Abstract
Target groups for human papillomavirus (HPV) vaccination are controversial. We evaluated vaccine efficacy (VE) against 1-year persistent infection, stratified by age and sexual behavior, among young women in Costa Rica. We randomized 7,466 healthy women 18 to 25 years of age to HPV16/18 or hepatitis A vaccine (follow-up, 50.4 months). According-to-protocol (ATP) cohorts included compliant HPV-negative women; intention-to-treat (ITT) included all randomized women. ATP VE was 90.9% (95% CI, 82.0–95.9) against HPV16/18 infections, 44.5% against HPV31/33/45 (95% CI, 17.5–63.1), and 12.4% (95% CI, −3.2 to 25.6) against any oncogenic infection. Overall ITT VE against HPV16/18 infections was 49.0%, but ATP and ITT VE almost reached 100% in year 4 of follow-up. ATP efficacy against HPV16/18 was similar by age, but ITT VE was greatest among youngest women (68.9% among those 18–19 years of age; 21.8% among those 24–25 years of age) and 79.8% among virgins. Among previously unexposed women, vaccination is highly efficacious against HPV16/18 and partially against HPV31/33/45. Vaccination is most effective in women and girls before they initiate sexual activity, with programmatic and individual decision implications.
Introduction
Human papillomavirus (HPV) vaccines have enormous potential in the control of cervical cancer, and developed countries are vaccinating adolescent girls to prevent cervical neoplasia. However, the worldwide cervical cancer burden (500,000 cases annually) will only decrease with high vaccination coverage in developing countries, where most cases (85%) occur (1). In this context, target ages and population groups to maximize reduction in morbidity, treatment, and mortality are still controversial.
The two vaccines based on L1 virus-like particles licensed worldwide, quadrivalent human papillomavirus (types 6, 11, 16, 18) vaccine (Gardasil; Merck and Co., Inc.) (2, 3) and bivalent human papillomavirus (types 16 and 18) vaccine (Cervarix; GlaxoSmithKline Biologicals) (4, 5), are highly protective against cervical neoplasia caused by HPV types 16 and 18 (HPV16 and 18) among women without current or past infection with these types. There is also evidence of limited cross-protection against HPV31, 33, 45, and possibly others (5–7). However, vaccination does not increase clearance or decrease progression of established infections (8, 9).
The ultimate goal is the prevention of cervical cancer, but trials with that end point are impractical. The choice of trial end points has been intensively debated (10). Regulatory authorities required histopathological outcomes, namely cervical intraepithelial neoplasia grade 2 or greater (CIN2+) (in effect, mainly CIN2), as cancer surrogates in licensure trials.
Although HPV infection is a necessary cause of cervical cancer, acute infection is extremely common and usually clears within months (11). Persistent oncogenic HPV infection, which is less frequent, is a much better end point than incident infection and, in some respects, a better surrogate marker than CIN2 because infection can be measured with high reproducibility (12), whereas CIN2 is subject to significant histologic misclassification (13). Also, attribution of the HPV type that caused a CIN2+ is difficult when multiple types of infections are present, as is common (14).
The evaluation of vaccine efficacy (VE) and potential impact in population subgroups can assure maximum benefit from high-cost programs in different settings. Developing countries with limited resources are considering whether investment in this preventive measure is worthwhile. In developed countries, a benefit is uncertain for older women born in earlier cohorts and those who miss vaccination as adolescents, particularly in the United States, where uptake of the vaccine in adolescents is limited (15).
We report here on efficacy of an HPV16/18 ASO4-adjuvanted vaccine (Cervarix) in a large community-based clinical trial [registered at clinicaltrials.gov (NCT00128661)] in a high-incidence area of Costa Rica (16), with 1-year persistence of a cervical HPV infection as an end point, including estimates of VE by age, sexual behavior, and previous exposure to individual HPV types. We present results for both intention-to-treat (ITT) cohorts, reflecting real-world efficacy, and according-to-protocol (ATP) cohorts, as a proxy for an ideal in which women are fully vaccinated before exposure so they can receive maximum benefit.
