Abstract
Atopic dermatitis (AD) in young children is often followed by the development of asthma (atopic march). The role of environmental exposures is unclear in this high-risk population. We aimed to determine the predictive relationship between indoor allergen exposures, particularly pets, rodents, and cockroaches, to the development of asthma in a prospective pediatric cohort. Children with AD and a family history of allergy were followed prospectively with questionnaire ascertainment of environmental exposure to cats, dogs, cockroaches, rats, and mice. Asthma was diagnosed by study physicians based on caregiver reports of symptoms continually assessed over the course of the study period. Fifty-five of the 299 children developed asthma by the end of the study. Cat exposure had a strong and independent effect to reduce the risk of developing asthma across all analyses (odds ratio [OR], 0.16; 95% confidence interval [CI], 0.05–0.53). Dog, mouse, rat, and cockroach exposures did not significantly influence the development of asthma. Daycare exposure had the largest risk reduction for the development of asthma (OR, 0.08; 95% CI, 0.03–0.19). Maternal asthma (OR, 2.93; 95% CI, 1.29–6.67), baseline body mass index (OR, 1.23; 95% CI, 1.08–1.42), and specific immunoglobulin E to house-dust mix at 3 years were each independent risk factors for the development of asthma. In children with AD, cat and daycare exposure may reduce the risk of developing early childhood asthma.
Keywords: Allergy, asthma, atopic dermatitis, atopic march, atopy, cat allergy, daycare, environmental exposure, epidemiology, pediatric, pet allergy
Atopic diseases such as atopic dermatitis (AD), asthma, and allergic rhinitis are among the most common chronic diseases in the developed world. Asthma, alone, affects 300 million people worldwide of all ages.1 The natural history of atopic diseases in childhood is defined by peak manifestations at different ages, with AD predating asthma and allergic rhinitis. The risk of developing asthma in children with AD is 34–43%.2
Childhood atopic diseases are associated with sensitization to environmental allergens, and indoor allergen exposure, particularly pets and cockroach, may be important in disease development3,4 and clearly influence morbidity.5 Despite many population and high-risk birth cohort studies, the role of environmental exposures in the development of asthma has been inconsistent6–9 and few studies have addressed children who already have atopic manifestations.10–12
In this study we aimed to determine the influence of exposure to indoor allergens, particularly to mouse, rat, cockroach, cat, and dog, on the development of asthma in a cohort of young children with AD and family history of atopy. This prospective epidemiological study was a substudy of a large randomized, double-blind, placebo-controlled trial of a topical AD therapy in children with AD.
METHODS
Study Participants
Subjects participating in a multicenter randomized control trial for AD were approached to participate in this substudy. Recruitment occurred at eight academic medical centers across the United States. The parent study enrolled over 1000 subjects 3–18 months of age with a diagnosis of AD according to the American Academy of Dermatology Consensus Conference Criteria,13 clinical evidence of AD ≤3 months' duration, a family history of atopy (at least one parent or sibling), and at least mild AD by Investigator Global Assessment score.14 Patients were excluded if they had received treatment with topical calcineurin inhibitor within 7 days before enrollment or were receiving daily treatment with antihistamines, systemic therapy (such as corticosteroids), or leukotriene antagonists within 1 month of first application of study drug.
All subjects enrolled in the parent study at the substudy sites were eligible for entry into this study without regard to their treatment assignment. No additional inclusion or exclusion criteria were applied.
The Institutional Review Board at each participating institution approved this study protocol. Written informed consent was obtained for all subjects.
Study Design
Children participated in a 3-year double-blind treatment phase of topical AD calcineurin inhibitor, and were then followed through an additional open-label phase that concluded when the child developed asthma or at study termination. Validated questionnaires collected baseline and follow-up data on home and environmental allergen exposure in addition to potential confounders. Follow-up questionnaires reassessed the child's environmental exposures at ∼18-month intervals from the first study visit.
