent need to diversify and to combine different strategies with non-overlapping anti-cancer modes of action to achieve a potentially successful anti-cancer therapy. Among the diversified arsenal of weapons against cancer is a particular class of agents that could bring much needed help: viruses. It is ironic that we have spent the last few million years fighting these infectious agents by developing innate and active immunity against them and then turn to them, recently, to enlist them in the fight against cancer. The specific lysis of cancer cells following viral infection is an opportunistic event that favors viral cycle completion in cancer cells due to their propensity to progress through the S phase, which is often induced by viruses themselves.
The idea of using viruses as an anti-cancer drug was first proposed in 1904 when patients with malignancies who underwent viral infections or rabies vaccination were found to experience transient remissions [9, 10]. This finding led to a broad-range investigation identifying a large arsenal of novel oncolytic viruses with anti-tumor activity—38 viruses, including adenovirus, Bunyamwara, coxsackie, dengue, feline panleukemia, Ilheus, mumps Newcastle disease, vaccinia, and West Nile virus [11–14], which were tested in vivo in both animals and man. Although all of these viruses replicate in cancer cells to a certain extent, none show the ideal attributes of a successful anti-cancer virus. Thus they fail to 1) infect only cancer cells, due to the ubiquity of their receptors on normal as well as tumor cell surfaces, 2) they do not replicate specifically in cancer cells, because of the high constitutive promoter expression of viral genes necessary for viral replication, and 3) they are unable to avoid the detection and elimination by the immune system. Positive attributes of adenoviruses include the findings that they do not cause serious human illnesses and have moderate side effects. Moreover, virus production can be safe and efficient to allow for the prospect of large-scale preparation and use.
Early studies using oncolytic viruses could not provide conclusive findings regarding the clinical utility of these agents. Indeed most of the studies used non-concentrated crude cell lysates, which limited the amount of virus to a suboptimal dose. The development of virology techniques and in particular of large-scale purification protocols allowed for subsequent more rigorous studies. The extensive studies of potential oncolytic viruses in the years between 1950 and 1975 and in particular the landmark study performed by the National Cancer Institute [14] indicated the feasibility of using adenoviruses as oncolytic viruses for cancer treatment. Sixty-five percent of the patients who were treated locally thus, showed moderate to marked local responses, translating into the ulceration and liquefaction of injected tumors, while no response was reported in patients whose tumors were injected with heat-inactivated adenoviruses. While the same study revealed that patients treated with replication-competent adenoviruses raised an immune response within 7 days after viral inoculation, viral particles were present in tumors even 17 days post-inoculation indicating viral replication in immune-competent hosts. Other oncolytic viruses did not show such potential due mainly to their lack of selectivity and high toxicity [12]. Other compelling reasons to use adenoviruses for the purpose of gene therapy [15, 16] in general and cancer therapy in particular [17–19] are its dramatic transduction efficiency in vivo, its ease of preparation, use and safety profile, and the possibility of enhancing and modifying adenovirus tropism and oncolytic effect for specific applications in cancer therapy. Moreover, since their first description in the early 1950’s [20], adenoviruses have been widely studied, and much is now known about the mechanism of cell entry and tropism as well as their replication cycle [21–26], making it possible to reroute their entry into specific cellular targets and to control the transcription of viral genes after viral infection. In brief, we know a great deal more about adenoviruses than any other oncolytic virus. As a result such knowledge enables the ability to engineer safer and more useful conditionally replicating adenoviruses for the purpose of cancer gene therapy.
Adenoviral vectors and adenovirus-transduced cells are susceptible both to cytotoxic T-lymphocyte and humoral immune responses. Additionally, adenoviral-based vectors do not integrate their genome into the cellular, chromosomal DNA of transduced cell populations and therefore do not allow for long-term transgene expression. For these reasons, adenoviral vectors are uniquely useful for genetic immunization programs against infectious diseases and for cancer therapy. The latter is the subject of this review.
ADENOVIRUSES
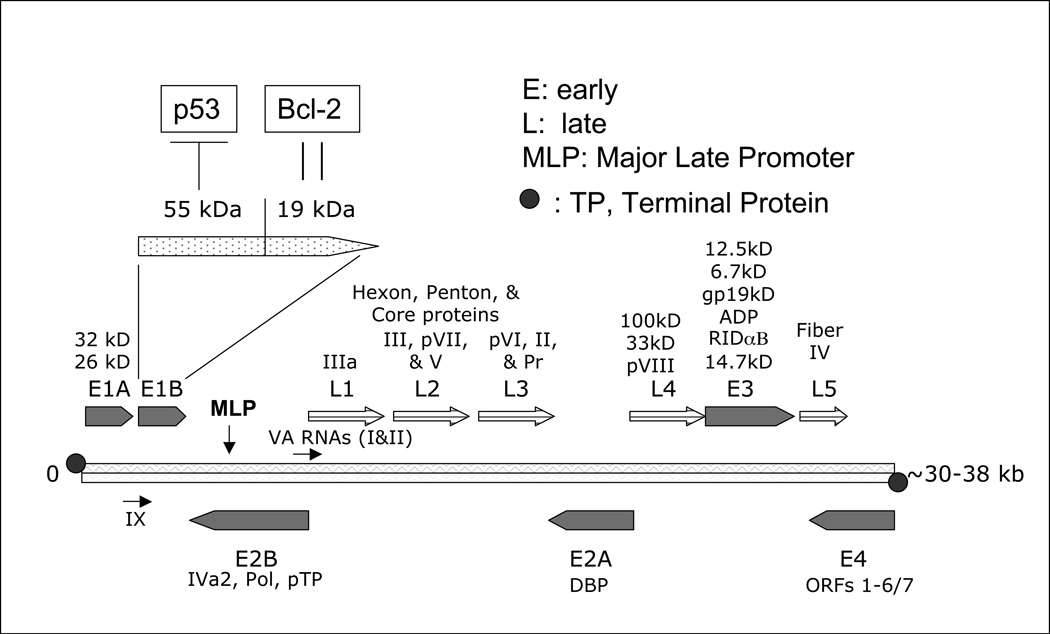
Members of the Adenovirus family are non-enveloped, non-integrating, lytic double-stranded DNA viruses with a genome ranging in size from 30–38 Kilobases and encoding a total of 30–40 genes “Fig. (1)”. Adenovirus infections occur primarily in children [27] and can infect a wide variety of well-differentiated dividing and resting cells, including liver, brain, lung, heart and skeletal muscle. Adenovirus infection in immunocompetent individuals is often mild and does not require medical treatment. However, adenovirus infection could be life-threatening in immunocompromised individuals, such as AIDS patients, transplant recipients, and patients with hereditary immunodeficiency. The classification of adenoviruses is based mainly on immunological criteria such as serotypes[28]. To date, 51 human adenovirus serotypes have been categorized into 6 species: A to F. Adenovirus species differ in their usage of the adenovirus receptor [29–31] and show a preference for specific organs. For example, adenovirus species D infects the eyes, species A and F target the gastrointestinal tract while adenovirus species C, E, and some of members of species B typically infect the respiratory tract, while others from B species infect the urinary tract as well [32].
Figure 1.
Adenovirus genome. The 30–38 Kb adenovirus genome is organized into multiple early (E) and late (L) regions of transcription. Initial induction of E1A within the E1 region leads to the downstream transcription and subsequent replication of the entire adenoviral genome. The majority of the late gene regions encode for structural virion elements (Figure 2) which are necessary for repackaging, lysis, and subsequent infection of other cells.
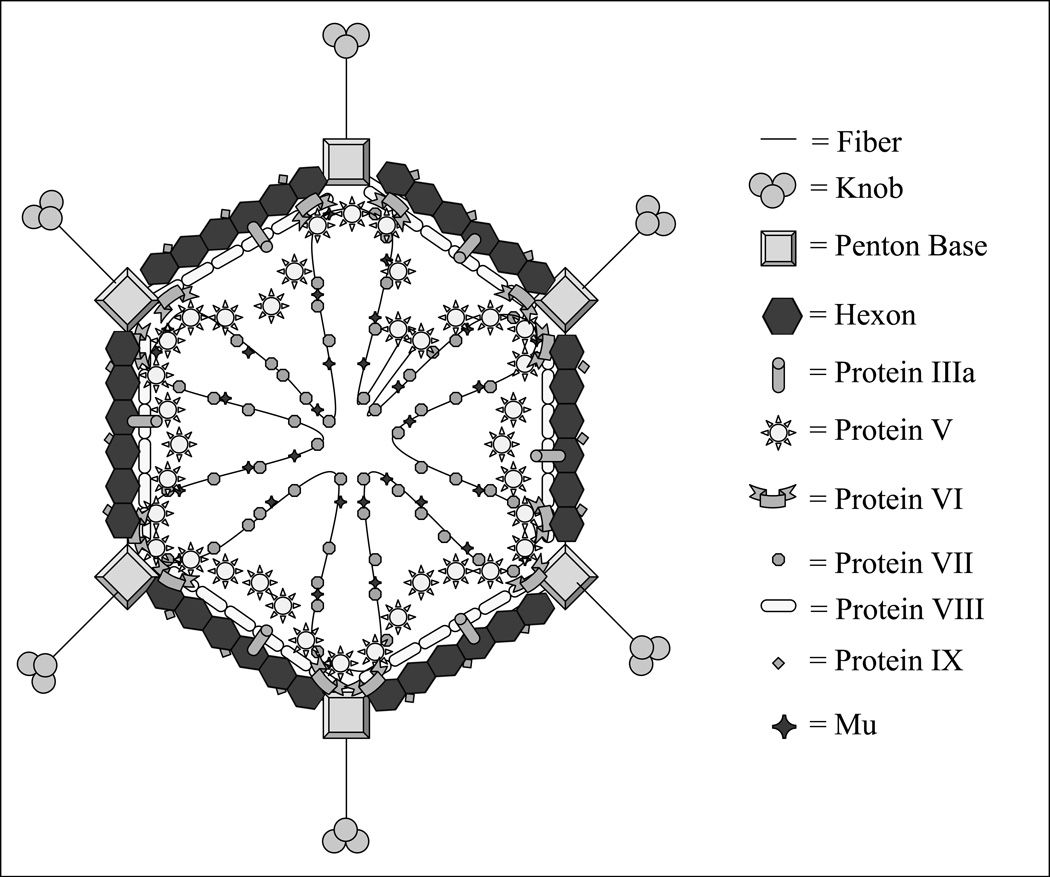
The 5’ ends of the adenovirus double-stranded genome are covalently attached to a terminal protein (TP) [33]. Inside the capsid the adenoviral DNA is wrapped around but non-covalently bound to the highly basic arginine-rich protein VII and the small (4 kDa) peptide mu [34] “Fig. (2)”. An additional arginine-rich protein, V, is attached to the DNA and provides an anchor between the whole DNA-protein complex and the hexon of the capsid via yet another protein VI [35]. Adding to the complexity of this intricate structure is another crucial component, a virus encoded protease, which is needed to process the structural protein forming the capsid of mature viral particles [36, 37]. On the outside, adenoviruses have an icosahedral-shaped capsid of a particulate size of 80–100nm [38] and therefore are potentially capable of reaching tumor cells via tumor blood vessels’ leaky pores, which have an estimated diameter of 400–600nm [39]. The viral capsid consists of three major structural proteins: the hexon, the fiber, and the penton base, along with other minor proteins: VI, VIII, and IX, which are associated with the hexon, and IIIa, which is associated with the penton, and IVa2.
Figure 2.
Adenovirus structure. The viral particle is icosahedral in shape and is approximately 80 – 100 nm in diameter. In order to efficiently package the entire viral genome the capsid is a highly ordered structure, containing many external as well as core components.