Results
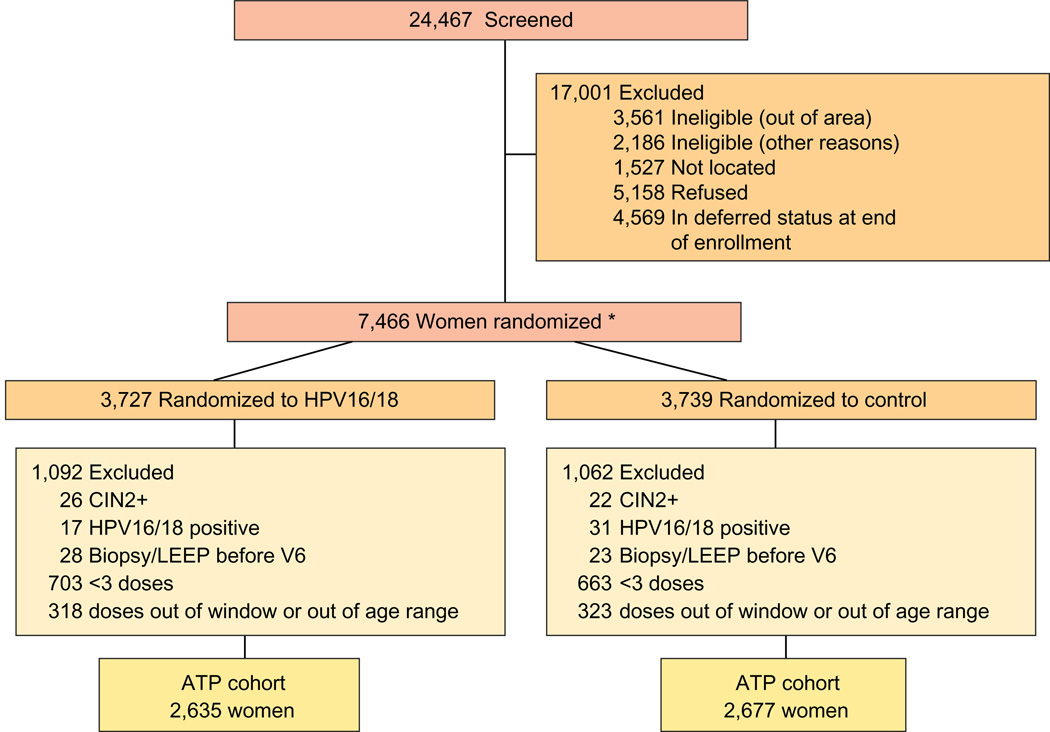
Figure 1 presents the trial profile diagram. Of nearly 25,000 women screened, 7,466 women were randomized, with 3,727 in the vaccine arm and 3,739 in the control arm (ITT cohort for HPV16/18). The ATP cohort comprised 2,635 and 2,677 women in the vaccine and control arms, respectively. The 7,466 women represented 59.1% of 12,624 potentially eligible women (considering those with recruitment deferred beyond the enrollment period for different reasons as noneligible) and 30.5% of all 24,467 women screened from the census.
Figure 1. Trial profile.
*Four women received discordant vaccines (one woman was enrolled twice and received 3 doses of each vaccine and three women received 2 doses of one vaccine and one dose of the other vaccine). For the aim of this analysis, the women were assigned to the group for which the first dose was given. LEEP, loop electrosurgical excision procedure.
Age, study clinic, presence and number of individual HPV types detected, and baseline cytology were similar in the two arms (Supplementary Table S1). As noted previously (8), HPV16 was more common at baseline in the vaccine than in the control arm (6.0% vs. 7.1%, P = 0.05). For this analysis, women in the ATP cohort for HPV16/18 had accumulated 10,268 and 10,472 person years in the vaccine and control arm, respectively, with a median follow-up time of 50.4 months. Total follow-up time, number of visits, maximum time between tests, and number of annual, semiannual, or colposcopy visits were similar by arm (data not shown). More than 90% of eligible women attended their corresponding visits and provided specimens.