Definition of Predictor Variables
Primary predictor variables captured through baseline and follow-up questionnaires were those that indicated exposure to mice, rats, cockroaches, cats, and dogs at home, determined by caregiver report. For these, questions ascertained, “in the past 18 months have you had any of the following pets: dogs, cats” with responses ‘yes,’ ‘no,’ or ‘don't know’ for each. Caregivers were also asked,” in the past 18 months, have you seen or noticed signs of the following pests: mice, rats, cockroaches” with responses “yes,” “no,” or “don't know” for each. Variables describing environmental exposures were categorized as positive only if the caregiver answered yes to the predictor on a questionnaire that predated the development of the primary outcome, diagnosis of asthma.
Several covariates were considered because of their previously described association with asthma development or as potential confounders to the relationship of the primary predictors to the outcome. Age, race, gender, body mass index (BMI) at study entry, age at entry to the study, maternal history of asthma, treatment assignment, day care attendance ascertained as “does your child attend day care or have frequent babysitting (>3 times per week) outside of the home,” environmental tobacco smoke exposure (greater than four times per week), history of breast-feeding, and estimated family annual income (>$50,000 annually) were considered for inclusion.
Total IgE and specific IgE (sIgE) for house-dust mix (HDM) and animal mix were determined from blood obtained at baseline and at 3 years using the ImmunoCAP assay (Phadia, Portage, MI). The limit of detection was 0.1 kU/L and lower limit of quantification was 0.35 kU/L. Serological tests were not specific to the primary predictors because they were part of the parent study assessment and were not intended as a measure of main effect in the current study.
Definition of Outcome Variables
The diagnosis of asthma, the primary outcome, was determined by the study physician and triggered by review of the primary caregiver's report of nocturnal cough with sleep disturbance or wheezing on an electronic diary (e-diary, Palm, M515, CRF; Health, Lansdale, PA). The criteria for making a diagnosis of asthma were three episodes of either nocturnal cough with sleep disturbance lasting for at least three consecutive nights, wheezing of any duration, or a combination of the two symptoms, separated by at least 7 days,11 in a clinical setting where asthma was likely and conditions other than allergy had been excluded (i.e., viral illness).12 Individual asthma episodes were also tabulated. Primary caregivers entered data on the e-diary on a daily to weekly basis. Subjects who did not have a diagnosis of asthma recorded by the time of study completion or last follow-up were considered not to have developed asthma.
Statistical Analysis
Exposures to binary predictors were categorized as positive if the subject answered yes to the predictor at any time before the outcome. Odds ratios were calculated and statistical significance was determined by the chi-squared test or Fisher's exact test in the case of sparse data. Continuous predictors were used in their native form and compared by t-test or Wilcoxon two-sample test for nonnormally distributed data. The relative risk of each predictor variable on the outcome of asthma diagnosis was determined by univariate analysis.
Multiple regression models assessed the influence of the primary predictors (exposure to dogs, cats, rats, and mice) and potential confounders on the development of asthma. Each primary predictor and covariate of clinical significance was included to determine the relative contribution of each to the development of asthma. Cockroach was not included in the models because it was not informative on univariate analysis. A second model also included serologic data (Total IgE and specific IgE at 3 years). This time point was chosen to evaluate serological response at a time when it may be more robust, given the age of the participant. Although the serological tests were not specific to the exposures of interest, they were included as potential confounders of the relationship of allergen exposure to asthma development. Because there were five a priori predictors, significant p value was determined as that ≤0.01 (0.05 ÷ 5 predictors) to correct for multiple testing.
Similar Poisson regression modeling was used to assess the significance of predictors on the number of asthma episodes. SAS Version 9.2 (SAS Institute, Inc., Cary, NC) was used for all statistical analysis.
RESULTS
Three hundred twenty-one subjects were enrolled, of which 299 subjects had data available for the primary exposures of interest and outcome of asthma. These subjects were included in all analyses. Fifty-five (18%) subjects developed asthma during the study period. The average length of time in study was 3 years and 11 months.
Table 1 describes the characteristics of this cohort. The average age of entry to the parent study was 6 months. Sixty-three percent of the cohort was boys, 78% were white and 10% black. Although all participants had a family history of atopy in a first-degree relative, approximately one-quarter had a maternal history of asthma. Dog, cat, and mouse exposure was reported in 30–40% of subjects, and rat and cockroach exposure were only reported in 5% of the cohort. Sixty-three percent of children attended day care.