Adenovirus cell entry
Except for members of adenovirus species B, which recognizes and binds to a distinct receptor [40], adenoviruses bind to target cells by the knob of the fiber. This binding involves a high affinity interaction with the adenovirus receptor termed CAR (coxsackie/adenovirus receptor). CAR is a 46 kDa trans-membrane protein of the immunoglobulin superfamily [41], and is identical to coxsackie B virus receptor [42]. Additionally, adenovirus C (type 2 and type 5) can bind to the histocompatibility class I molecule, a member of the immunoglobulin superfamily [43]. Following receptor recognition, the penton base interacts, via its Arg-Gly-Asp (RGD) motif, with αv integrins [44, 45], especially αvβ3 and αvβ5 integrins. These initial interactions are followed by the activation of signaling pathways that enforce adenovirus cellular entry of the adenovirus but, potentially, may simultaneously trigger the host’s immune system. The enforced entry signaling pathway is induced by the activation of phosphoinositide-3-OH kinase (PI-3K), which in turn activates the Rho family of GTPases and subsequently leads to the polymerization and reorganization of actin with the apparent goal of facilitating endocytosis [46, 47]. The other signaling pathway is responsible for the activation of the Raf/mitogen-activated protein kinase (MAPK), which is followed by IL-8 production as early as 20 min post-infection, potentially acting as a chemoattractant for leukocytes. Clathrin-coated endosomes containing the engulfed adenoviruses are then shuttled to the cytoplasm [48]. Inside the virus, the virus-encoded protease disrupts the association between the capsid and the complexed DNA/core proteins by proteolytic cleavage of the protein anchor VI [49]. After disruption of the capsid, the viral DNA is injected into the nucleus through a nuclear pore. This passage to the nucleus requires the intervention of dynein and microtubules [50, 51]. One or two hours after infection, adenoviral DNA and proteins V and VII as well as viral particles can be detected in the nucleus [52, 53].
Replication of adenoviruses
Adenoviral replication events are quite common to all species and start by the active transcription of a battery of genes classified temporally as early and late genes. The early phase of replication is initiated by the transcription of several cassettes termed E1, E2, E3 and E4.
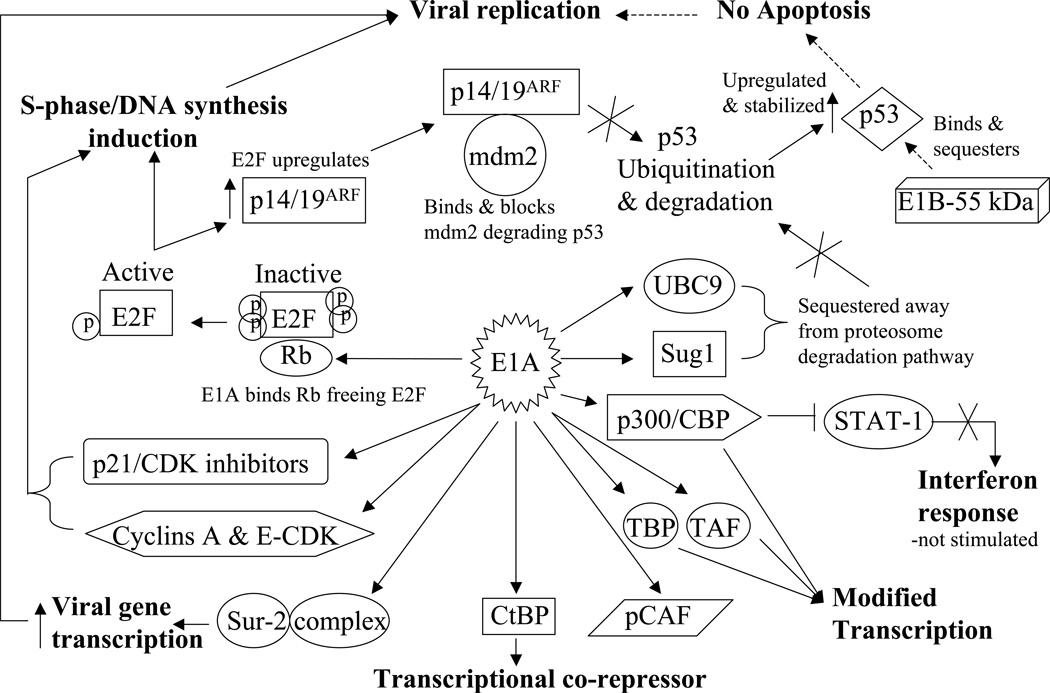
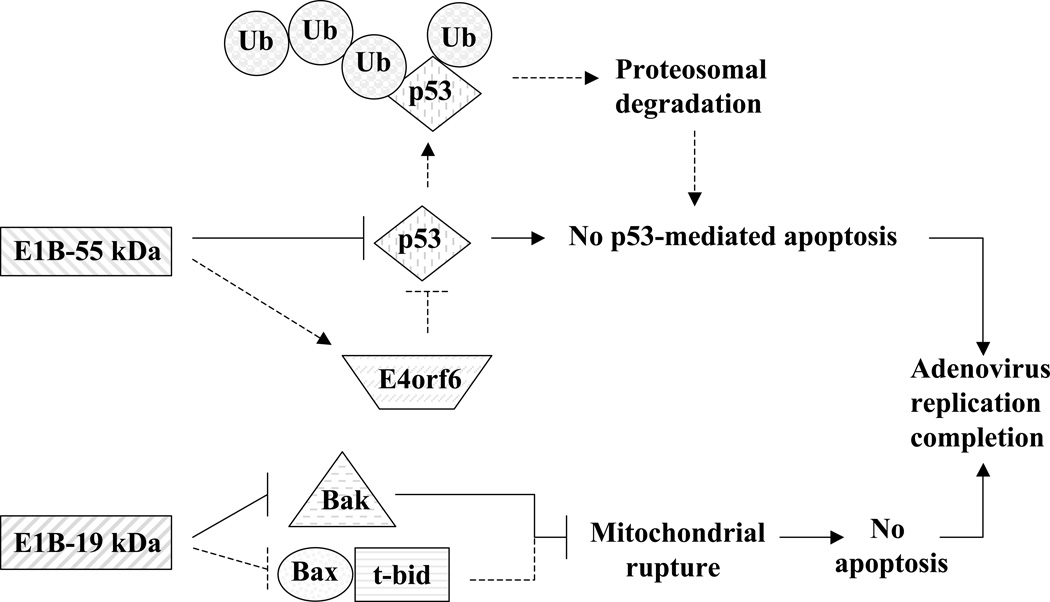
E1 transcripts are mainly designed to subdue cellular components that could hamper adenoviral replication and cycle completion. These transcripts are the E1A and E1B. E1A itself encodes two major proteins termed 32 kD and 26 kD “Fig. (1)”. E1A proteins are designed to modulate the functions of several major cellular proteins “Fig. (3)”, which have far reaching roles in cell division and cell fate. E1A proteins will bind to p21 and CDK inhibitors [54], cyclin A and E-CDK complexes [55], and p300/CBP transactivators thus interfering with transcriptional activities, modulating associated acetyltransferase activities associated with pCAF, and affecting the activity of STAT-1 which is required for interferon response and blocking caspase activation in p53-independent apoptosis [56–60]. E1A will also interfere with transcription by binding directly to the TATA-box-binding protein (TBP) and TBP-associated protein TAF [61]. Like T antigen (SV40), and E7 (HPV), E1A binds to and inactivates the retinoblastoma (Rb) tumor suppressor protein by using an E1A conserved LXCXE sequence motif [62]. The binding of E1A to Rb releases the transcription factor E2F which leads to the transcription of p53 thus promoting apoptosis [63] and of p19ARF which interacts with mdm2, thus preventing mdm2 from interacting with p53 [64] leading to a stabilization of p53 by avoiding its proteolysis by ubiquitination [65, 66]. The stabilization of p53 is further enhanced by the interaction of E1A with Sug1, a subunit of the proteasome complex that is required for p53 degradation [67]. All of these interactions are directed at subduing the transcriptional machinery of the infected cell to allow for adenovirus replication, and they seem to drive infected cells toward apoptosis since several interactions are aimed at enhancing p53 stability and activity. However, the second transcript group of the E1 region, E1B seems to counter this pro-apoptotic trend “Fig. (4)”. The E1B product E1B-55 kDa seems to interact directly with p53 and mediates its inactivation [68] and translocation to the cytoplasm [69]. E1B-55 kDa does help mediate the ubiquitination and degradation of p53 in concert with another adenoviral protein E4orf6 [70, 71]. The interaction of E1B-55 kDa and p53 does in fact transform p53 into a very potent repressor while increasing p53 affinity to its binding site [72]. This observed relationship suggests that wild type adenoviruses reduce the tumor suppressor activity of p53. Furthermore, another product of the E1B gene, E1B-19 kDa, blocks the downstream effects of p53 to prevent apoptosis [73]. In this respect E1B-19 kDa functions as an analogous form of Bcl-2 and can inactivate the pro-apoptotic factor Bax [74]. To further antagonize the E1A proapoptotic functions, E1B-19 kDa can bind much like Bcl-2 to the powerful transcription repressor btf which promotes cell death by inducing mitochondrial membrane permeabilization [75, 76]. The anti-apoptotic function of E1B-19 kDa is truly revealed when deleted from the adenovirus genome itself. E1B-19 kDa deletion was shown to yield a highly oncolytic adenovirus [77], which lyses infected cells and spreads much faster than the wild type adenovirus. The deletion of both E1B products (E1B-55 kDa and E1B-19 kDa) in another adenovirus, termed Ad337, [78] resulted in similar increases in oncolytic effect and virus cytotoxicity.
Figure 3.
E1A protein interaction network. E1A is the first protein translated from the viral genome. As such, it is responsible for the successful downstream transcription as well as replication of the entire viral genome in order to ensure viral infection and propagation.
Figure 4.
E1B protein anti-apoptotic mechanisms. The two E1B region proteins, E1B-55 kDa and E1B-19 kDa, both result in apoptotic-prevention mechanisms to help ensure successful viral infection and spread. The E1B-55 kDa predominantly functions to sequester and inhibit p53 but can also indirectly act through E4orf6 to ubiquitinate and degrade p53 to prevent p53-activated apoptosis. The E1B-19 kDa protein acts as a Bcl-2 analog and inhibits Bax and/or Bak from signaling mitochondrial rupture, cytochrome c release, caspase activation, and ultimate apoptosis.
This interplay provides a counter measure to E1A activation of p53. A simplistic view of these complex interactions would seem to indicate that in order for adenoviruses to replicate they must first modulate the transcriptional machinery of the infected cell at the cost of triggering a pro-apoptotic program by the E1A gene product and then manage this crisis by the products of the E1B genes. The E2 gene products are necessary for the replication of virus DNA and provide the tools for DNA replication and transcription in collaboration with other cellular components [79]. The transcription of the E2 genes is dependent on the E1 products, which act as trans-acting transcriptional factors. The genes encoded by the E3 regions are not necessary to the replication of adenoviruses. However, the sophisticated function of some of its products is worth mentioning, in particular of the 19 kDa glycoprotein termed E3 gp19K. This protein is anchored to the ER and binds to the heavy chain of MHC class 1 but also delays its expression, thus preventing its shuttling to the cell surface for presentation and recognition by CTLs as well as an ultimate confrontation with the immune system [80]. Another protein termed adenovirus death protein (ADP) or E3-11.6K is known to promote cell lysis and the release of adenoviral particles [81]. Adenoviruses overexpressing ADP were found to induce early cell lysis, increased viral spread [82] and to provoke cell death by both caspase-dependent and independent mechanisms [83]. Due to the dispensable nature of these functions in relation to the replication of the adenovirus, the E3 region is commonly deleted from its genome to allow for the creation of valuable “real estate space” in order to incorporate large transgenes for the purpose of cancer gene therapy. The quintessential example of an adenovirus with a deletion-modified E3 region is the famous Onyx-015 [84]. The E4 gene products main function is to shut-off cellular protein synthesis [85] and possibly cooperate with the E1B-55 kDa to allow the replication of the adenovirus in a cell cycle independent manner [86]. Finally, many transcripts are encoded toward the end of the virus cycle and are termed late genes L1 to L5, which result mostly in transcripts whose products correspond to the structural proteins of the capsid. Their transcription is delayed due to an attenuation of the major late promoter (MLP) in part because of fierce competition for transcriptional activators which are limited [87].