Estimated VE against HPV16/18 was 90.9% (95% CI, 82.0–95.9) in the ATP cohort and 49.0% (95% CI, 38.1–58.1) in the ITT cohort (Table 1). The efficacy against HPV31/33/45, for which previous evidence of protection exists, was 44.5% (95% CI, 17.5–63.1) in the ATP cohort. Efficacies against other oncogenic types combined were not significant. The overall efficacy against all oncogenic types was approximately 10% in both the ATP and ITT analyses. Considering individual A9-species HPV types in ATP cohorts, protection against the HPV16 target was 86.5% (95% CI, 72.9–94.0), with significant cross-protection against HPV31 (45.7%; 95% CI, 8.2–68.6). There was a nonsignificant cross-protection (37.3%; 95% CI, −51.4 to 75.3) against HPV33 but not against other types in this species (Supplementary Table S2). In species A7, efficacy against HPV18 persistent infection was 100% (95% CI, 90.7–100.0), with nonsignificant cross-protection for closely related HPV45 (52.0%; 95% CI, −9.8 to 80.4). The other types in this species had nonsignificant negative estimates of efficacy. In species A5 and A6, the only noteworthy finding was an increase in persistent infection with HPV51 (A5) in the HPV arm (VE: −63.9%; 95% CI, −150.7 to −8.2).
Table 1.
VE against 1-year persistence of different combinations of HPV types
| HPV type | ATP analysisa | ITT analysisb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm | Women in ATP cohort, N |
Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
Efficacy, % (95% CI) |
Women in ITT cohort, N |
Women with events, n |
Rate per 100 women, n (95%CI) |
Rate reduction/ 100 women, n (95%CI) |
VE, % (95% CI) |
|
| H PV16,18 | Vaccine | 2,635 | 8 | 0.3 (0.1–0.6) | 3.0 (2.5–3.3) | 90.9c (82.0–95.9) | 3,727 | 153 | 4.1 (3.5–4.8) | 3.9 (2. 9–5.0) | 49.0d (38.1–58.1) |
| Control | 2,677 | 89 | 3.3 (27–4.1) | 3,739 | 301 | 8.1 (7.2–9.0) | |||||
| HPV31, 33, 45 | Vaccine | 2,642 | 37 | 1.4 (1.0–1.9) | 1.1 (0.4–1.8) | 44.5 (17.5–63.1) | 3,727 | 150 | 4.0 (3.4–4.7) | 0.7 (−0.2 to 1.7) | 15.5 (−5.0 to 32.0) |
| Control | 2,695 | 68 | 2.5 (2.0–3.2) | 3,739 | 178 | 4.8 (4.1–5.5) | |||||
| Other | Vaccine | 2,643 | 230 | 8.7 (7.7–9.8) | −1.0 (−2.6 to 0.5) | −13.4 (−36.9 to 6.0) | 3,727 | 559 | 15.0 (13.9–16.2) | −0.2 (−2.0 to 1.5) | −1.4 (−14.1 to 9.8) |
| oncogenic types | Control | 2,697 | 207 | 7.7 (6.7–8.7) | 3,739 | 553 | 14.8 (13.7–16.0) | ||||
| Any | Vaccine | 2,643 | 267 | 10.1 (9.0–11.3) | 1.4 (−0.3 to 3.2) | 12.4 (−3.2 to 25.6) | 3,727 | 764 | 20.5 (19.2–21.8) | 2.6 (0.5–4.7) | 11.3 (2.2–19.5) |
| oncogenic type | Control | 2,697 | 311 | 11.5 (10.4–12.8) | 3,739 | 864 | 23.1 (21.8–24.5) | ||||
The ATP cohort includes women who received all 3 doses within protocol-defined windows, complied with the protocol during the vaccination period, did not have a biopsy or treatment (loop electrosurgical excision procedure [LEEP]) before the 6-month visit, and were HPV DNA negative (by PCR) for at least one of the HPV types in the end point at enrollment and at the 6-month visit.
ITT cohorts include all women randomized and vaccinated, regardless of prevalence of infection and follow-up visits.
One-sided P-value for test of VE equals 0 against the alternative that VE is greater than 0 is less than 10−17.
One-sided P-value for test of VE equals 0 against the alternative that VE is greater than 0 is less than 10−11.
Table 2 presents ATP efficacy against HPV16 by baseline HPV16 serology status. Rates of “breakthrough” persistent infections in the HPV arm were greater among seropositive patients than seronegative patients, although in the control arm, the rate of infections was lower in the seropositive patients. Thus, efficacy was greater than 90% among HPV16 seronegative women but only 50% among the seropositive women. Interestingly, efficacy against HPV16 was similar among HPV18 seronegative and seropositive women.