Table 1.
Characteristics of children in the cohort
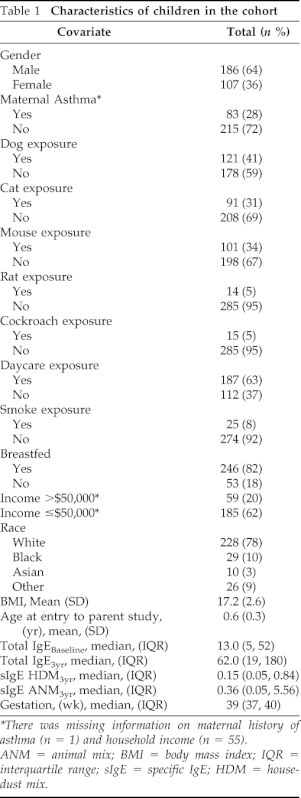
*There was missing information on maternal history of asthma (n = 1) and household income (n = 55).
ANM = animal mix; BMI = body mass index; IQR = interquartile range; sIgE = specific IgE; HDM = house-dust mix.
Univariate analysis exploring the relationship between each predictor and the outcome of diagnosis of asthma is reported in Table 2. Exposure to dogs, cats, and mice were inversely related to the development if asthma. Exposure to rats was not significantly associated with asthma. No children who developed asthma reported exposure to cockroaches or smoke at any time before their diagnosis. Maternal history of asthma was a positive predictor of asthma. Serological testing showed significantly greater sensitization to house-dust mites at 3 years in the group of children who developed asthma. There were no differences between those that developed asthma and those who did not based on age, race, gender, or family income.
Table 2.
Univariate analysis of home exposure and association with asthma
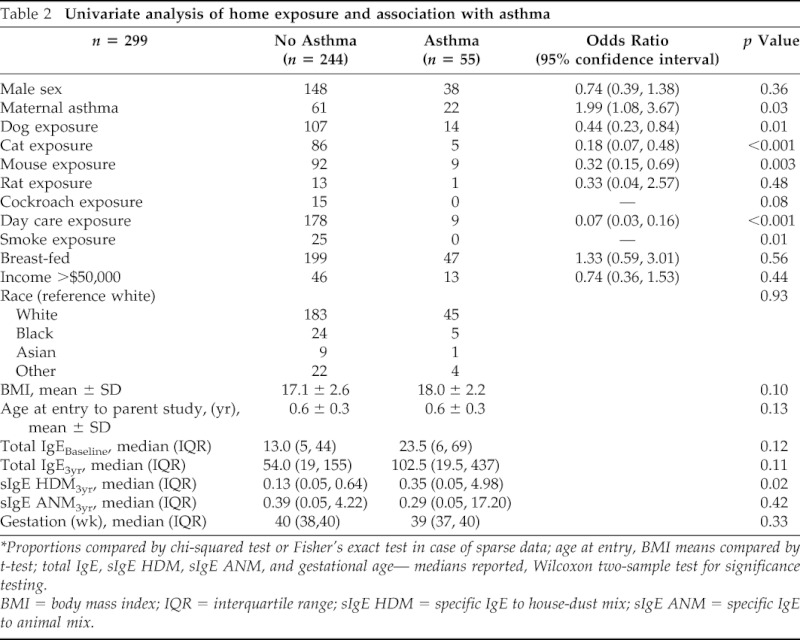
*Proportions compared by chi-squared test or Fisher's exact test in case of sparse data; age at entry, BMI means compared by t-test; total IgE, sIgE HDM, sIgE ANM, and gestational age— medians reported, Wilcoxon two-sample test for significance testing.
BMI = body mass index; IQR = interquartile range; sIgE HDM = specific IgE to house-dust mix; sIgE ANM = specific IgE to animal mix.