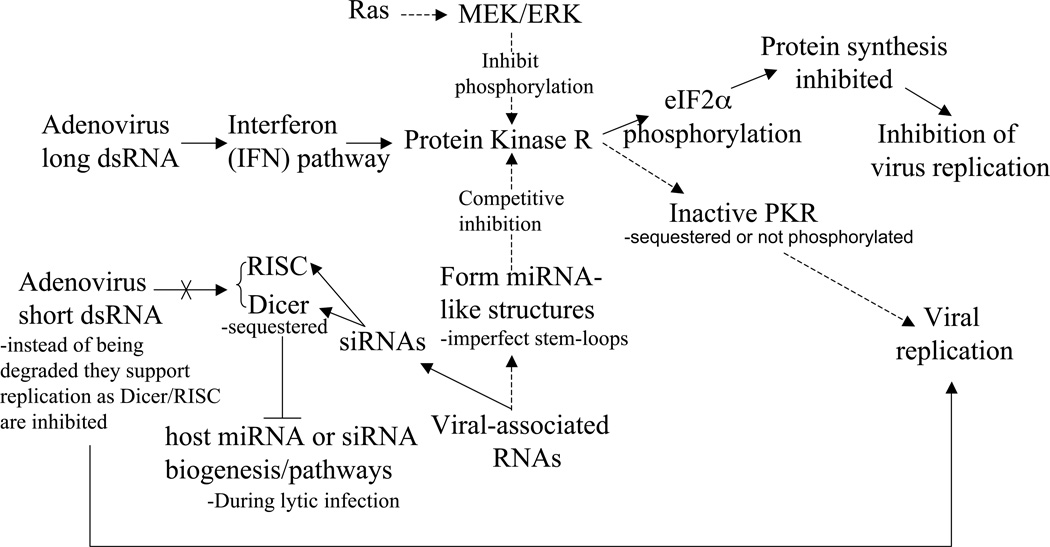
It is of interest to note that few adenoviral transcripts do not yield proteins. Among them are the Virus-associated (VA) RNAs which are noncoding, polymerase III-transcribed, 160-nucleotide single-stranded RNA molecules that fold into dsRNA and accumulate in the cytoplasm of adenovirus-infected cells [88] “Fig. (5)”. For example, Adenovirus type 5 expresses two VA RNAs, VAI and VAII. Though dsRNAs usually activate an RNA-dependent protein kinase (PKR), an interferon-inducible serine-threonine protein kinase which leads to protein synthesis shut-off as a response to viral infection [89], the main function of adenoviral VAI RNAs is the inhibition of PKR activation [90]. A recent report suggested that VAI RNAs are processed to small RNAs and could behave as functional siRNAs or miRNAs to regulate viral components [91]. Interestingly, the Ras oncogene, which is overexpressed in many tumors is also able to inhibit PKR. This property was used to build an adenovirus without the viral associated RNA, thus enabling its replication only in Ras overexpressing cells [92]. Another report indicates that VA RNAs suppress RNA interference (RNAi) later after infection by suppressing the activity of two key enzyme, Dicer and RNA-induced silencing complex (RISC) [93].
Figure 5.
Adenovirus VA RNA inhibition of both the Interferon/PKR pathway. VA RNAs (with imperfect-stemloop secondary structure) inhibit IFN-induced PKR signaling leading to downstream eIF2α phosphorylation and ultimate protein translation inhibition. Ras signaling via MEK and its substrate ERK may also inhibit IFN-induced PKR activation by preventing PKR phosphorylation. Additionally, VA RNAs may also be processed into siRNAs and inhibit the functionality of the host cell’s Dicer/RISC RNAi pathway, thus blocking degradation of short viral dsRNAs as well as the cell’s endogenous miRNA/siRNA synthesis pathway.
The assembly of the adenovirus is triggered by the encapsidation of viral DNA which contains an AT-rich packaging signal at its left end [94]. The exit of adenovirus particles from the nucleus where they matured is facilitated by the disruption of the nuclear membrane followed by the collapse of the plasma membrane without necessarily showing signs of apoptosis [81].
DESIGNING USEFUL ADENOVIRAL CONSTRUCTS FOR CANCER GENE THERAPY
Several properties of adenoviruses make them useful as vectors for cancer gene therapy. These include, the mild nature of illness resulting from adenovirus infections, the lack of integration into the host genome, their high transduction ability. A wild type adenovirus has a “cargo” capacity of 2 kb for potential gene transfer, corresponding to ~ 105% of the original size of the genome [95]. This capacity is limited by the intra-capsid volume and steric interactions with the viral core proteins inside it. However, depending on the application, certain genes such as E1 and E3 may be deleted to increase this capacity to 7.5 kb [96]. A further increase in capacity to 11 kb may be achieved with an additional deletion in the E2 region [97]. For replication incompetent adenoviruses, also termed first generation adenoviruses, deletions of adenoviral genes to yield replication defective viruses are also an effort to make the virus less susceptible to generating wild type virion particles. On one hand the deletion of the E1A genes will lead to a reduced transcription of the E2 genes, while deletion of E1B will enhance the pro-apoptotic signals in infected cells and finally the deletion of E3 will reduce the chances of virus-infected cells escaping immune responses [98, 99]. These deletions thus limit adenovirus usage in long-term gene therapy protocols but potentially enhance their application in vaccine development. Adenoviruses lacking E1 genes could be grown in a packaging cell line transformed with E1A and E1B genes [100]. This system provides viral preparation in excess of 1013 particles/ml, allowing for direct usage in in vivo applications [101]. Second generation replication-defective adenoviruses were later developed by excising some or all the adenoviral genes from the E2 and E4 regions [97, 102, 103]. However, eliminating all these adenoviral components does not abrogate the immune response to adenoviruses, as the basis of this response is not only triggered by the therapeutic transgene inserted into the adenoviral genome [104] but also by the required structural components of adenovirus [98, 105]. The following generations of adenovirus vectors were indeed aimed at removing the maximum number of genes from the genome to generate the so called “gutless” [106–108] vector. Gutless vectors contain ITRs and packaging signals but require a helper virus, which means that gutless adenoviruses needed special care for purification. This problem was solved by incapacitating the packaging of the helper virus through the use of the cre-lox system [109]. Another variety of vectors emerged, called high capacity adenoviruses (HC-Ad) and in which most viral DNA was replaced by “stuffers” in order to permit efficient adenovirus packaging [106, 109–115]. HC-Ad are more suitable for therapeutic gene delivery of large genes and by contrast to their first generation predecessors were reported to allow for transgene expression for a surprisingly longer period of time, over one year [116].
ADENOVIRUSES FOR CANCER GENE THERAPY
The use of adenoviruses for cancer gene therapy involving the delivery of a therapeutic gene could be classified into three categories: 1) adenoviruses expressing tumor suppressor genes; 2) oncolytic adenoviruses potentially armed with prodrug-activating enzymes and; 3) adenoviruses for DNA vaccines. However, it is conceivable to unify all of these classes into a single one. For example, it is possible to design an oncolytic adenovirus which will be armed with a prodrug-activating enzyme and which could elicit an immune response. Another design could also combine tumor suppressor genes and immunomodulatory genes. A wide array of other combinations are described in the literature. However, in this review we will only discuss the first two categories.
Adenoviruses expressing tumor suppressor genes
Mutations or deletions leading to the loss of function of tumor suppressor genes and other genes involved in checkpoints controlling the quality of genetic material, cell division, survival and the death of cells, often lead to an imbalance of cell growth, which becomes unregulated, leading to an uncontrolled cell cycle. Such imbalance often leads to the development of cancer cells. The re-introduction of tumor suppressor genes or other genes involved in cellular growth control is thought to restore normal cellular functions [117].
Targeting the p53 pathway: anti-cancer chemotherapy utilizing 60,000 compounds against a panel of 60 human cancer cell lines demonstrated that it is most efficacious in tumor cell lines expressing a functional p53 gene [118]. However, most human cancers harbor a defective p53 gene [119]. Consequently, it was demonstrated that the introduction of a wild type p53 gene into p53 defective colorectal carcinoma leads to cell growth suppression [120]. A recent clinical trial using adenovirus-mediated p53 gene transfer in patients with chemo-radiation-resistant advanced esophageal carcinoma showed a clear anti-tumor effect [121]. Combining the transfer of p53 with chemotherapy is a good practice, which synergizes their actions. For example, it was found that the introduction of wild-type p53 in non-small lung cancer cells sensitizes them to cisplatin [122], and sensitizes myeloid leukemia cells to etoposide [123] and thyroid cancer cell lines to adriamycin [124]. Combination with radiotherapy was equally effective on radioresistant colon [125], ovarian [126], and radioresistant head and neck cancer cell lines in vivo [127]. However, the introduction of wild type p53 function or its abrogation does not enhance or reduce the radiosensitivity of some cell lines [128, 129], indicating a cell-type specific p53 radiosensitization. The overexpression of mouse double-minute 2 (mdm2) in certain cancers could limit the efficacy of p53 gene transfer, for example 30% of osteosarcoma tumors overexpress mdm2 due to gene amplification [130]. This was resolved through the creation of a chimeric p53 in which the domains that mediate its inactivation were replaced [131]. This chimeric p53 induced apoptosis six-fold more efficiently than p53 wild type. Other modifications to overcome the inactivation by mdm2 are the substitutions of hydrophobic residues Leu-14 and Phe-19 on p53, both of which are involved in the interaction with mdm2 [66]. The modified p53(14/19) was delivered by Ad-p53(14/19) and was found to induce apoptosis more dramatically than the wild type p53 especially in osteosarcoma cells overexpressing mdm2 [132].
Wild type p53 transfer will probably be ineffective in HPV induced cervical cancer since E6 protein of HPV inactivates p53 through ubiquitin-mediated proteolysis [133], in the SV40 large T-antigen expressing tumor cells in which the T-antigen inactivates p53 [134], or in hepatitis induced liver cancer where the X-protein excludes p53 from the nucleus [135].
Other tumor suppressor genes: approaches involving inhibition of cyclin-dependent kinases were explored by adenoviral delivery of p21 waf/cip1, a universal inhibitor of CDK’s and a mediator for p53 G1 arrest. p21 transfer induces apoptosis [136] and would be useful for those cancer cells overexpressing mdm2 resulting in inactivated p53. Indeed it was shown that p21 was more effective than p53 in a rat glioma [137]. In some cases however the lack of p21 is behind the enhanced sensitivity to chemotherapy [138, 139], thus throwing some doubt about the utility of p21 gene transfer.
p16INK4 deletions are encountered in over 50% of gliomas [140]. When comparing the impact of adenovirus transfer of p53, p21 and p16, it was found that while p16 induced a prostate cancer growth delay similar to p21 it was weaker than p53 [141]. However, surprisingly, the introduction of p16 into a melanoma cell line lead to a dramatic chemoresistance to methotraxate, vinblastine, and cisplatin [142]. Another tumor suppressor that could be considered is the Retinoblastoma (Rb) gene, which binds the E2F transcription factor when hypophosphorylated but releases it when hyperphosphorylated, thus allowing cell-cycle entry into S phase [143]. Rb gene deletions are present in lung, bladder, breast, and osteosarcoma and other cancers. Upon adenoviral transfer of the Rb gene to these cancers, Rb showed a hypophosphorylated status and lead to a complete tumor suppression of the bladder treated tumor cells in nude mice when using a N-terminal truncated retinoblastoma (RB) protein (pRB94) [144, 145].
p27Kip1 is another universal CDK inhibitor from the same family as p21 and mediates a similar growth arrest at G1 phase [146]. p27 was compared to p21 following adenovirus-mediated transfer in breast cancer and was found to decrease CDK activities more than p21 [147] and to inhibit tumor growth of glioma in vivo [148]. It appears however that the approach of delivering tumor suppressor genes using replication-defective adenoviruses is of limited utility since not all tumor cells will be successfully infected.
Among all the tumor suppressors discussed here, it appears that the use of the p53 mutants, resistant to mdm2 inactivation [131, 132] are the most likely candidates to proceed toward advanced clinical use. However, due to the limited spread imposed on replication-defective adenoviruses, it seems logical to deliver p53 using conditionally replicating adenoviruses (CRADs). There is, however, a potential problem with this concept. Since most E1 gene products are antagonists of p53, especially E1B-55 kDa which interacts directly with p53 and mediates its inactivation [68] and translocation to the cytoplasm [69]. Again, E1B-55 kDa also mediates the ubiquitination and degradation of p53 in concert with the adenoviral protein E4orf6 [70, 71]. The interaction of E1B-55 kDa and p53 does in fact transform p53 into a very potent repressor while increasing p53 affinity to its binding site [72]. This interaction suggests that E1B-55 kDa reduces the tumor suppressor activity of p53, which is the goal of p53 transfer in cancer cells in the first place. However, since E1B-55 kDa is produced early during the replication cycle of adenoviruses it seems sensible to place p53 gene under the control of a late promoter. This goal was achieved by placing p53 cDNA into the fiber transcription cassette [149]. The late production of p53 did not impair the cycle of the replication competent adenovirus. However, this elegant engineering did not mediate a strong p53 tumor suppressor function in this context despite its strong nuclear accumulation. The solution to this problem was provided by a p53 mutant in which the protein domain interacting with E1B-55 kDa was removed [150, 151] or by the deletion of the domain interacting with mdm2 such as the modified p53(14/19), which was delivered by Ad-p53(14/19) and was found to induce apoptosis more dramatically than the wild type p53 especially in osteosarcoma cells overexpressing mdm2 [132].