Table 2.
VE (ATP) against 1-year persistence with HPV16 stratified by HPV16 and HPV18 serology at enrollment
| HPV serologya,b | Arm | Women, N | Women with events, n |
Rate per 100 women |
95% CI | Rate reduction per 100 women |
95% CI | VE, % | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| HPV16 serology negative | Vaccine | 1,875 | 4 | 0.2 | 0.1–0.5 | 2.5 | 2.0–2.8 | 92.2 | 80.3–97.6 |
| Control | 1,856 | 51 | 2.7 | 2.1–3.6 | |||||
| HPV16 serology positive | Vaccine | 558 | 4 | 0.7 | 0.2–1.7 | 0.7 | −0.5 to 1.7 | 50.6 | −63.3 to 87.0 |
| Control | 551 | 8 | 1.5 | 0.7–2.7 | |||||
| HPV18 serology negative | Vaccine | 1,853 | 4 | 0.2 | 0.1–0.5 | 2.2 | 1.6–2.4 | 90.9 | 76.7–97.2 |
| Control | 1,854 | 44 | 2.4 | 1.8–3.1 | |||||
| HPV18 serology positive | Vaccine | 563 | 3 | 0.5 | 0.1–1.4 | 2.1 | 0.6–2.9 | 79.4 | 33.5–95.3 |
| Control | 541 | 14 | 2.6 | 1.5–4.2 | |||||
The stratification by HPV16 serology excludes 31 and 45 subjects without HPV16 serology results from the vaccine and control arm, respectively.
The stratification by HPV18 serology excludes 48 and 57 subjects without HPV18 serology from the vaccine and control arm, respectively.
Efficacy in the ATP cohort was similar regardless of vaccination age (P for trend 0.362; Table 3); however, in the ITT cohort, VE decreased from 68.9% (95% CI, 53.1–79.9) for women 18–19 years of age to 21.8% (95% CI, −16.9 to 47.9) among 24- to 25-year-old women (P for trend = 0.005). Corresponding rate reductions per 100 women vaccinated decreased from 5.2 (95% CI, 3.6–6.6) to 1.6 (95% CI, −1.0 to 4.0). Similarly, in the ITT cohort, efficacy was greatest among virgins at enrollment (79.8%; 95% CI, 44.9–94.1), with decreasing efficacy with increasing time since first sexual intercourse (Table 4) and increasing number of sexual partners (Table 5). When we considered stratification of the ITT results by time since first sexual intercourse and number of sexual partners, we found that virgins, despite high VE, had a lower rate reduction than sexually active women because they can only contribute outcomes after the initiation of sexual activity and therefore have less observation time. Among sexually active women, rate reductions, such as VE, decreased with time since first sexual intercourse. However, rate reductions increased with the number of sexual partners despite decreasing VE, as a consequence of the greater attack rate with increasing number of partners.
Table 3.
VE against 1-year persistence with HPV16/18 stratified by age at enrollment
| Age | Arm | ATP analysisa | ITT analysisb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
Women, N | Women with events, n |
Rate 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
||
| 18–19 y | Vaccine | 825 | 1 | 0.1 (0.0–0.6) | 2.9 (2.0–3.1) | 95.9 (78.5–99.8) | 1,193 | 28 | 2.3 (1.6–3.3) | 5.2 (3.6–6.6) | 68.9 (53.1–79.9) |
| Control | 870 | 26 | 3.0 (2.0–4.3) | 1,244 | 94 | 7.6 (6.2–9.1) | |||||
| 2 0–21 y | Vaccine | 659 | 3 | 0.5 (0.1–1.2) | 2.9 (1.6–3.6) | 86.6 (59.2–96.8) | 946 | 46 | 4.9 (3.6–6.4) | 3.6 (1.3–5.8) | 42.8 (17.9–60.6) |
| Control | 649 | 22 | 3.4 (2.2–5.0) | 905 | 77 | 8.5 (6.8–10.5) | |||||
| 22–23 y | Vaccine | 588 | 1 | 0.2 (0.0–0.8) | 3.8 (2.7–4.1) | 95.7 (77.4–99.8) | 818 | 36 | 4.4 (3.1–6.0) | 4.7 (2.2–6.9) | 51.5 (28.4–67.7) |
| Control | 625 | 25 | 4.0 (2.7–5.8) | 848 | 77 | 9.1 (7.3–11.2) | |||||
| 24–25 y | Vaccine | 563 | 3 | 0.5 (0.1–1.4) | 2.5 (1.0–3.3) | 82.2 (43.9–95.9) | 770 | 43 | 5.6 (4.1–7.4) | 1.6 (−1.0 to 4.0) | 21.8 (−16.9 to 47.9) |
| Control | 533 | 16 | 3.0 (1.8–4.7) | 742 | 53 | 7.1 (5.5–9.2) | |||||
The ATP cohort includes women who received all 3 doses within protocol-defined windows, complied with the protocol during the vaccination period, did not have a biopsy or treatment (LEEP) before the 6-month visit, and were HPV DNA negative (by PCR) for at least one of the HPV types in the end point at enrollment and at the 6-month visit.