Multiple regression analysis included report of cat, dog, mouse, and rat exposure; day care attendance; BMI; household income level; maternal history of asthma; and treatment assignment. A second model also included Total IgE and sIgE to house-dust mite antigen at the 3-year time point. Table 3 shows the results of the multiple logistic regression models for the development of asthma. Children exposed to cat had significantly decreased risk of developing asthma. Similarly, day care exposure was found to greatly reduce the risk of asthma. Maternal history of asthma, higher BMI, and higher sIgE to HDM conferred higher risk of asthma diagnosis.
Table 3.
Multiple regression analysis of home exposure and association with asthma
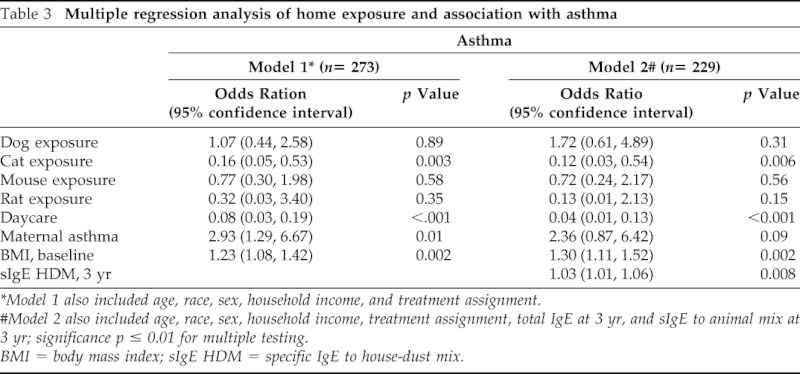
*Model 1 also included age, race, sex, household income, and treatment assignment.
#Model 2 also included age, race, sex, household income, treatment assignment, total IgE at 3 yr, and sIgE to animal mix at 3 yr; significance p ≤ 0.01 for multiple testing.
BMI = body mass index; sIgE HDM = specific IgE to house-dust mix.
Although dog and mouse exposures appeared to have a protective relationship with the development of asthma in univariate analysis, these relationships were significantly confounded by day care attendance, maternal history of asthma, and treatment assignment and therefore were not found to significantly predict asthma in the adjusted models.
When Poisson regression modeling was used to determine the influence of the same exposures on the number of asthma episodes, cat exposure and day care attendance continued to show a risk reduction, and HDM sensitization remained a significant risk factor (Table 4). Maternal asthma and BMI continued to trend toward risk. These results mirror those found in the logistic regression, although the degree of statistical significance was lessened for many of the exposures.
Table 4.
Poisson regression analysis of home exposure and association with quantity of asthma episodes
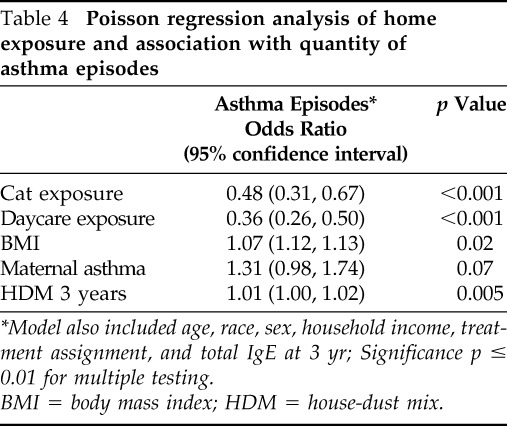
*Model also included age, race, sex, household income, treatment assignment, and total IgE at 3 yr; Significance p ≤ 0.01 for multiple testing.
BMI = body mass index; HDM = house-dust mix.
DISCUSSION
We aimed to determine the effect of environmental exposure to common indoor allergens, specifically mouse, rat, cockroach, cat, and dog, in a group of children with AD at high risk of developing asthma. Separate multiple regression analyses, looking first at the report of exposures and then including serological evidence of sensitization, confirmed that cat and day care exposure reduced the risk of the development of asthma in this cohort. Cat and day care exposure similarly reduced the risk of asthma episodes, corroborating the primary findings. This consistent relationship present through univariate analysis and multiple iterations of statistical modeling reinforces the strong and independent nature of cat and day care exposure to reduce the risk of developing asthma in this cohort.