Tumor suppressor targeting strategies should provide a multimodal treatment involving 1) the tumor suppressor function while insuring that such property will be exacted in target tumor cells; 2) the additional oncolytic activity of CRAD to allow for the spread of the tumor suppressor gene to tumor cells; and 3) the possibility to combine the first approaches with chemotherapy or radiation.
Conditionally replicating adenoviruses (CRADs)
Like many DNA viruses, adenoviruses have developed intricate mechanisms to disrupt major cellular checkpoint effectors, such as p53, mdm2, and Rb, thus affecting several cellular pathways including cell cycle progression in a manner conducive to bringing the adenoviral cycle to completion. Adenoviruses achieve this remarkable exploit by a very limited repertoire of genes whose products can achieve several protein-protein interactions via multiple domains within the same adenoviral protein “Fig. (3, 4)”. However normal cells rather than tumor cells are the evolutionary target of adenoviruses. Therefore, adenovirus biology was first dictated and shaped to function in normal cells. If the mechanisms used by wild-type adenoviruses to subvert the normal cells’ processes are altered by removal of specific genes from the adenovirus genome, then the replication of the modified adenovirus will be allowed only in cells with de facto subverted processes. It appears that in most tumor cells those adenoviral-targeted pathways are already defective thus giving the adenoviruses’ replication a green light signal.
CRADs generated by deletion of adenoviral genes
One of the first oncolytic adenoviruses that was developed for cancer therapy was Onyx-015 [84]. This adenovirus was built on a simple, yet very elegant premise. The wild type adenovirus after infecting a normal cell (say an airway, epithelium cell), will force cell entry into S phase via one of its early products, the E1A protein, which interacts with the Rb protein. Rb is usually closely associated with E2F transcription factors and this close association blocks cell cycle progression. The interaction of E1A and Rb releases E2F transcription factors thus lifting the blockade and allows infected cells to progress into the S phase. By eliciting such progression, the replication machinery of the infected cell is activated and the adenovirus’ replication is a direct beneficiary [152]. The infected cell’s response is swift and tries to abort this hijacking by triggering the expression of p53 (see earlier) which should lead to apoptosis [153], thus eliminating the infected cell and preventing subsequent viral spread. This cellular response is, however, countered by another adenoviral protein E1B-55 kDa (see earlier), which inactivates p53 [154]. Onyx-015 is devoid of a functional E1B-55 kDa and is therefore “theoretically” unable to complete its cycle in cells possessing a functional p53. However, Onyx-015 will be able to do so if p53 is not functional, such as in the case of most human cancers [119]. The initial reports showed a greater sensitivity of cells lacking p53 [84]. The p53-based specificity of Onyx-015 was later challenged by the finding that cells possessing functional p53 were permissive to Onyx-015 [155]. Although, this finding could be counter-challenged by the possibility of an indirect deficiency in the p53 pathway due to for example the loss of p14ARF [156]; p14ARF down regulates mdm2, a ubiquitin ligase which degrades p53 (see earlier), thus allowing cancer cells with defective p14ARF or overexpressed mdm2 to behave as p53 defective cells even with a wild type p53 gene [157]. Moreover and probably more damaging to Onyx-015 theory, some tumor cell lines required E1B-55 kDa protein to allow the replication of Onyx-015 regardless of their p53 status [158]. We should remember that in addition to inactivating the tumor suppressor p53, E1B-55 kDa protein has two late functions including host protein synthesis shut-off and the transport of late adenoviral mRNA [159, 160]. It was later found that the late mRNA transport is allowed in some tumor cell lines but not others and only where the export was allowed was Onyx-015 replication permissible, thus defining the true mechanism behind 0nyx-015 selectivity [161]. O’Shea and colleagues also showed that non-permissive cells would become permissive after a heat shock which allows late mRNA transport thus pheno-copying of E1B-55 kDa functions [162]. The anti-tumor mechanism of Onyx-015 is therefore not universal. Other oncolytic adenoviruses were generated by deleting both E1B-55 kDa and E1B-19 kDa for p53 and Rb null cancers [78, 163], by deletion of VA-RNA for Ras positive cancers [92], or by deleting E1B-55 kDa and modifying the E1A/Rb interaction domain in the constant region CR2 [164, 165] (Table 1).
Table 1.
Oncolytic Adenoviruses. Some viruses gain tumor-replication selectivity based upon novel gene deletions within their viral genome; others gain selectivity based upon cancer-associated, specific promoter induction.
| Adenoviruses | Tumor-specificity | Clinical Trial | Ref |
|---|---|---|---|
| Wild type | None | N/A | [14, 236] |
| dl1520 (ONYX-015) | E1B-55 kDa-deletion/p53 inhibition | Phases I–III | [84] |
| dl337 | None | N/A | [237] |
| dl118 | E1B-deletion/p53 inhibition | N/A | [238] |
| hTERT-Ad | hTERT promoter specific induction | N/A | [239] |
| ONYX-411 | E2F-1 promoter | N/A | [240] |
| AD.DF3-E1 | DF3 promoter | N/A | [167] |
| CV787 | Rat probasin & PSA promoters | Phase I | [241] |
| CV764 | Human glandular kallikrein & PSA promoters | N/A | [242] |
| CN706 | PSA promoter | Phase I | [19] |
CRADS generated by modified transcriptional control
The efforts to generate selective replication of adenoviruses were achieved by the replacement of adenoviral promoters in particular E1A’s with tumor or tissue-specific promoter sequences. Utilized promoters include the α-fetoprotein promoter [166], the prostate-specific antigen (PSA) promoter [19], and the MUC1/DF3 promoter [167]. These tissue-specific adenoviruses showed tumor specificity for the corresponding tissues in vitro, in liver, prostate, breast and ovarian cancer cell lines respectively, and showed anti-tumor activity in vivo. However, the tissue specificity of these promoters does not preclude transcriptional leakiness, which will lead to minimal levels of E1A protein sufficient to ensure adenovirus replication in other tissues especially at higher MOI’s (multiplicity of infection). This basal expression of tissue-specific promoters from the adenovirus genome is due to potential viral enhancer sequences and the lack of insulation of the adenoviral genome with histones. Another limitation to tissue-specific approaches is the heterogeneity of tumors whereby different populations will provide different transcriptional profiles. Tumor specificity was also achieved by the use of the human telomerase reverse transcriptase (hTERT) promoter [168] and the E2F promoter [169]. Several other promoters were used to achieve tumor specificity by placing the E1A under the control of the hypoxia responsive element [170], the early growth response gene 1 (EGR-1) which is radiation inducible [171], the L-plastin promoter for estrogen dependent cancers [172], the cyclooxygenase-2 (COX-2) promoter [173], or the Osteocalcin promoter (OC), which is transcriptionally active in primary prostate tumors as well as in bone metastases [174].
Some promoters are more likely to offer broad expression in several cancers. For example, studies showed that DF3/MUC1 antigen is overexpressed in a variety of carcinomas, including lung [175] ovarian [176], prostate [177], and pancreatic cancers [178]. This occurrence in diverse cancers could in fact enhance the usefulness of the proposed targeting strategy thus benefiting a very large population of cancer patients. Similarly, over 90% of human cancer cells express hTERT [179]. hTERT expression is regulated at the transcriptional level [180]. While the hTERT promoter lacks a TATA box it is G/C rich and contains numerous other binding motifs for transcription factors involved in cell proliferation and tumorigenesis. Interestingly, hTERT promoter activity is repressed by overexpression of functional p53 protein [181]. Telomerase overexpression is tumor specific and not tissue-specific thus offering a universal property which could target a multitude of cancers (Table 1).
Another interesting approach for transcriptional control was used to target endothelial cells (EC) using E-selectin, the expression of which is minimal in normal vasculature but high in tumor vasculature. In the context of adenoviral transfer the expression of E-selectin could be further increased by the use of TNF-α [182]. This direct targeting is reminiscent of anti-angiogenic therapy. Another type of transcriptional control involves the control of the E4 region in concert with the E1 region with a synthetic tyrosinase enhancer/promoter, which confers an augmented selective adenoviral replication in melanomas [183]. In an attempt to take into account several cancer defects, a more complex transcriptional control was achieved by placing the E1A under the control of a minimal dual-specificity promoter that responds to estrogens and hypoxia. Furthermore, the E4 region in this adenovirus was placed under the control of the E2F-1 promoter. The resulting adenovirus was attenuated in non-transformed quiescent cells growing under normoxic conditions [184]. A similar approach was reported for an adenovirus which showed a restricted replication to pancreatic and colorectal cell lines when using corresponding tissue specific promoters for these tissues [185].
CRADs with modified tropism
All generations of adenoviral vectors discussed so far rely on the presence of the CAR receptor. However, there is evidence that the CAR receptor is scarce on the surface of cancer cells [186, 187]. This limitation could be detrimental to the whole concept of CRAD vectors since no meaningful spread will occur in the absence of the CAR receptor. This problem could be compounded by the fact that most adenovirus particles injected intravenously will be trapped in the liver, spleen, heart, lung and kidney [188]. The liver in particular, due to the abundance of the CAR receptor on hepatocytes will provide a sink effect. This problem led to the modification of adenovirus tropism in order to bypass the CAR receptor. The initial interaction between the adenovirus and its receptor occurs at the level of the fiber-knob followed by the interaction of the penton motif RGD and the αvβ3 and αvβ5 integrins. Unlike CAR, integrins are commonly expressed on tumor cells and vasculature. An elegant modification was the integration of an RGD motif on the C-terminus and H1 loop regions of the fiber knob, which allowed the adenovirus to attach directly to the αvβ3 and αvβ5 integrins. This modification substantially improved the infection of glioma where less than 50% of cells express the CAR receptor [189] as well as of osteosarcoma xenografts [190]. Equally effective was the insertion of polylysine residues in the fiber knob, which improves binding to negatively charged cell surface proteins and heparan sulfate thus enhancing the efficacy of Onyx-015 in vitro and in vivo [191]. Another interesting modification to the tropism was realized by the coexpression of the fiber tail and shaft domains from adenovirus serotype 5 and the knob domain of serotype 3. This modification was shown to alter the receptor recognition profile of the resulting virus [192].
CRADS IN COMBINATION WITH CANCER THERAPIES
Combining CRADs with chemotherapy
Several advanced clinical studies have shown the safety of Onyx-015 when administered systemically (intravenous, intra-arterial) or locally (intratumoral), and a moderate anti-tumor effect was reported [193–196]. However, oncolytic adenoviruses are more effective when associated with chemotherapy [38, 196–198] (Table 2). This indicates a synergy between the oncolytic effect of the virus and the cytotoxic effect of chemotherapy. Since the cell killing mechanisms of these therapies are not identical it is thought that these combinations will not lead to tumor resistance in the course of the treatment. Another possible explanation of the enhanced efficacy of the combination treatment is the possibility of immunosuppressing the immune system by the chemotherapeutic drugs thus allowing the replication of adenoviruses in the host as suggested earlier [199].
Table 2.
Prodrug armed Adenoviruses. Diverse strategies allow for the incorporation of prodrug-activating enzymes into treatment schemes to produce an improved anti-tumor combination therapy.
| Adenovirus | Tumor- specificity |
Mode of Action | Prodrug | Clinical Trial(s) |
Ref |
|---|---|---|---|---|---|
| AdTKRC | E1B-55 kDa | Oncolysis & suicide (TK) gene therapy | Ganciclovir | In vivo | [243] |
| Ad-5-CD-Tkrep or FGR | E1B-55 kDa | Oncolysis & suicide (CD & TK) gene therapy | 5-Fluorocytosine & Ganciclovir | Phase I | [206] |
| ONYX-710→-713 (dl1520-CE) | E1B-55 kDa | Oncolysis & suicide (Carboxylesterase) | Irinotecan or CPT-11 | In vivo | [244] |
| Onyx-015, CD/HSV-1, TK | E1B-55 kDa | Oncolysis & suicide (CD & TK) gene therapy | 5-Fluorocytosine & Ganciclovir | In vitro | [206] |
This combination with chemotherapy could be further enhanced by the use of a prodrug-activating enzyme system, also known as suicide gene therapy. This will allow for a high concentration of locally activated drugs thus minimizing systemic toxicity. Several prodrug-activating systems were introduced in the last two decades and some of them have been extensively tested in clinical trials. Examples of prodrug activating enzymes include the herpes simplex virus thymidine kinase (HSVtk), which convert the anti-viral nucleoside analog ganciclovir to a DNA replication inhibitor [200], the bacterial cytosine deaminase (CD) which converts 5-Flurocytosine (5-FC) to 5-fluorouracil (5-FU) [201], cytochrome P450 and the NADPH P450 enzymes which activate a wide variety of anti-cancer prodrugs, including cyclophosphamide (CPA), and the bioreductive prodrug Tirapazamine (TPZ), which is activated under hypoxia [187, 199, 202, 203]. While the activation of prodrugs could synergize with the oncolytic effect of CRADs, it is important however to avoid prodrugs that could inhibit the replication of adenoviruses. Indeed it was reported that high concentrations of topoisomerase inhibitors campothecins could inhibit adenovirus replication [204]. This possibility will be dependent on the mechanism of action for each drug. However, in the context of prodrug-activating enzymes with a very low Km, the amount of active metabolites in infected cells could reach critical levels capable of interfering with adenovirus replication and spread in the tumor.