ITT cohorts include all women randomized and vaccinated, regardless of prevalence of infection and follow-up visits.
Table 4.
ATP and ITT efficacy estimates against HPV16/18 by time since first sexual intercourse at enrollment
| Time since first sex |
Arm | ATP analysisa | ITT analysisb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
||
| Virgin | Vaccine | 566 | 1 | 0.2 (0.0–0.9) | 2.6 (1.4–2.9) | 93.6 (64.8–99.7) | 773 | 4 | 0.5 (0.2–1.2) | 2.0 (0.9–2.7) | 79.8 (44.9–94.1) |
| Control | 615 | 17 | 2.8 (1.7–4.3) | 819 | 21 | 2.6 (1.6–3.8) | |||||
| <2 y | Vaccine | 227 | 1 | 0.4 (0.0–2.2) | 4.5 (1.7–5.3) | 91.0 (48.3–99.6) | 352 | 12 | 3.4 (1.9–5.7) | 7.5 (3.7–10.4) | 68.7 (41.2–84.3) |
| Control | 244 | 12 | 4.9 (2.7–8.2) | 349 | 38 | 10.9 (7.9–14.5) | |||||
| 2 y | Vaccine | 233 | 0 | 0.0 (0.0–1.3) | 4.1 (1.8–4.1) | 100.0 (62.5–100.0) | 335 | 19 | 5.7 (3.6–8.6) | 7.3 (2.7–11.2) | 56.1 (25.2–75.0) |
| Control | 221 | 9 | 4.1 (2.0–7.3) | 325 | 42 | 12.9 (9.6–16.9) | |||||
| 3 y | Vaccine | 279 | 0 | 0.0 (0.0–1.1) | 5.1 (3.1–5.1) | 100.0 (76.2–100.0) | 395 | 19 | 4.8 (3.6–7.3) | 5.8 (2.0–9.1) | 54.9 (23.1–74.3) |
| Control | 256 | 13 | 5.1 (2.9–8.3) | 394 | 42 | 10.7 (7.9–14.0) | |||||
| 4+ y | Vaccine | 1,330 | 6 | 0.5 (0.2–0.9) | 2.4 (1.6–2.9) | 84.1 (64.2–93.9) | 1,872 | 99 | 5.3 (4.3–6.4) | 3.2 (1.6–4.8) | 38.0 (20.4–51.9) |
| Control | 1,341 | 38 | 2.8 (2.0–3.8) | 1,852 | 158 | 8.5 (7.3–9.9) | |||||
The ATP cohort includes women who received all 3 doses within protocol-defined windows, complied with the protocol during the vaccination period, did not have a biopsy or treatment (LEEP) before the 6-month visit, and were HPV DNA negative (by PCR) for at least one of the HPV types in the end point at enrollment and at the 6-month visit.
ITT cohorts include all women randomized and vaccinated, regardless of prevalence of infection and follow-up visits.
Table 5.