Cat exposure has been described as both a risk factor for the development of asthma9 and, as here, a protective.6,15 Data from the Asthma Multicenter Infant Cohort Study9 and others16,17 have strongly suggested that cat allergen exposure is associated with the development of allergic sensitivity and asthma. Exposure to cat allergen measured during the child's first 3 months of life and sensitivity and asthma outcomes at 6 years old showed a dose-dependent relationship up to a plateau of 1 μg of fel d1/g of dust and even stronger association in a high-risk subgroup.9 However, others have found just the opposite effect. In the German Multicenter Allergy Study15 the infants exposed to the highest levels of cat allergen (fel d1) had decreased cat-specific IgE levels and high IgG levels with corresponding low-risk phenotype for wheeze. Early exposure to cats has been found to reduce the risk of asthma in other cohorts as well.18–21
In AD cohorts such as ours, the results are equally varied. Warner11 reported that evidence of allergic sensitization to cats in children with AD increased the odds for the development of asthma by 1.5 in the placebo arm of the Early Treatment of the Atopic Child study. However, sIgE to cats was not a significant factor in asthma development in the prospective AD cohort followed by Ricci et al.10 When environmental exposure was investigated as an explanatory factor in the development of asthma in a cohort of young children with AD, Gustafsson et al.2 found furred pets to have a nonsignificant protective effect (odds ratio, 0.4; confidence interval, 0.2–1.1). Our study, which had much greater power to determine such a relationship by enrolling three times the number of subjects, corroborates cat exposure as a risk-reducing factor in the development of asthma. This effect may suggest that tolerance, rather than allergy, develops in children with prior history of atopy when exposed to high levels of exposure to cat allergen as well.15,18,19 It has been suggested that IL-10 may play a key role in allergen tolerance to such exposures.22 The asthma risk may only be relevant in those exposed to just enough cat allergen (or for infrequent enough exposure) to induce sensitization.23
In an attempt to differentiate overall allergic sensitization from the exposures of interest, our second model tested the effects of total IgE and sIgE to HDM and animal mix measured at 3 years. Including these variables in the model reduced the significance of cat exposure on asthma development but did not change the magnitude and direction of the effect size, suggesting the effect is independent of overall sensitization.
Day care attendance indicated the strongest reduction in risk for asthma in this study. Day care exposure has been implicated as protective for asthma development in a large cohort of Canadian children24 and is associated with decreased wheeze in early school age children but not subsequent asthma in another study.25 Similarly, Celedon et al.26 showed that day care exposure in the 1st year of life in children of atopic, but not asthmatic, mothers had a greatly reduced risk of asthma and recurrent wheezing at 6 years old. The relative risk was 0.3 (95% confidence interval, 0.1–0.7) showing a sizeable effect. These protective effects are presumably caused by the early exposure to viral illness27 or other beneficial effects related to exposure to children.28 Both infantile28 and toddler exposure29 to day care have been reported to have similar effects. Recent evidence suggests that a genetic polymorphism in the Toll-like receptor 2 may play a role in determining the development of asthma in children with day care exposure early in life.30 The magnitude of risk reduction we found in this cohort is substantial and will need to be validated further.
The association of body habits and asthma has had mixed results in young children.31,32 In this study we found BMI at study entry to be positively associated with asthma development. The very young age of sampling and the known variability in calculating BMI in infants and toddlers may limit the precision of this association. Additionally, our findings extend the important role of maternal history of asthma33 as a risk factor for the development of asthma in children with AD.