Combination with radiotherapy
Unlike chemotherapy, radiation therapy is a localized treatment and as such it could be applied in combination with local adenovirus treatment. This modality is clearly less toxic than chemotherapy but still with a great efficacy. Radiation therapy that was used in combination with oncolytic adenoviruses such as Onyx-015 translated into higher tumor free periods and longer survival than in animals receiving monotherapies [205]. Similar indicators of enhanced anti-cancer benefit were reported with other oncolytic adenoviruses [206–208]. Like chemotherapy, radiation did not impede adenovirus replication, and it seemed to potentiate the oncolytic effect of adenoviruses indicating that the order of administration is important.
CRADs with anti-angiogenic therapy
Several angiogenesis inhibitors have been approved for clinical use in 29 countries including the United States. However, anti-angiogenic drugs administered as single agents can produce only modest objective responses [2, 3], are not able to enhance patients survival in clinical trials [4], and may require chemotherapy to exert their therapeutic benefit [5, 6]. It was even suggested that anti-angiogenic resistance mechanisms could be developed by reducing tumor response to hypoxia through the loss of p53 function [7] or switching proangiogenic factors. For example, some cancers initially produce vascular endothelium growth factor (VEGF), but will later express other angiogenic factors due to new mutations or express several angiogenic factors at the same time [209]. To date 27 endogenous angiogenesis inhibitors have been identified [210]. Difficulty in manufacturing—which will ultimately translate into high costs to patients, and the poor efficacy of angiogenesis inhibitors as monotherapies suggests that multimodal treatments should be considered for this class of therapeutic. Delivery of angiogenic inhibitors using replicating adenoviruses will preclude manufacturing difficulties and ensure a continuous production of these inhibitors in cancer tissues. Indeed, several angiogenesis inhibitors have been delivered using adenoviruses: endostatin and angiostatin [211, 212], a thrompospondin peptide [213], and a soluble VEGF receptor for lung metastasis of renal cell carcinoma and colon tumor xenograft [214, 215]. The use of an angiogenesis inhibitor in the context of CRADs is a very attractive modality and could be the standard protocol for administering these costly and difficult to manufacture molecules.
ADMINISTRATION OF ONCOLYTIC ADENOVIRUSES FOR CANCER THERAPY
Onyx-015 was the first conditionally-replicating oncolytic adenovirus to be tested in a clinical trial. The safety and anti-tumor efficacy of this type of oncolytic adenoviruses is now evident. The therapeutic efficiency of oncolytic adenoviruses will be dependent on the route of administration. Intratumoral administration is by far the most common modality for administering these agents. This modality increases targeting and enhances the chances of the initial tumor cells’ infection. Intratumoral injection would require far less adenovirus than if injected intravenously, intra-arterial or intraperitoneally. These last three modalities require an enormous amount of adenoviral particles: ~2×1013 pfu. This enormous load could trigger an immune response and is often behind the high frequency of flu-like symptoms observed for patients receiving the oncolytic adenoviruses via these routes. Other symptoms accompanying adenovirus injections are the elevation of liver transaminases, hyperbilirubnemia, and increases in inflammatory cytokines (TNFα) and interleukins IL-1 and 6 as well as interferon γ [193, 196]. The tragic death of Jesse Gelsinger 7 years ago during a clinical trial at the University of Pennsylvania in which he received 4×1013 pfu for the treatment of ornithine decarboxylase deficiency [216] shows the need to generate adenoviruses with reduced immunogenicity and higher oncolytic effect. Intratumoral injection was 1000-fold more effective than systemic injections in tumor xenografts [217]. While intratumoral injection would be ideal for localized solid tumors, for metastatic tumors a systemic injection would be required in which case the presence of pre-existing neutralizing antibodies will be an impediment to the success of this strategy and a combination with another treatment such as chemotherapy would be advisable. This problem is compounded by the absence of an animal model and therefore only studies of human patients in clinical trials will help understand how to solve this problem.
MODELS FOR STUDYING ADENOVIRAL SPREAD
Most in vitro studies of oncolytic adenoviruses are carried out in a monolayer system. This model is far from reflecting the tumor environment, which is often hypoxic, necrotic, with heterogeneous cell populations, and with high interstitial fluid pressure (IFP). While the size of an adenovirus would not be a problem limiting its entry into tumors and leaking through blood vessel pores to reach tumors, it is however the higher interstitial pressure of tumors that limits even small molecule entry [218]. IFP was described as uniform in the center of tumors with values as high as 60 mm Hg and dropping towards the periphery of tumors [219]. This would suggest that adenoviral infections will most likely follow a gradient decreasing toward the center of the tumor with higher infection at the periphery. One approach to lowering IFP is the normalization of tumor vasculature by chemotherapy. The concept of normalization was introduced to explain the surge of drug entry into tumors after anti-angiogenic therapy [220]. Normalization is hypothesized to occur after elimination of leaky blood vessels, which are thought to be behind the high IFP. The tumor vasculature is “pruned” and retains only normal vasculature “normalization.” This phenomenon could be well used to improve adenovirus delivery into tumors in a similar fashion as that for drugs. The other missing parameter in the current model is the heterogeneity of tumor cell populations. These diverse populations develop under the specific selective pressure encountered in tumors only. Current oncolytic adenoviruses are designed to take advantage of particular traits found in tumors, such as p53 deficiency, telomerase activation, DF3/MUC1 antigen, Rb, etc. However, targeting a single trait will probably fail and would only be of limited therapeutic utility. Strategies that will target several traits at once will most likely emerge as a better alternative.
Another important aspect of tumor heterogeneity is the presence of cancer stem cells (CSC) with the ability to self renew and to recapitulate tumor growth in vivo. Properties of CSC such as slow growth are thought to be behind the chemoresistance of this population [221]. Therefore, any strategy that does not target CSC will fail. Regarding oncolytic adenoviruses, it is not clear if CSC will be infected and lysed less or more than daughter cells. It may be possible to answer some of these questions by utilizing the spheroid model, which could mimic most of tumor properties including CSC. Models such as the neurospheres [222, 223] could provide the complexity of a 3D structure, CSC with a potentially heterogeneous population, and a pressure gradient changing from the periphery to the center. The model of spheroids is particularly important for tumor cells isolated from clinical samples with the intent to preserve the characteristics found in the original tumors [224].
CONCLUSIONS AND FUTURE DIRECTIONS
A recent assessment showed a decline of 1.1% in the death rate from cancer between 1995 and 2002. This decline constitutes a trend observed in the last 70 years [225]. One can hope that these predictions will apply to the rest of the world. However, in this country this predicted decline could be offset by the increase of aging population in the following decades. Despite the fact that chemotherapy was introduced a half century ago it is the most used treatment for cancers, indicating the clear lack of better alternatives. There is a clear need to diversify the therapeutic options in the fight against cancer, and adenoviruses could be of extreme utility in this regard.
While adenoviruses provide one such possible therapeutic option, their design and application will require many improvements. This will probably be possible as we learn more about adenovirus biology. One pressing problem that needs to be circumvented is that of immunogenicity. Indeed, the vast majority of humans has previous exposure to adenovirus infections and possesses neutralizing antibodies. Nonetheless, it has been shown in clinical trials involving Onyx-015 that repeated intratumoral administrations lead to effective tumor responses despite high titers of neutralizing antibody [198]. This result could be explained by the possibility that large molecules such as immunoglobulins are unable to penetrate deep into tumors as was already suggested [226, 227]. While the anti-tumor efficacy after intratumoral adenovirus delivery may be negligibly affected by preexisting neutralizing antibodies, systemic adenovirus injection schemes will most likely be impeded. Thus, a next step towards adenoviruses improvement involves directly modifying adenoviral protein structure in order to reduce its immunogenicity. A recent study showed that pre-existing adenovirus 5 (Ad5) anti-vector immunity could be circumvented when the seven short hypervariable regions (HVRs) on the surface of the Ad5 hexon protein were replaced with the corresponding HVRs from the rare adenovirus serotype Ad48, [228]. These data also indicate that Ad5-specific neutralizing antibodies are raised against epitopes located within the hexon HVRs [229]. Another emerging concept is that of “shielded” adenoviruses [230]. This strategy is based on the genetic modification of capsid protein pIX, by inserting a large protein [231], which would provide uniformly shielded Ad vectors by concealing the Adenovirus from pre-existing neutralizing antibodies. Such chimaeric adenoviral vectors may have important practical implications for cancer gene therapy and could bring adenoviruses closer to mainstream cancer therapies. Adenoviruses have shown great potential in numerous studies, especially when combined with cytokines [232–235]. However, the curative potential of adenoviruses in those studies was mostly seen in immuno-deficient animals, thus limiting the conclusions regarding their efficacy in immuno-competent hosts. The extent to which animal studies will help in the implementation of adenovirus mediated cancer gene therapy in humans remains to be seen.
