Summary
A missense mutation (R43Q) in the γ2 subunit of the GABAA receptor is associated with generalised (genetic) epilepsy with febrile seizures plus (GEFS+). Heterozygous GABAAγ2(R43Q) mice displayed a lower temperature threshold for thermal seizures as compared to wild type littermates. Temperature-dependent internalization of GABAAγ2(R43Q) containing receptors has been proposed as a mechanism underlying febrile seizure genesis in patients with this mutation. We tested this idea using the GABAAγ2(R43Q) knockin mouse model and analysed GABAergic miniature post synaptic inhibitory currents (mIPSCs) in acute brain slices after exposure to varying temperatures. Incubation of slices at an elevated temperature increased mIPSC amplitude in neurons from heterozygous mice with no change seen in wild type controls. [3H]Flumazenil binding measured in whole brain homogenates from mutant and control mice following elevation of body temperature showed no temperature-dependent differences in γ2-containing receptor density. Therefore, in vivo mouse data does not support earlier in vitro observations that proposed temperature-dependent internalization of γ2 R43Q containing GABAA receptors as the cellular mechanism underlying febrile seizure genesis in patients with the GABAAγ2(R43Q) mutation.
Keywords: Febrile seizures, GABAA receptor, Temperature, Receptor trafficking, Cortex, Mice
Introduction
Febrile seizures (FS) are a common form of epilepsy that impact 3% of children. As many as 30–50% of patients exhibiting recurrent seizures including temporal lobe epilepsy have a history of FS (Cendes & Andermann 2002). The GABRG2R43/Q43 (R43Q) mutation was identified in a large Australian family presenting with generalized (genetic) epilepsy with febrile seizures plus (GEFS+) (Wallace, et al. 2001). The GABAA γ2 subunit plays an important role in subcellular receptor trafficking and is essential for postsynaptic clustering of receptors (Jacob et al., 2008).
In vitro data suggest that the R43Q mutation confers temperature sensitivity to the GABAA receptor complex such that even a brief (30 minute) increase in temperature to 40°C results in a marked decrease in cell surface expression and reduced current responses to GABA application (Kang et al., 2006). Based on these findings the authors proposed that a heat-mediated reduction in GABAA receptor cell surface expression is responsible for seizure genesis in patients with the R43Q mutation.
We used a genetically engineered knockin mouse model harbouring the R43Q mutation (Tan, et al. 2007) and characterized the effect of this mutation on receptor trafficking in situ by investigating phasic cortical GABAergic inhibition at elevated temperatures in brain slices. Additionally, we conducted a radioligand binding assay to assess for changes in the number of membrane-bound GABAA receptor complexes in R43Q mice after thermal stress. Thermal seizure thresholds were also assessed in these mice. In contrast to studies in heterologous systems we observed no heat-dependent decreases in either of these markers of GABAA receptor cell surface expression. In contrast, pre-incubating slices from R43Q animals at 38°C increased the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) compared to that of wild type suggesting that internalization of R43Q containing GABAA receptors is unlikely to be responsible for FS in patients harbouring this mutation.
Methods
All experimental protocols were approved by the Howard Florey Institute Animal Ethics Committee. Thermal Seizure threshold: P14-17 wild-type and heterozygous mice body temperatures were maintained at ~ 40–41.5°C for 30 minutes using a warm air stream (hairdryer sound pressure level of approximately 65dB) with core temperature monitoring as outlined by Baram, et al. (1997). Seizure activity was determined by behavioural analysis and experiments were conducted blind to genotype.
Brain slice electrophysiology: Following anaesthesia with 1–3% isoflurane (inhalation), postnatal day 14–17 mice were decapitated, cortical slices were cut (300 µm thick) and miniature inhibitory postsynaptic currents (mIPSCs) were recorded and analysed as described in Tan et al. (2007). Drugs and salts were obtained from Sigma (Sigma-Aldrich Pty. Ltd., Castle Hill, NSW, Australia). Miniature IPSCs in layer 2/3 cortical pyramidal neurons were recorded in voltage clamp mode at 34°C from wild-type (Gabrg2R43R; wt) and heterozygous (Gabrg2R43Q; het) slices incubated at 22°C or 38°C for 1 hr. Only cells recorded within 40 minutes of being transferred to 34°C were included for analysis. For each cell recorded, the number of events and event amplitudes were averaged over three 90 s recording periods.