ATP and ITT efficacy estimates against HPV16/18 by number of sexual partners at enrollment
| Number of sex partners |
Arm | ATP analysisa | ITT analysis b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
||
| Virgin | Vaccine | 566 | 1 | 0.2 (0.0–0.9) | 2.6 (1.4–2.9) | 93.6 (64.8–99.7) | 773 | 4 | 0.5 (0.2–1.2) | 2.0 (0.9–2.7) | 79.8 (44.9–94.1) |
| Control | 615 | 17 | 2.8 (1.7–4.3) | 819 | 21 | 2.6 (1.6–3.8) | |||||
| 1 partner | Vaccine | 904 | 3 | 0.3 (0.1–0.9) | 2.6 (1.6–3.1) | 88.8 (66.5–97.3) | 1,237 | 40 | 3.2 (2.4–4.3) | 3.4 (1.7–4.9) | 51.1 (28.9–66.7) |
| Control | 915 | 27 | 3.0 (2.0–4.2) | 1.256 | 83 | 6.6 (5.3–8.1) | |||||
| 2 partners | Vaccine | 544 | 1 | 0.2 (0.0–0.9) | 3.1 (1.8–3.4) | 94.4 (69.1–99.7) | 777 | 38 | 4.9 (3.5–6.6) | 5.9 (3.1–8.3) | 54.5 (33.5–69.3) |
| Control | 519 | 17 | 3.3 (2.0–5.1) | 753 | 81 | 10.8 (8.7–13.1) | |||||
| 3+ partners | Vaccine | 621 | 3 | 0.5 (0.1–1.3) | 4.0 (2.5–4.7) | 89.2 (67.9–97.4) | 940 | 71 | 7.6 (6.0–9.4) | 5.2 (2.3–7.9) | 40.7 (20.4–56.0) |
| Control | 628 | 28 | 4.5 (3.0–6.3) | 911 | 116 | 12.7 (10.7–15.0) | |||||
The ATP cohort includes women who received all 3 doses within protocol-defined windows, complied with the protocol during the vaccination period, did not have a biopsy or treatment (LEEP) before the 6-month visit, and were HPV DNA negative (by PCR) for at least one of the HPV types in the end point at enrollment and at the 6-month visit.
ITT cohorts include all women randomized and vaccinated, regardless of prevalence of infection and follow-up visits.
We also investigated VE according to time between vaccination and incidence of persistent infections (Table 6). In the ATP analysis, VE against HPV16/18 increased with time since enrollment to 100% after 34 months. In the ITT analysis, efficacy also increased with follow-up from only 16% in the first 2 years to more than 90% after 46 months. A similar effect was observed when we considered efficacy against HPV31, 33, and 45 combined: ATP efficacy changed from 41.7% (95% CI, −31.3 to 75.4) 10–22 months after vaccination to 57.6% (95%CI, −31.9 to 88.5) after 46 months. In the ITT analysis, corresponding VE went from −19.4% (95% CI, −64.9 to 13.3) to 53.1% (95% CI, 8.0 to 77.1).
Table 6.
Rates of persistent infection with HPV16/18 and VE (ATP and ITT) against HPV16/18 by time since enrollment
| Time since enrollment |
Arm | ATP analysisa | ITT analysisb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, % (95% CI) |
Women, N | Women with events, n |
Rate per 100 women, n (95% CI) |
Rate reduction/ 100 women, n (95% CI) |
VE, n (95% CI) |
||
| 10–22 mo | Vaccine | 1,599 | 5 | 0.3 (0.1–0.7) | 0.8 (0.2–1.2) | 71.2 (25.6–90.5) | 3,056 | 115 | 3.8 (3.1–4.5) | 0.7 (−0.3 to 1.7) | 15.6 (−8.1 to 34.2) |
| Control | 1,655 | 18 | 1.1 (0.7–1.7) | 3,071 | 137 | 4.5 (3.8–5.2) | |||||
| 22–34 mo | Vaccine | 2,190 | 3 | 0.1 (0.0–0.4) | 1.6 (1.1–1.8) | 91.9 (76.6–98.0) | 2,870 | 25 | 0.9 (0.6–1.3) | 1.3 (0.7–1.8) | 59.7 (36.5–75.0) |
| Control | 2,239 | 38 | 1.7 (1.2–2.3) | 2,913 | 63 | 2.2 (1.7–2.7) | |||||
| 34–46 mo | Vaccine | 1,258 | 0 | 0.0 (0.0–0.2) | 1.4 (0.9–1.4) | 100.0 (81.0–100.0) | 3,031 | 11 | 0.4 (0.2–0.6) | 1.8 (1.4–2.2) | 83.5 (69.6–91.7) |
| Control | 1,240 | 17 | 1.4 (0.8–2.1) | 3,001 | 66 | 2.2 (1.7–2.8) | |||||
| 46+ mo | Vaccine | 973 | 0 | 0.0 (0.0–0.3) | 1.6 (1.0–1.6) | 100.0 (78.6–100.0) | 2,101 | 2 | 0.1 (0.0–0.3) | 1.6 (1.2–1.7) | 94.3 (80.1–99.1) |
| Control | 1,011 | 16 | 1.6 (0.9–2.5) | 2,083 | 35 | 1.7 (1.2–2.3) | |||||
The ATP cohort includes women who received all 3 doses within protocol-defined windows, complied with the protocol during the vaccination period, did not have a biopsy or treatment (LEEP) before the 6-month visit, and were HPV DNA negative (by PCR) for at least one of the HPV types in the end point at enrollment and at the 6-month visit.