Difficulty separating asthma from transient wheeze in young children may be a limitation to this study. However, the definition of asthma used in this study has been used in other large epidemiological studies12,15 and was further limited to attempt to exclude cough and wheeze associated with viral respiratory infections. Furthermore, in young children with AD, concomitant wheeze is highly predictive of current wheeze at age 7 years.34 We recognize that our study doctor's diagnosis of asthma may reflect bias of parental reporting of symptoms. However, parental reports of both wheeze and asthma have consistently been associated with lower lung function, skin test reactivity, and airways responsiveness in the pediatric epidemiological literature.35 Based on a variety of reports, physicians tend to underdiagnose asthma in early childhood, and our results could be conservative.36 Environmental exposure in this study is based on caregiver report, which is subject to recall bias and may be biased by perceived social stigma related to rodent or cockroach infestation. However, reported exposure to cats and rodents has been shown to be highly correlated with measured allergen concentrations in the home.37–39 Chew et al.37 found a 90% predictive value for detectable mouse allergen in homes in which there was a reported exposure to mice. Similarly, Waser and colleagues39 found that current report of cat contact was highly correlated with mattress fel d1 concentrations in rural school-age children participating in the Allergy and Endotoxin study. Across studies, exposure to cats is 22–40%39 and to mice is 25–33%,38,40 which lends credibility to the similar prevalence found in our study. Misclassification bias must be considered for participants who may have developed asthma after discontinuation from the study.
Exclusion of children who previously had a diagnosis of asthma at the time of enrollment may limit the generalizability of these results; however, because of the young age at recruitment it is unlikely that many potential subjects fit this profile. Exclusion of those already on calcineurin inhibitors or systemic therapies for AD may also limit the applicability of these results for those with severe AD in early childhood.
These findings suggest a strong and temporally relevant association between day care attendance and cat exposure and the development of asthma. Although it is impossible to determine direct causality in this study, the exposure to the predictors was only assessed before the development of asthma, arguing against reverse causality. The decision of a family to place a child in day care or own a pet may be influenced by several factors that may bias the distribution of exposures in this study and confound the relationship between the exposures and outcome. We adjusted the multiple regression analysis for what are likely the strongest confounders to the relationship of pet and day care to asthma, socioeconomic status, and maternal history of asthma; however, other unmeasured confounding factors may have influenced these results.
In children with AD, cat exposure and day care attendance may independently reduce the risk of developing early childhood asthma. Additional study of measured environmental exposures on the development of asthma in children with AD is needed.
ACKNOWLEDGMENTS
The authors thank to Jaclyn Morrill and Irene M. Borras-Coughlin from Children's Hospital, Boston, for their support coordinating this study.
Footnotes
Funded by Novartis Pharmaceuticals Corp.
J.M. Gaffin is funded by NIH KL2 RR025757-0 1, Harvard Clinical and Translational Science Center (KL1) and the American Thoracic Society Fellows Career Development Award. W. Phipatanakul is funded by NIH/NIAID R-01grant (AI-073964) and NIH/NHLBI AsthmaNet 1U10HL098102. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 59:469–478, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis—A prospective follow-up to 7 years of age. Allergy 55:240–245, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Gold DR. Environmental tobacco smoke, indoor allergens, and childhood asthma. Environ Health Perspect 108(suppl 4):643–651, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McHugh BM, MacGinnitie AJ. Indoor allergen sensitization and the risk of asthma and eczema in children in Pittsburgh. Allergy Asthma Proc 32:372–376, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Ownby DR. Pet dander and difficult-to-control asthma: The burden of illness. Allergy Asthma Proc 31:381–384, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Remes ST, Castro-Rodriguez JA, Holberg CJ, et al. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol 108:509–515, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med 166:696–702, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 288:963–972, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Torrent M, Sunyer J, Garcia R, et al. Early-life allergen exposure and atopy, asthma, and wheeze up to 6 years of age. Am J Respir Crit Care Med 176:446–453, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Ricci G, Patrizi A, Baldi E, et al. Long-term follow-up of atopic dermatitis: Retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol 55:765–771, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Warner JO. A double-blinded, randomized, placebo-controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 Months' treatment and 18 months' posttreatment follow-up. J Allergy Clin Immunol 108:929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Allergic factors associated with the development of asthma and the influence of cetirizine in a double-blind, randomised, placebo-controlled trial: First results of ETAC. Early treatment of the atopic child. Pediatr Allergy Immunol 9:116–124, 1998. [PubMed] [Google Scholar]