Acknowledgement
Supported by a grant from the Susan Komen Breast Cancer Foundation to Youssef Jounaidi. Grant # BCTR0504032
Abbreviations
- Ad
Adenovirus
- CPA
Cyclophosphamide
- TPZ
Tirapazamine
- CRAD
Conditionally Replicating Adenoviruses
- GCV
Ganciclovir
- HSV-TK
Herpes Simplex Virus-Thymidine Kinase
- CYP
Cytochrome P450
- NADPH P450 reductase
Nicotineamide dinucleotide phosphate reduced
- CD
Cytosine Deaminase
- CPG2
Carboxypeptidase G2
- HVR
Hypervariable region of Hexon
- E1A
E1A gene
- HC-Ad
High Capacity Adenovirus
- E1B
E1B gene
- L
Late
- E
Early
- MLP
Major Late Promoter
- PKR pathway
Protein Kinase R pathway
- RGD
Arginine, Glycine, Aspartic acid
- CAR
Coxsackie Adenovirus Receptor
- Rb
Retinobalstoma gene
- VA RNAs
Virus-associated RNAs
References
- 1.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, Reimann JD, Vassel A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Mayer RJ. Two steps forward in the treatment of colorectal cancer. N Engl J Med. 2004;350:2406–2408. doi: 10.1056/NEJMe048098. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 7.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 9.Sinkovics J, Horvath J. New developments in the virus therapy of cancer: a historical review. Intervirology. 1993;36:193–214. doi: 10.1159/000150339. [DOI] [PubMed] [Google Scholar]
- 10.Dock G. Rabies virus vaccination in a patient with cervical carcinoma. Amer J Med Sci. 1904;127:563. [Google Scholar]
- 11.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34:1907–1928. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Southam CM, Moore AE. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–1034. doi: 10.1002/1097-0142(195209)5:5<1025::aid-cncr2820050518>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Webb HE, Smith CE. Viruses in the treatment of cancer. Lancet. 1970;1:1206–1208. doi: 10.1016/s0140-6736(70)91790-3. [DOI] [PubMed] [Google Scholar]
- 14.Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Shen J, Mital D, Hong Y, Alemany R, Zhong WW, Jensik SC, Williams JW. Efficient gene transfer and expression in islets by an adenoviral vector that lacks all viral genes. Cell Transplant. 1999;8:661–671. doi: 10.1177/096368979900800612. [DOI] [PubMed] [Google Scholar]
- 16.Zhang WW, Josephs SF, Zhou J, Fang X, Alemany R, Balague C, Dai Y, Ayares D, Prokopenko E, Lou YC, Sethi E, Hubert-Leslie D, Kennedy M, Ruiz L, Rockow-Magnone S. Development and application of a minimal-adenoviral vector system for gene therapy of hemophilia A. Thromb Haemost. 1999;82:562–571. [PubMed] [Google Scholar]
- 17.Alemany R, Gomez-Manzano C, Balague C, Yung WK, Curiel DT, Kyritsis AP, Fueyo J. Gene therapy for gliomas: molecular targets, adenoviral vectors, and oncolytic adenoviruses. Exp Cell Res. 1999;252:1–12. doi: 10.1006/excr.1999.4623. [DOI] [PubMed] [Google Scholar]
- 18.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 20.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84:570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 21.Nemerow GR, Stewart PL. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S, Reddy V, Dasgupta N, Nemerow GR. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol. 1999;73:2798–2802. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 24.Nevins JR. Control of cellular and viral transcription during adenovirus infection. CRC Crit Rev Biochem. 1986;19:307–322. doi: 10.3109/10409238609082543. [DOI] [PubMed] [Google Scholar]
- 25.de Jong RN, van der Vliet PC. Mechanism of DNA replication in eukaryotic cells: cellular host factors stimulating adenovirus DNA replication. Gene. 1999;236:1–12. doi: 10.1016/s0378-1119(99)00249-8. [DOI] [PubMed] [Google Scholar]
- 26.Yeh P, Perricaudet M. Advances in adenoviral vectors: from genetic engineering to their biology. Faseb J. 1997;11:615–623. doi: 10.1096/fasebj.11.8.9240963. [DOI] [PubMed] [Google Scholar]
- 27.Brandt CD, Kim HW, Vargosko AJ, Jeffries BC, Arrobio JO, Rindge B, Parrott RH, Chanock RM. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am J Epidemiol. 1969;90:484–500. doi: 10.1093/oxfordjournals.aje.a121094. [DOI] [PubMed] [Google Scholar]
- 28.Madisch I, Harste G, Pommer H, Heim A. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J Virol. 2005;79:15265–15276. doi: 10.1128/JVI.79.24.15265-15276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei YF, Lindman K, Wadell G. Two closely related adenovirus genome types with kidney or respiratory tract tropism differ in their binding to epithelial cells of various origins. Virology. 1998;240:254–266. doi: 10.1006/viro.1997.8904. [DOI] [PubMed] [Google Scholar]
- 30.Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao J, Natarajan K, Rajala MS, Astley RA, Ramadan RT, Chodosh J. Vitronectin: a possible determinant of adenovirus type 19 tropism for human corneal epithelium. Am J Ophthalmol. 2005;140:363–369. doi: 10.1016/j.ajo.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 32.Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 33.Rekosh DM, Russell WC, Bellet AJ, Robinson AJ. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977;11:283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- 34.Anderson CW, Young ME, Flint SJ. Characterization of the adenovirus 2 virion protein, mu. Virology. 1989;172:506–512. doi: 10.1016/0042-6822(89)90193-1. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DA, Russell WC. Adenovirus protein-protein interactions: molecular parameters governing the binding of protein VI to hexon and the activation of the adenovirus 23K protease. J Gen Virol. 1995;76(Pt 8):1959–1969. doi: 10.1099/0022-1317-76-8-1959. [DOI] [PubMed] [Google Scholar]
- 36.Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976;17:462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster A, Russell S, Talbot P, Russell WC, Kemp GD. Characterization of the adenovirus proteinase: substrate specificity. J Gen Virol. 1989;70(Pt 12):3225–3234. doi: 10.1099/0022-1317-70-12-3225. [DOI] [PubMed] [Google Scholar]
- 38.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [see comments]. [DOI] [PubMed] [Google Scholar]
- 39.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 40.Stevenson SC, Rollence M, White B, Weaver L, McClelland A. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J Virol. 1995;69:2850–2857. doi: 10.1128/jvi.69.5.2850-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 43.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. Embo J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart PL, Chiu CY, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow GR. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. Embo J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 46.Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauma T, Tuukkanen J, Bergelson JM, Denning G, Hautala T. rab5 GTPase regulates adenovirus endocytosis. J Virol. 1999;73:9664–9668. doi: 10.1128/jvi.73.11.9664-9668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greber UF, Webster P, Weber J, Helenius A. The role of the adenovirus protease on virus entry into cells. Embo J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- 50.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 51.Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greber UF, Suomalainen M, Stidwill RP, Boucke K, Ebersold MW, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. Embo J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dales S, Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973;56:465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- 54.Keblusek P, Dorsman JC, Teunisse AF, Teunissen H, van der Eb AJ, Zantema A. The adenoviral E1A oncoproteins interfere with the growth-inhibiting effect of the cdk-inhibitor p21(CIP1/WAF1) J Gen Virol. 1999;80(Pt 2):381–390. doi: 10.1099/0022-1317-80-2-381. [DOI] [PubMed] [Google Scholar]
- 55.Faha B, Harlow E, Lees E. The adenovirus E1A-associated kinase consists of cyclin E-p33cdk2 and cyclin A-p33cdk2. J Virol. 1993;67:2456–2465. doi: 10.1128/jvi.67.5.2456-2465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arany Z, Newsome D, Oldread E, Livingston DM, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 57.Reid JL, Bannister AJ, Zegerman P, Martinez-Balbas MA, Kouzarides T. E1A directly binds and regulates the P/CAF acetyltransferase. Embo J. 1998;17:4469–4477. doi: 10.1093/emboj/17.15.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Putzer BM, Stiewe T, Parssanedjad K, Rega S, Esche H. E1A is sufficient by itself to induce apoptosis independent of p53 and other adenoviral gene products. Cell Death Differ. 2000;7:177–188. doi: 10.1038/sj.cdd.4400618. [DOI] [PubMed] [Google Scholar]
- 59.Paulson M, Pisharody S, Pan L, Guadagno S, Mui AL, Levy DE. Stat protein transactivation domains recruit p300/CBP through widely divergent sequences. J Biol Chem. 1999;274:25343–25349. doi: 10.1074/jbc.274.36.25343. [DOI] [PubMed] [Google Scholar]
- 60.Look DC, Roswit WT, Frick AG, Gris-Alevy Y, Dickhaus DM, Walter MJ, Holtzman MJ. Direct suppression of Stat1 function during adenoviral infection. Immunity. 1998;9:871–880. doi: 10.1016/s1074-7613(00)80652-4. [DOI] [PubMed] [Google Scholar]
- 61.Mazzarelli JM, Mengus G, Davidson I, Ricciardi RP. The transactivation domain of adenovirus E1A interacts with the C terminus of human TAF(II)135. J Virol. 1997;71:7978–7983. doi: 10.1128/jvi.71.10.7978-7983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JO, Russo AA, Pavletich NP. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391:859–865. doi: 10.1038/36038. [DOI] [PubMed] [Google Scholar]
- 63.Hale TK, Braithwaite AW. The adenovirus oncoprotein E1a stimulates binding of transcription factor ETF to transcriptionally activate the p53 gene. J Biol Chem. 1999;274:23777–23786. doi: 10.1074/jbc.274.34.23777. [DOI] [PubMed] [Google Scholar]
- 64.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. Embo J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao W, Levine AJ. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci U S A. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin J, Chen J, Elenbaas B, Levine AJ. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 67.Grand RJ, Turnell AS, Mason GG, Wang W, Milner AE, Mymryk JS, Rookes SM, Rivett AJ, Gallimore PH. Adenovirus early region 1A protein binds to mammalian SUG1-a regulatory component of the proteasome. Oncogene. 1999;18:449–458. doi: 10.1038/sj.onc.1202304. [DOI] [PubMed] [Google Scholar]
- 68.Sarnow P, Ho YS, Williams J, Levine AJ. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 69.Grand RJ, Parkhill J, Szestak T, Rookes SM, Roberts S, Gallimore PH. Definition of a major p53 binding site on Ad2E1B58K protein and a possible nuclear localization signal on the Ad12E1B54K protein. Oncogene. 1999;18:955–965. doi: 10.1038/sj.onc.1202358. [DOI] [PubMed] [Google Scholar]
- 70.Querido E, Marcellus RC, Lai A, Charbonneau R, Teodoro JG, Ketner G, Branton PE. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steegenga WT, Riteco N, Jochemsen AG, Fallaux FJ, Bos JL. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 72.Martin ME, Berk AJ. Adenovirus E1B 55K represses p53 activation in vitro. J Virol. 1998;72:3146–3154. doi: 10.1128/jvi.72.4.3146-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White E, Sabbatini P, Debbas M, Wold WS, Kusher DI, Gooding LR. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 75.Kasof GM, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;19:4390–4404. doi: 10.1128/mcb.19.6.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imazu T, Shimizu S, Tagami S, Matsushima M, Nakamura Y, Miki T, Okuyama A, Tsujimoto Y. Bcl-2/E1B 19 kDa-interacting protein 3-like protein (Bnip3L) interacts with bcl-2/Bcl-xL and induces apoptosis by altering mitochondrial membrane permeability. Oncogene. 1999;18:4523–4529. doi: 10.1038/sj.onc.1202722. [DOI] [PubMed] [Google Scholar]
- 77.Harrison D, Sauthoff H, Heitner S, Jagirdar J, Rom WN, Hay JG. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved--deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum Gene Ther. 2001;12:1323–1332. doi: 10.1089/104303401750270977. [DOI] [PubMed] [Google Scholar]
- 78.Duque PM, Alonso C, Sanchez-Prieto R, Lleonart M, Martinez C, de Buitrago GG, Cano A, Quintanilla M, Ramon y Cajal S. Adenovirus lacking the 19-kDa and 55-kDa E1B genes exerts a marked cytotoxic effect in human malignant cells. Cancer Gene Ther. 1999;6:554–563. doi: 10.1038/sj.cgt.7700077. [DOI] [PubMed] [Google Scholar]
- 79.Hay RT, Freeman A, Leith I, Monaghan A, Webster A. Molecular interactions during adenovirus DNA replication. Curr Top Microbiol Immunol. 1995;199(Pt 2):31–48. doi: 10.1007/978-3-642-79499-5_2. [DOI] [PubMed] [Google Scholar]
- 80.Bennett EM, Bennink JR, Yewdell JW, Brodsky FM. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J Immunol. 1999;162:5049–5052. [PubMed] [Google Scholar]
- 81.Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WS. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 82.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 83.Zou A, Atencio I, Huang WM, Horn M, Ramachandra M. Overexpression of adenovirus E3-11.6K protein induces cell killing by both caspase-dependent and caspase-independent mechanisms. Virology. 2004;326:240–249. doi: 10.1016/j.virol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [see comments]. [DOI] [PubMed] [Google Scholar]