Radioligand binding assay experiments: FMZ ([3H]Flumazenil (N-methyl-[3H]-Ro 15–1788, 78.6 Ci/mol)) was obtained from Perkin Elmer Life Sciences (Boston, USA). P14-17 animals were heated as described above. Animals were decapitated following anaesthesia with isoflurane and whole brains (excluding cerebellum) quickly removed and homogenised individually for 30 s in approximately 10 tissue volumes of 0.32mM sucrose at 4° C. The resulting supernatant was centrifuged (1240g for 10 min at 4°C) yielding a pellet that was resuspended in 2ml Tris-HCl (50mM, pH7.4). Pellet protein concentration was determined using the BCA assay kit (Sigma-Aldrich) with BSA as a standard. The binding assay of Sihver, et al. (1997) was modified for 96 well filter plates of the Millipore MultiScreen system (Millipore, USA). Brain homogenates were incubated for 30 min in flumazenil at saturated binding concentrations as a measure of Bmax (0.2 and 30nM, 78.6Ci/mmol). Filters were next washed with Tris-HCl buffer. Flumazenil binding on filters was read by a Beckman Ls6500 scintillation counter after a 20h equilibration period with scintillant (5 ml, Ultima Gold, Packard Bioscience, USA). Cold flumazenil was used to measure non-specific binding. All group data are expressed as mean ± s.e.m. and comparisons were made using a 2-way ANOVA test unless otherwise indicated. p< 0.05 was taken as statistical significance.
Results
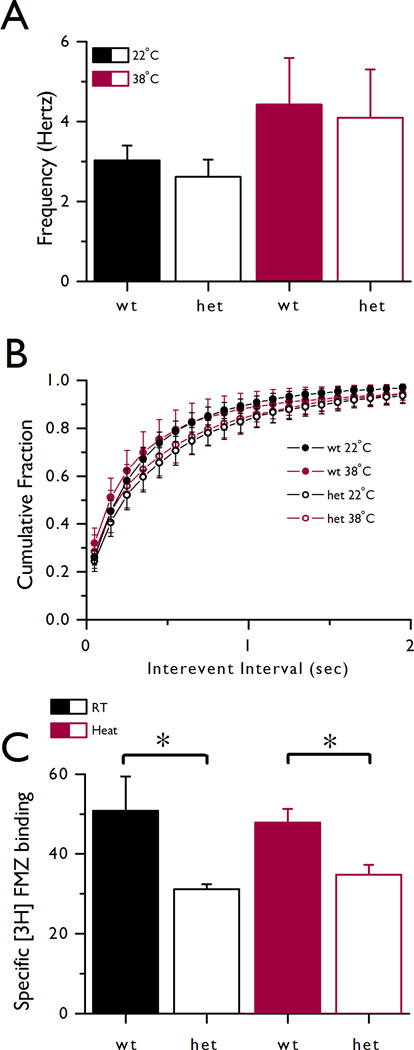
Upon heating, mice underwent seizures that included hypotonic spells similar to those described by Baram et al., (1997). Control experiments in which the hairdryer fan was on but without heat did not result in hypotonic behavior (n=3 heterozygotes and n=3 wild type mice). Mice heterozygous for the mutation (R43Q) displayed a seizure threshold of 38.00 ± 0.13 °C (n = 44) whereas the threshold for wild type mice was 38.44 + 0.13 °C (n = 47; students t-test, p = 0.013, mean ± sem). We investigated the effect of temperature on GABAA receptor-mediated mIPSCs in cortical slices from wild type and R43Q (heterozygous) animals. Cortical brain slices were preheated at either 22°C or 38°C for 1 hour prior to recording at 34°C. No overt differences were observed in the quality of the tissue or voltage clamp recordings obtained from either the 22°C or 38°C slices (Figure 1A-D). The current study confirmed our earlier observations (Tan et al., 2007) that mean mIPSC amplitude is significantly lower in layer 2/3 pyramidal neurons from heterozygous mice compared to wild type counterparts (Figures 1E and 1F). We next compared the effect of pre-incubation at either 22°C or 38°C. Curiously, the only temperature-dependent change we observed was in het mice where mIPSC mean amplitudes were significantly greater following incubation at 38°C for 1 hour (Figures 1E and 1F). The increase in mean mIPSC amplitude may be explained by either an increase in mutant receptor function or cell surface expression following a brief exposure to increased temperature. Cumulative probability histograms confirmed these observations (Figures 1G and 1H) and further suggested that for 38°C pre-incubated het slices, the distribution curve was right shifted, indicating fewer small amplitude events contributing to the total event population (Figure 1H). No change in mIPSC frequency following temperature elevation was observed (p>0.05, Figure 2A). Frequency histograms showed no significant difference between genotypes or treatment groups (p>0.05; Kolmogorov-Smirnoff test; Figure 2B).