- 13. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 49:1088–1095, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Eichenfield LF, Lucky AW, Langley RG, et al. Use of pimecrolimus cream 1% (Elidel) in the treatment of atopic dermatitis in infants and children: The effects of ethnic origin and baseline disease severity on treatment outcome. Int J Dermatol 44:70–75, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Lau S, Illi S, Platts-Mills TA, et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat-specific IgG and development of asthma in childhood—Report of the German Multicentre Allergy Study (MAS 90). Allergy 60:766–773, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Sears MR, Herbison GP, Holdaway MD, et al. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy 19:419–424, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Polk S, Sunyer J, Munoz-Ortiz L, et al. A prospective study of Fel d1 and Der p1 exposure in infancy and childhood wheezing. Am J Respir Crit Care Med 170:273–278, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Hesselmar B, Aberg N, Aberg B, et al. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy 29:611–617, 1999. [DOI] [PubMed] [Google Scholar]
- 19. Takkouche B, Gonzalez-Barcala FJ, Etminan M, Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: A meta-analysis. Allergy 63:857–864, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Kelly LA, Erwin EA, Platts-Mills TA. The indoor air and asthma: The role of cat allergens. Curr Opin Pulm Med 18:29–34, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lodge CJ, Allen KJ, Lowe AJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: A systematic review of longitudinal studies. Clin Dev Immunol 176484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Platts-Mills TA, Woodfolk JA. Allergens and their role in the allergic immune response. Immunol Rev 242:51–68, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Celedon JC, Litonjua AA, Ryan L, et al. Exposure to cat allergen, maternal history of asthma, and wheezing in first 5 years of life. Lancet 360:781–782, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Midodzi WK, Rowe BH, Majaesic CM, et al. Early life factors associated with incidence of physician-diagnosed asthma in preschool children: Results from the Canadian Early Childhood Development cohort study. J Asthma 47:7–13, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Caudri D, Wijga A, Scholtens S, et al. Early daycare is associated with an increase in airway symptoms in early childhood but is no protection against asthma or atopy at 8 years. Am J Respir Crit Care Med 180:491–498, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Celedon JC, Wright RJ, Litonjua AA, et al. Day care attendance in early life, maternal history of asthma, and asthma at the age of 6 years. Am J Respir Crit Care Med 167:1239–1243, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Lemanske RF. Viral infections and asthma inception. J Allergy Clin Immunol 114:1023–1026, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Ball TM, Castro-Rodriguez JA, Griffith KA, et al. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 343:538–543, 2000. [DOI] [PubMed] [Google Scholar]
- 29. Gurka MJ, Blackman JA, Heymann PW. Risk of childhood asthma in relation to the timing of early child care exposures. J Pediatr 155:781–787, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Custovic A, Rothers J, Stern D, et al. Effect of day care attendance on sensitization and atopic wheezing differs by Toll-like receptor 2 genotype in 2 population-based birth cohort studies. J Allergy Clin Immunol 127:390–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwon HL, Ortiz B, Swaner R, et al. Childhood asthma and extreme values of body mass index: The Harlem Children's Zone Asthma Initiative. J Urban Health 83:421–433, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP). Thorax 58:1036–1041, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 332:133–138, 1995. [DOI] [PubMed] [Google Scholar]
- 34. Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 113:925–931, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Weiss ST. Epidemiology and heterogeneity of asthma. Ann Allergy Asthma Immunol 87:5–8, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Gold DR, Wypij D, Wang X, et al. Gender- and race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. Am J Respir Crit Care Med 149:1198–1208, 1994. [DOI] [PubMed] [Google Scholar]
- 37. Chew GL, Perzanowski MS, Miller RL, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect 111:1348–1351, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol 106:1070–1074, 2000. [DOI] [PubMed] [Google Scholar]
- 39. Waser M, von Mutius E, Riedler J, et al. Exposure to pets, and the association with hay fever, asthma, and atopic sensitization in rural children. Allergy 60:177–184, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Welch JE, Hogan MB, Wilson NW. Mouse allergy among asthmatic children from rural Appalachia. Ann Allergy Asthma Immunol 90:223–225, 2003. [DOI] [PubMed] [Google Scholar]