- 85.Halbert DN, Cutt JR, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodrum FD, Ornelles DA. Roles for the E4 orf6, orf3, and E1B 55-kilodalton proteins in cell cycle-independent adenovirus replication. J Virol. 1999;73:7474–7488. doi: 10.1128/jvi.73.9.7474-7488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fessler SP, Young CS. Control of adenovirus early gene expression during the late phase of infection. J Virol. 1998;72:4049–4056. doi: 10.1128/jvi.72.5.4049-4056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma Y, Mathews MB. Structure, function, and evolution of adenovirus-associated RNA: a phylogenetic approach. J Virol. 1996;70:5083–5099. doi: 10.1128/jvi.70.8.5083-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 90.O'Malley RP, Mariano TM, Siekierka J, Mathews MB. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986;44:391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- 91.Aparicio O, Razquin N, Zaratiegui M, Narvaiza I, Fortes P. Adenovirus virus-associated RNA is processed to functional interfering RNAs involved in virus production. J Virol. 2006;80:1376–1384. doi: 10.1128/JVI.80.3.1376-1384.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cascallo M, Capella G, Mazo A, Alemany R. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 2003;63:5544–5550. [PubMed] [Google Scholar]
- 93.Andersson MG, Haasnoot PC, Xu N, Berenjian S, Berkhout B, Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hearing P, Samulski RJ, Wishart WL, Shenk T. Identification of a repeated sequence element required for efficient encapsidation of the adenovirus type 5 chromosome. J Virol. 1987;61:2555–2558. doi: 10.1128/jvi.61.8.2555-2558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bett AJ, Prevec L, Graham FL. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilgenkrantz H, Duboc D, Juillard V, Couton D, Pavirani A, Guillet JG, Briand P, Kahn A. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum Gene Ther. 1995;6:1265–1274. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- 99.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Graham FL, Smiley J, Russel WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 101.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan JC, Perricaudet M, et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 102.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Pavirani A, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moorhead JW, Clayton GH, Smith RL, Schaack J. A replication-incompetent adenovirus vector with the preterminal protein gene deleted efficiently transduces mouse ears. J Virol. 1999;73:1046–1053. doi: 10.1128/jvi.73.2.1046-1053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Su Q, Wilson JM. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks RJ, Graham FL, Kochanek S, Bett AJ, Caskey CT. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steinwaerder DS, Carlson CA, Lieber A. Generation of adenovirus vectors devoid of all viral genes by recombination between inverted repeats. J Virol. 1999;73:9303–9313. doi: 10.1128/jvi.73.11.9303-9313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci U S A. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fisher KJ, Choi H, Burda J, Chen SJ, Wilson JM. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 112.Clemens PR, Kochanek S, Sunada Y, Chan S, Chen HH, Campbell KP, Caskey CT. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- 113.Bilbao R, Reay DP, Wu E, Zheng H, Biermann V, Kochanek S, Clemens PR. Comparison of high-capacity and first-generation adenoviral vector gene delivery to murine muscle in utero. Gene Ther. 2005;12:39–47. doi: 10.1038/sj.gt.3302392. [DOI] [PubMed] [Google Scholar]
- 114.Xiong W, Goverdhana S, Sciascia SA, Candolfi M, Zirger JM, Barcia C, Curtin JF, King GD, Jaita G, Liu C, Kroeger K, Agadjanian H, Medina-Kauwe L, Palmer D, Ng P, Lowenstein PR, Castro MG. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Candolfi M, Curtin JF, Xiong WD, Kroeger KM, Liu C, Rentsendorj A, Agadjanian H, Medina-Kauwe L, Palmer D, Ng P, Lowenstein PR, Castro MG. Effective High-Capacity Gutless Adenoviral Vectors Mediate Transgene Expression in Human Glioma Cells. Mol Ther. 2006 doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD, Beaudet AL. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci U S A. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McNeish IA, Bell SJ, Lemoine NR. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Ther. 2004;11:497–503. doi: 10.1038/sj.gt.3302238. [DOI] [PubMed] [Google Scholar]
- 118.Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 119.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 120.Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 121.Shimada H, Matsubara H, Shiratori T, Shimizu T, Miyazaki S, Okazumi S, Nabeya Y, Shuto K, Hayashi H, Tanizawa T, Nakatani Y, Nakasa H, Kitada M, Ochiai T. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci. 2006;97:554–561. doi: 10.1111/j.1349-7006.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fujiwara T, Grimm EA, Mukhopadhyay T, Zhang WW, Owen-Schaub LB, Roth JA. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]
- 123.Skladanowski A, Larsen AK. Expression of wild-type p53 increases etoposide cytotoxicity in M1 myeloid leukemia cells by facilitated G2 to M transition: implications for gene therapy. Cancer Res. 1997;57:818–823. [PubMed] [Google Scholar]
- 124.Blagosklonny MV, Giannakakou P, Wojtowicz M, Romanova LY, Ain KB, Bates SE, Fojo T. Effects of p53-expressing adenovirus on the chemosensitivity and differentiation of anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 1998;83:2516–2522. doi: 10.1210/jcem.83.7.4984. [DOI] [PubMed] [Google Scholar]
- 125.Spitz FR, Nguyen D, Skibber JM, Meyn RE, Cristiano RJ, Roth JA. Adenoviral-mediated wild-type p53 gene expression sensitizes colorectal cancer cells to ionizing radiation. Clin Cancer Res. 1996;2:1665–1671. [PubMed] [Google Scholar]
- 126.Gallardo D, Drazan KE, McBride WH. Adenovirus-based transfer of wild-type p53 gene increases ovarian tumor radiosensitivity. Cancer Res. 1996;56:4891–4893. [PubMed] [Google Scholar]
- 127.Chang EH, Jang YJ, Hao Z, Murphy G, Rait A, Fee WE, Jr, Sussman HH, Ryan P, Chiang Y, Pirollo KF. Restoration of the G1 checkpoint and the apoptotic pathway mediated by wild-type p53 sensitizes squamous cell carcinoma of the head and neck to radiotherapy. Arch Otolaryngol Head Neck Surg. 1997;123:507–512. doi: 10.1001/archotol.1997.01900050055007. [DOI] [PubMed] [Google Scholar]
- 128.Bracey TS, Miller JC, Preece A, Paraskeva C. Gamma-radiation-induced apoptosis in human colorectal adenoma and carcinoma cell lines can occur in the absence of wild type p53. Oncogene. 1995;10:2391–2396. [PubMed] [Google Scholar]
- 129.Huang H, Li CY, Little JB. Abrogation of P53 function by transfection of HPV16 E6 gene does not enhance resistance of human tumour cells to ionizing radiation. Int J Radiat Biol. 1996;70:151–160. doi: 10.1080/095530096145148. [DOI] [PubMed] [Google Scholar]
- 130.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 131.Conseiller E, Debussche L, Landais D, Venot C, Maratrat M, Sierra V, Tocque B, Bracco L. CTS1: a p53-derived chimeric tumor suppressor gene with enhanced in vitro apoptotic properties. J Clin Invest. 1998;101:120–127. doi: 10.1172/JCI1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang HJ, Qian D, Sondak VK, Stachura S, Lin J. A modified p53 enhances apoptosis in sarcoma cell lines mediated by doxorubicin. Br J Cancer. 2004;90:1285–1292. doi: 10.1038/sj.bjc.6601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 134.Tiemann F, Zerrahn J, Deppert W. Cooperation of simian virus 40 large and small T antigens in metabolic stabilization of tumor suppressor p53 during cellular transformation. J Virol. 1995;69:6115–6121. doi: 10.1128/jvi.69.10.6115-6121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takada S, Kaneniwa N, Tsuchida N, Koike K. Cytoplasmic retention of the p53 tumor suppressor gene product is observed in the hepatitis B virus X gene-transfected cells. Oncogene. 1997;15:1895–1901. doi: 10.1038/sj.onc.1201369. [DOI] [PubMed] [Google Scholar]
- 136.Tsao YP, Huang SJ, Chang JL, Hsieh JT, Pong RC, Chen SL. Adenovirus-mediated p21((WAF1/SDII/CIP1)) gene transfer induces apoptosis of human cervical cancer cell lines. J Virol. 1999;73:4983–4990. doi: 10.1128/jvi.73.6.4983-4990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsiao M, Tse V, Carmel J, Costanzi E, Strauss B, Haas M, Silverberg GD. Functional expression of human p21(WAF1/CIP1) gene in rat glioma cells suppresses tumor growth in vivo and induces radiosensitivity. Biochem Biophys Res Commun. 1997;233:329–335. doi: 10.1006/bbrc.1997.6450. [DOI] [PubMed] [Google Scholar]
- 138.Fan S, Chang JK, Smith ML, Duba D, Fornace AJ, Jr, O'Connor PM. Cells lacking CIP1/WAF1 genes exhibit preferential sensitivity to cisplatin and nitrogen mustard. Oncogene. 1997;14:2127–2136. doi: 10.1038/sj.onc.1201052. [DOI] [PubMed] [Google Scholar]
- 139.Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 140.Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 141.Gotoh A, Kao C, Ko SC, Hamada K, Liu TJ, Chung LW. Cytotoxic effects of recombinant adenovirus p53 and cell cycle regulator genes (p21 WAF1/CIP1 and p16CDKN4) in human prostate cancers. J Urol. 1997;158:636–641. [PubMed] [Google Scholar]
- 142.Stone S, Dayananth P, Kamb A. Reversible, p16-mediated cell cycle arrest as protection from chemotherapy. Cancer Res. 1996;56:3199–3202. [PubMed] [Google Scholar]
- 143.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 144.Xu HJ, Xu K, Zhou Y, Li J, Benedict WF, Hu SX. Enhanced tumor cell growth suppression by an N-terminal truncated retinoblastoma protein. Proc Natl Acad Sci U S A. 1994;91:9837–9841. doi: 10.1073/pnas.91.21.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu HJ, Zhou Y, Seigne J, Perng GS, Mixon M, Zhang C, Li J, Benedict WF, Hu SX. Enhanced tumor suppressor gene therapy via replication-deficient adenovirus vectors expressing an N-terminal truncated retinoblastoma protein. Cancer Res. 1996;56:2245–2249. [PubMed] [Google Scholar]
- 146.Ponce-Castaneda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J, et al. p27Kip1: chromosomal mapping to 12p12–12p13.1 and absence of mutations in human tumors. Cancer Res. 1995;55:1211–1214. [PubMed] [Google Scholar]
- 147.Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee SJ, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997;14:2283–2289. doi: 10.1038/sj.onc.1201064. [DOI] [PubMed] [Google Scholar]
- 148.Chen J, Willingham T, Shuford M, Nisen PD. Tumor suppression and inhibition of aneuploid cell accumulation in human brain tumor cells by ectopic overexpression of the cyclin-dependent kinase inhibitor p27KIP1. J Clin Invest. 1996;97:1983–1988. doi: 10.1172/JCI118631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sauthoff H, Pipiya T, Heitner S, Chen S, Norman RG, Rom WN, Hay JG. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum Gene Ther. 2002;13:1859–1871. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]
- 150.Roth J, Konig C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. Inactivation of p53 but not p73 by adenovirus type 5 E1B 55-kilodalton and E4 34-kilodalton oncoproteins. J Virol. 1998;72:8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koch P, Gatfield J, Lober C, Hobom U, Lenz-Stoppler C, Roth J, Dobbelstein M. Efficient replication of adenovirus despite the overexpression of active and nondegradable p53. Cancer Res. 2001;61:5941–5947. [PubMed] [Google Scholar]
- 152.Flint J, Shenk T. Viral transactivating proteins. Annu Rev Genet. 1997;31:177–212. doi: 10.1146/annurev.genet.31.1.177. [DOI] [PubMed] [Google Scholar]
- 153.Wu X, Levine AJ. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yew PR, Berk AJ. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 155.Rothmann T, Hengstermann A, Whitaker NJ, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ries SJ, Brandts CH, Chung AS, Biederer CH, Hann BC, Lipner EM, McCormick F, Korn WM. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015) Nat Med. 2000;6:1128–1133. doi: 10.1038/80466. [DOI] [PubMed] [Google Scholar]
- 157.Yang CT, You L, Uematsu K, Yeh CC, McCormick F, Jablons DM. p14(ARF) modulates the cytolytic effect of ONYX-015 in mesothelioma cells with wild-type p53. Cancer Res. 2001;61:5959–5963. [PubMed] [Google Scholar]
- 158.Harada JN, Berk AJ. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Babiss LE, Ginsberg HS, Darnell JE., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, Shen Y, Habets G, Ginzinger D, McCormick F. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 162.O'Shea CC, Soria C, Bagus B, McCormick F. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell. 2005;8:61–74. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 163.Fabra A, Parada C, Vinyals A, Martin Duque P, Fernandez V, Sanchez-Prieto R, Ramon y Cajal S. Intravascular injections of a conditional replicative adenovirus (adl118) prevent metastatic disease in human breast carcinoma xenografts. Gene Ther. 2001;8:1627–1634. doi: 10.1038/sj.gt.3301567. [DOI] [PubMed] [Google Scholar]
- 164.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 165.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 166.Hallenbeck PL, Chang YN, Hay C, Golightly D, Stewart D, Lin J, Phipps S, Chiang YL. A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Hum Gene Ther. 1999;10:1721–1733. doi: 10.1089/10430349950017725. [DOI] [PubMed] [Google Scholar]
- 167.Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Irving J, Wang Z, Powell S, O'Sullivan C, Mok M, Murphy B, Cardoza L, Lebkowski JS, Majumdar AS. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11:174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- 169.Tsukuda K, Wiewrodt R, Molnar-Kimber K, Jovanovic VP, Amin KM. An E2F-responsive replication-selective adenovirus targeted to the defective cell cycle in cancer cells: potent antitumoral efficacy but no toxicity to normal cell. Cancer Res. 2002;62:3438–3447. [PubMed] [Google Scholar]