Figure 1.
A–D: Representative examples of traces recorded from pyramidal neurons in acute cortical slices taken from wild-type (wt) and heterozygous (het) animals following incubation at 22°C (A, B) or 38°C (C, D) for 1 hr. All recordings were performed at 34°C. E–H: Effect of elevated temperature on mIPSC amplitude. E: Mean IPSCs (averaged traces) following incubation at 22°C or 38°C. E and F: Amplitudes of mIPSCs from heterozygous slices incubated at 22°C were reduced compared to wild type (40.76± 1.50 pA, 50.30± 2.42 pA; P = 0.02, students t-test; black traces and columns). G and H: Cumulative probability histograms illustrating event amplitudes recorded from wt or het, following incubation at 22°C or 38°C. No change in mean amplitude was observed for events recorded from wild type slices preincubated at 22°C or 38°C (50.30 ± 2.42 pA, 49.60 ± 7.43 pA; n=18 and 10 respectively; p=0.91); G. In contrast, mIPSCs recorded from neurons heterozygous for the R43Q mutation showed a statistically significant increase in mean current amplitude following incubation at 38°C degrees compared with 22°C (61.22 ± 3.83 pA, 40.76 ± 1.50 pA; n=11 and 13 respectively, p<0.05); F, asterisk. G: Cumulative probability histograms showed no difference between the distributions of event amplitudes recorded from wt tissue under both conditions (p>0.05, Kolmogorov-Smirnoff test). H: For het slices, the distribution curve was right shifted following pre-incubation at 38°C (p = 0.001, Kolmogorov-Smirnoff test). E–H: Number of cells compared: 18 (wt 22°C), 10 (wt 38°C), 13 (het 22°C), 11 (het 38°C) cells.
Figure 2.
Mean frequency of GABAergic mIPSCs and receptor density were unchanged with heating. A: Mean event frequencies for each condition were (Hz): wt 22°C: 3.03 ± 0.37, het 22°C: 2.61 ± 0.43, wt 38°C: 4.42 ± 1.17, het 38°C: 4.10 ± 1.21; total n(cells)=52; p>0.05). B: Cumulative probability histogram for mIPSC interevent interval showing no significant difference between groups (p>0.05; Kolmogorov-Smirnoff test); wt (22°C and 38°C) and het (22°C and 38°C). Data from 18 (wt 22°C), 10 (wt 38°C), 13 (het 22°C) and 11 (het 38°C) cells (A, B). C: Mean [3H]FMZ binding was reduced in het compared with wt homogenates independent of temperature stress. [3H]FMZ binding (pM/mg protein) was reduced in het (31.20 ± 1.22, n=8) compared to wt samples (50.82 ± 8.60, n=9; p<0.001) obtained from animals maintained at room temperature. Similarly, following heating, binding was reduced in heterozygous tissue (34.85 ± 2.45, n=8) compared to wild type (47.85 ± 3.44, n=11; p<0.05). No temperature dependent changes were observed for either genotype (p>0.05). Asterisks indicate statistical difference between wt and het only (p<0.05). Whole brain homogenates were obtained from non-heated (RT; n=9 wt, n=8 het) or heated (heat; n=11 wt, n=8 het) animals.
In order to investigate the effect of temperature on GABAA receptor binding, a radioligand binding assay using the competitive GABAA antagonist [3H]Flumazenil (FMZ) was conducted. The density of membrane-bound γ2 subunit containing receptors was determined from whole brain homogenates collected from heated or non-heated (RT) animals. We observed a genotype effect whereby [3H]FMZ binding was reduced in R43Q samples compared to wild type. However, this reduction in [3H]FMZ binding was evident in samples from both the heated and RT animals (Figure 2C) indicating no temperature dependent change in the total number of membrane-bound γ2 subunit containing receptors.
Discussion
The current study demonstrates that the GABAAγ2(R43Q) mouse model recapitulates the excitable phenotype seen in patients, providing a unique tool with which to investigate pathophysiological mechanisms of epilepsy. We further demonstrate a significant increase in mIPSC amplitude measured in het brain slices following heating. In contrast, previous work in heterologous systems has shown a temperature dependent decrease in GABA-mediated current for R43Q containing GABAA receptors (Kang et al. 2006). Differences between our mouse model data and those reported by Kang et al. (2006) could be due to limitations imposed by cell culture models. The increase in mIPSC amplitude following 38°C pre-incubation seen only in het neurons may be explained by an availability of an extrasynaptic reserve pool of wt GABAA receptors (for review see Jacob et al. 2008) which could preferentially replace synaptic R43Q GABAA receptors at elevated temperatures. In vitro systems may lack the necessary complexity to model these critical aspects of receptor trafficking.
We show a reduction in mean [3H]FMZ binding in heterozygous compared with wild-type homogenates, in agreement with [11C]FMZ binding data in patients heterozygous for the R43Q mutation (Fedi et al., 2006). We and others report a reduction in cell surface expression of the γ2 subunit expressing the R43Q mutation using affinity purification of total and cell surface receptor fractions at RT (Sancar & Czajkowski 2004, Tan et al. 2007). Historical analysis suggests that these animals are seizure naïve prior to this intervention (ie. spontaneous absence seizures are not evident in these animals prior to P21; Tan et al. 2007), implying that a role for seizure activity in influencing GABAA receptor membrane expression is unlikely. While we cannot discount specific effects of anesthesia, the lack of temperature dependent changes in our FMZ data suggest that the total amounts of γ2 subunit containing receptors remain unchanged during the higher temperature pre-incubation and further suggests that mutant receptors are not preferentially degraded.
Increased inhibitory neurotransmission has previously been reported in animal models of epilepsy (Cohen, et al. 2003, Cope, et al. 2009) as well as after experimental febrile seizures (Chen et al., 1999). Specifically, Cohen et al. (2003) reported an increase in mIPSC amplitude in dentate granule cells with concurrent changes in GABAA receptor properties. These authors propose a role for disinhibition of neural networks and altered subunit composition of GABAA receptors in seizure susceptibility.
In conclusion, we observed an increase in mIPSC amplitude following a transient increase in temperature in acute slices taken from het mice. These observations do not support a reduction in receptor expression at the cell surface following heating, as observed in heterologous systems (Kang et al., 2006). Contrasting properties of slice and cell culture environments may contribute to differences between our observations and those reported by Kang et al., (2006). Moreover, [3H]FMZ binding data show no temperature dependent change in heterozygote receptor density further contradicting the idea that temperature-dependent receptor trafficking underlies FS genesis in the R43Q mouse or patients.
Acknowledgements
This study was supported by a New Investigator project grant from the National Health and Medical Research Council (NHMRC) of Australia (509224; to EH), an NHMRC program grant (400121; to SP), the Caitlin’s Fund for Epilepsy Research (to EH) and the National Institute of Health (NS35439; to TZB and CMD)
Footnotes
Disclosure of Conflicts of interest:
SP served as a paid consultant for Bionomics Limited (Thebarton, SA, Australia), which holds the intellectual property surrounding the R43Q mouse. None of the other authors has any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Baram TZ, Gerth A, Schultz L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Res Dev Brain Res. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendes F, Andermann F. Do febrile seizures promote temporal lobe epilepsy? Retrospective studies. In: Baram TZ, Shinnar S, editors. Febrile Seizures. Elsevier; 2002. pp. 78–83. [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABA(A) receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi M, Berkovic SF, Marini C, Mulligan R, Tochon-Danguy H, Reutens DC. A GABAA receptor mutation causing generalized epilepsy reduces benzodiazepine receptor binding. Neuroimage. 2006;32:995–1000. doi: 10.1016/j.neuroimage.2006.05.059. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590–2597. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. A GABAA receptor mutation linked to human epilepsy (gamma2R43Q) impairs cell surface expression of alphabetagamma receptors. J Biol Chem. 2004;279:47034–47039. doi: 10.1074/jbc.M403388200. [DOI] [PubMed] [Google Scholar]
- Sihver W, Sihver S, Bergstrom M, Murata T, Matsumura K, Onoe H, Andersson Y, Bjurling P, Fasth KJ, Westerberg G, Ogren M, Jacobsson G, Lundqvist H, Oreland L, Watanabe Y, Langstrom B. Methodological aspects for in vitro characterization of receptor binding using 11C-labeled receptor ligands: a detailed study with the benzodiazepine receptor antagonist [11C]Ro 15-1788. Nucl Med Biol. 1997;24:723–731. doi: 10.1016/s0969-8051(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Tan HO, Reid CA, Single FN, Davies PJ, Chiu C, Murphy S, Clarke AL, Dibbens L, Krestel H, Mulley JC, Jones MV, Seeburg PH, Sakmann B, Berkovic SF, Sprengel R, Petrou S. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc Natl Acad Sci U S A. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]