- 170.Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 171.Manome Y, Kunieda T, Wen PY, Koga T, Kufe DW, Ohno T. Transgene expression in malignant glioma using a replication-defective adenoviral vector containing the Egr-1 promoter: activation by ionizing radiation or uptake of radioactive iododeoxyuridine. Hum Gene Ther. 1998;9:1409–1417. doi: 10.1089/hum.1998.9.10-1409. [DOI] [PubMed] [Google Scholar]
- 172.Zhang L, Akbulut H, Tang Y, Peng X, Pizzorno G, Sapi E, Manegold S, Deisseroth A. Adenoviral vectors with E1A regulated by tumor-specific promoters are selectively cytolytic for breast cancer and melanoma. Mol Ther. 2002;6:386–393. doi: 10.1006/mthe.2002.0680. [DOI] [PubMed] [Google Scholar]
- 173.Shirakawa T, Hamada K, Zhang Z, Okada H, Tagawa M, Kamidono S, Kawabata M, Gotoh A. A cox-2 promoter-based replication-selective adenoviral vector to target the cox-2-expressing human bladder cancer cells. Clin Cancer Res. 2004;10:4342–4348. doi: 10.1158/1078-0432.CCR-03-0267. [DOI] [PubMed] [Google Scholar]
- 174.Matsubara S, Wada Y, Gardner TA, Egawa M, Park MS, Hsieh CL, Zhau HE, Kao C, Kamidono S, Gillenwater JY, Chung LW. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–6019. [PubMed] [Google Scholar]
- 175.Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, Szabo E. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 1998;58:5582–5589. [PubMed] [Google Scholar]
- 176.Friedman EL, Hayes DF, Kufe DW. Reactivity of monoclonal antibody DF3 with a high molecular weight antigen expressed in human ovarian carcinomas. Cancer Res. 1986;46:5189–5194. [PubMed] [Google Scholar]
- 177.Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, Dahiya R, Kim YS. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- 178.Metzgar RS, Rodriguez N, Finn OJ, Lan MS, Daasch VN, Fernsten PD, Meyers WC, Sindelar WF, Sandler RS, Seigler HF. Detection of a pancreatic cancer-associated antigen (DU-PAN-2 antigen) in serum and ascites of patients with adenocarcinoma. Proc Natl Acad Sci U S A. 1984;81:5242–5246. doi: 10.1073/pnas.81.16.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 180.Poole JC, Andrews LG, Tollefsbol TO. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT) Gene. 2001;269:1–12. doi: 10.1016/s0378-1119(01)00440-1. [DOI] [PubMed] [Google Scholar]
- 181.Kanaya T, Kyo S, Hamada K, Takakura M, Kitagawa Y, Harada H, Inoue M. Adenoviral expression of p53 represses telomerase activity through down-regulation of human telomerase reverse transcriptase transcription. Clin Cancer Res. 2000;6:1239–1247. [PubMed] [Google Scholar]
- 182.Walton T, Wang JL, Ribas A, Barsky SH, Economou J, Nguyen M. Endothelium-specific expression of an E-selectin promoter recombinant adenoviral vector. Anticancer Res. 1998;18:1357–1360. [PubMed] [Google Scholar]
- 183.Banerjee NS, Rivera AA, Wang M, Chow LT, Broker TR, Curiel DT, Nettelbeck DM. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol Cancer Ther. 2004;3:437–449. [PubMed] [Google Scholar]
- 184.Hernandez-Alcoceba R, Pihalja M, Qian D, Clarke MF. New oncolytic adenoviruses with hypoxia- and estrogen receptor-regulated replication. Hum Gene Ther. 2002;13:1737–1750. doi: 10.1089/104303402760293574. [DOI] [PubMed] [Google Scholar]
- 185.Hoffmann D, Wildner O. Restriction of adenoviral replication to the transcriptional intersection of two different promoters for colorectal and pancreatic cancer treatment. Mol Cancer Ther. 2006;5:374–381. doi: 10.1158/1535-7163.MCT-05-0374. [DOI] [PubMed] [Google Scholar]
- 186.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jounaidi Y, Waxman DJ. Use of replication-conditional adenovirus as a helper system to enhance delivery of P450 prodrug-activation genes for cancer therapy. Cancer Res. 2004;64:292–303. doi: 10.1158/0008-5472.can-03-1798. [DOI] [PubMed] [Google Scholar]
- 188.Wood M, Perrotte P, Onishi E, Harper ME, Dinney C, Pagliaro L, Wilson DR. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- 189.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, Sawaya R, Curiel DT, Yung WK, Lang FF. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 190.Witlox AM, Van Beusechem VW, Molenaar B, Bras H, Schaap GR, Alemany R, Curiel DT, Pinedo HM, Wuisman PI, Gerritsen WR. Conditionally replicative adenovirus with tropism expanded towards integrins inhibits osteosarcoma tumor growth in vitro and in vivo. Clin Cancer Res. 2004;10:61–67. doi: 10.1158/1078-0432.ccr-0609-03. [DOI] [PubMed] [Google Scholar]
- 191.Shinoura N, Yoshida Y, Tsunoda R, Ohashi M, Zhang W, Asai A, Kirino T, Hamada H. Highly augmented cytopathic effect of a fiber-mutant E1B-defective adenovirus for gene therapy of gliomas. Cancer Res. 1999;59:3411–3416. [PubMed] [Google Scholar]
- 192.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, Netto G, Tong A, Randlev B, Olson S, Kirn D. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 194.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A, Romel L, Randlev B, Kaye S, Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 195.Nemunaitis J, Cunningham C, Tong AW, Post L, Netto G, Paulson AS, Rich D, Blackburn A, Sands B, Gibson B, Randlev B, Freeman S. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther. 2003;10:341–352. doi: 10.1038/sj.cgt.7700585. [DOI] [PubMed] [Google Scholar]
- 196.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, Randlev B, Heise C, Uprichard M, Hatfield M, Rome L, Rubin J, Kirn D. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- 197.Yu DC, Chen Y, Dilley J, Li Y, Embry M, Zhang H, Nguyen N, Amin P, Oh J, Henderson DR. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–525. [PubMed] [Google Scholar]
- 198.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 199.Jounaidi Y. Cytochrome P450-based Gene Therapy for Cancer Treatment: From Concept to the Clinic. Curr Drug Metab. 2002;3:609–622. doi: 10.2174/1389200023337027. [DOI] [PubMed] [Google Scholar]
- 200.Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc. Natl. Acad. Sci. USA. 1994;91:3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mullen CA, Coale MM, Lowe R, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994;54:1503–1506. [PubMed] [Google Scholar]
- 202.Jounaidi Y, Hecht JED, Waxman DJ. Retroviral transfer of human cytochrome P450 genes for oxazaphosphorine-based cancer gene therapy. Cancer Res. 1998;58:4391–4401. [PubMed] [Google Scholar]
- 203.Jounaidi Y, Waxman DJ. Combination of the bioreductive drug tirapazamine with the chemotherapeutic prodrug cyclophosphamide for P450/P450-reductase-based cancer gene therapy. Cancer Res. 2000;60:3761–3769. [PubMed] [Google Scholar]
- 204.Schaack J, Schedl P, Shenk T. Topoisomerase I and II cleavage of adenovirus DNA in vivo: both topoisomerase activities appear to be required for adenovirus DNA replication. J Virol. 1990;64:78–85. doi: 10.1128/jvi.64.1.78-85.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rogulski KR, Freytag SO, Zhang K, Gilbert JD, Paielli DL, Kim JH, Heise CC, Kirn DH. In vivo antitumor activity of ONYX-015 is influenced by p53 status and is augmented by radiotherapy. Cancer Res. 2000;60:1193–1196. [PubMed] [Google Scholar]
- 206.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 207.Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, Lee J, Pennathur-Das R, Radzyminski J, Wypych J, Brignetti D, Scott S, Stephens J, Karpf DB, Henderson DR, Yu DC. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453–5460. [PubMed] [Google Scholar]
- 208.Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, Alemany R, Fueyo J, Curiel DT, Vassal G, Pinedo HM, Vandertop WP, Gerritsen WR. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- 209.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 210.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 211.Li GC, Nie MM, Yang JM, Su CQ, Sun LC, Qian YZ, Sham J, Fang GE, Wu MC, Qian QJ. [Treatment of hepatocellular carcinoma with a novel gene-viral therapeutic system CNHK300-murine endostatin] Zhonghua Yi Xue Za Zhi. 2004;84:943–948. [PubMed] [Google Scholar]
- 212.Schmitz V, Wang L, Barajas M, Gomar C, Prieto J, Qian C. Treatment of colorectal and hepatocellular carcinomas by adenoviral mediated gene transfer of endostatin and angiostatin-like molecule in mice. Gut. 2004;53:561–567. doi: 10.1136/gut.2003.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu P, Wang Y, Li YH, Yang C, Zhou YL, Li B, Lu SH, Yang RC, Cai YL, Tobelem G, Caen J, Han ZC. Adenovirus-mediated gene therapy with an antiangiogenic fragment of thrombospondin-1 inhibits human leukemia xenograft growth in nude mice. Leuk Res. 2003;27:701–708. doi: 10.1016/s0145-2126(02)00346-6. [DOI] [PubMed] [Google Scholar]
- 214.Yoshimura I, Mizuguchi Y, Miyajima A, Asano T, Tadakuma T, Hayakawa M. Suppression of lung metastasis of renal cell carcinoma by the intramuscular gene transfer of a soluble form of vascular endothelial growth factor receptor I. J Urol. 2004;171:2467–2470. doi: 10.1097/01.ju.0000117801.04926.a8. [DOI] [PubMed] [Google Scholar]
- 215.Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B, Zhao L, Qian C, Qian Q, Liu X. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol Ther. 2005;11:553–562. doi: 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 216.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 217.Demers GW, Johnson DE, Tsai V, Wen SF, Quijano E, Machemer T, Philopena J, Ramachandra M, Howe JA, Shabram P, Ralston R, Engler H. Pharmacologic indicators of antitumor efficacy for oncolytic virotherapy. Cancer Res. 2003;63:4003–4008. [PubMed] [Google Scholar]
- 218.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 219.DiResta GR, Lee J, Larson SM, Arbit E. Characterization of neuroblastoma xenograft in rat flank. I. Growth, interstitial fluid pressure, and interstitial fluid velocity distribution profiles. Microvasc Res. 1993;46:158–177. doi: 10.1006/mvre.1993.1044. [DOI] [PubMed] [Google Scholar]
- 220.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 221.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 222.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 224.Lamfers ML, Hemminki A. Multicellular tumor spheroids in gene therapy and oncolytic virus therapy. Curr Opin Mol Ther. 2004;6:403–411. [PubMed] [Google Scholar]
- 225.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 226.Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55:4611–4622. [PubMed] [Google Scholar]
- 227.Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–1528. [PubMed] [Google Scholar]
- 228.Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 229.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, Jackson SS, Gorgone DA, Lifton MA, Essex M, Walker BD, Goudsmit J, Havenga MJ, Barouch DH. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 230.Hedley SJ, Chen J, Mountz JD, Li J, Curiel DT, Korokhov N, Kovesdi I. Targeted and shielded adenovectors for cancer therapy. Cancer Immunol Immunother. 2006 doi: 10.1007/s00262-006-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li J, Le L, Sibley DA, Mathis JM, Curiel DT. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology. 2005;338:247–258. doi: 10.1016/j.virol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 232.Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, Fisher PB. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- 234.Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, Wang Y, Qian Q, Qian C, Wu J, Liu XY. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–4298. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- 235.Su C, Peng L, Sham J, Wang X, Zhang Q, Chua D, Liu C, Cui Z, Xue H, Wu H, Yang Q, Zhang B, Liu X, Wu M, Qian Q. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-gamma gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 236.Khoobyarian N, Barone F, Sabet T, El-Domeiri AA, Das Gupta TK. Inhibition of melanoma growth in hamsters by type-2 adenovirus. J Surg Oncol. 1975;7:421–425. doi: 10.1002/jso.2930070514. [DOI] [PubMed] [Google Scholar]
- 237.Sauthoff H, Heitner S, Rom WN, Hay JG. Deletion of the adenoviral E1b-19kD gene enhances tumor cell killing of a replicating adenoviral vector. Hum Gene Ther. 2000;11:379–388. doi: 10.1089/10430340050015851. [DOI] [PubMed] [Google Scholar]
- 238.Duque PM, Alonso C, Sanchez-Prieto R, Quintanilla M, Ramon S, Ramon y Cajal S. Antitumoral effect of E1B defective adenoviruses in human malignant cells. Gene Ther. 1998;5:286–287. doi: 10.1038/sj.gt.3300585. [DOI] [PubMed] [Google Scholar]
- 239.Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, Manns M, Kubicka S, Kuhnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. [PubMed] [Google Scholar]
- 240.Johnson L, Shen A, Boyle L, Kunich J, Pandey K, Lemmon M, Hermiston T, Giedlin M, McCormick F, Fattaey A. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–337. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 241.Yu DC, Chen Y, Seng M, Dilley J, Henderson DR. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59:4200–4203. [PubMed] [Google Scholar]
- 242.Yu DC, Sakamoto GT, Henderson DR. Identification of the transcriptional regulatory sequences of human kallikrein 2 and their use in the construction of calydon virus 764, an attenuated replication competent adenovirus for prostate cancer therapy. Cancer Res. 1999;59:1498–1504. [PubMed] [Google Scholar]
- 243.Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- 244.Stubdal H, Perin N, Lemmon M, Holman P, Bauzon M, Potter PM, Danks MK, Fattaey A, Dubensky T, Johnson L. A prodrug strategy using ONYX-015-based replicating adenoviruses to deliver rabbit carboxylesterase to tumor cells for conversion of CPT-11 to SN-38. Cancer Res. 2003;63:6900–6908. [PubMed] [Google Scholar]