ITT cohorts include all women randomized and vaccinated, regardless of prevalence of infection and follow-up visits.
In an effort to compare the VE to prevent 12-month persistent infections with VE to prevent 6-month persistent infections, we also calculated VE against that outcome, including the same stratified analyses (Supplementary Tables S3–S9). The results were very similar, although there is more statistical power. In this context, it is noteworthy that HPV31 was no longer the only nonvaccine HPV type with significant protection. The VE to prevent 6-month infection with HPV45 was 73.0% (95% CI, 45.3–87.8). Among women who were HPV DNA positive at enrollment, we did not detect significant efficacy against persistent infection with any of the HPV types investigated (Supplementary Table S10).
We also analyzed the 600 subjects excluded from ATP because they received at least 1 of the 3 vaccine doses outside the ATP windows. Estimates of VE against HPV16/18 with the use of similar exclusion criteria as those for the ATP analysis and among all women (ITT) produced results similar to the respective analyses among women who received their 3 doses within the windows (Supplementary Table S11).
Discussion
Results from this independent trial support the strong protective effect of Cervarix against 12-month HPV16/18 persistent infections in the ATP cohort (5). Protection was close to 90% against these two types, which are responsible for approximately 70% of cervical cancers (17). In addition, we observed nearly 50% cross-protection against HPV31/33/45, which are associated with approximately 10% of cancers. The VE against HPV16/18 was only 50% when we considered all vaccinated women (ITT) and just 12% when we considered persistent infections with any oncogenic HPV type, even in ATP cohorts.
For these analyses, we chose the surrogate outcome of persistent infection, which is highly reproducible (18), unlike histopathologic end points emphasized in previous reports. In previous work we have reported from Guanacaste, we compared the relative reproducibility and validity of CIN2 and CIN3 diagnoses by comparing community pathologists’ diagnoses with two independent reviewers from the United States (total, N = 357). Two review pathologists agreed with 84% and 81%, respectively, of initial diagnoses of CIN3 compared with 13% and 31% of CIN2. Although CIN3 is a substantially more reproducible diagnosis than CIN2, the latter constitutes an important fraction of lesions in reported clinical trials (13). In addition, the virologic outcome provides direct assessment of causality in the presence of multiple infections and has a relatively high positive predictive value for subsequent development of lesions (19). The ITT analyses incorporate the reality of incomplete vaccination in mostly sexually active adults and can be extrapolated to other populations of similar age, sexual behavior, and compliance. In contrast, most women in the ATP analyses are probably naïve to HPV infection, allowing extrapolation to women vaccinated before sexual debut and who comply with vaccination regimens.
We observed statistically significant cross-protection against HPV31/33/45 as a group. There was no apparent efficacy against the very common persistent infections with HPV types other than HPV16, 18, 31, 33, and 45, an association that attenuated the overall efficacy against persistent infections with all oncogenic types down to 12%. The nominally significant deleterious effect on HPV51 may be a chance finding among many comparisons made and was not observed in the other large trials of Cervarix (20). The 4-year follow up of our study was too short to observe whether other HPV types replace vaccine types in vaccinated cohorts. Natural history data do not indicate that one HPV type modifies the epidemiology of the other (21, 22), but we did not investigate whether the presence of a nonvaccine type modifies the vaccine's protection against infection with HPV16 or HPV18.
Inclusion of women regardless of serostatus, which is imperfectly measured, allowed us to observe the full impact of the vaccine in a population, including presumably immune women. The ATP VE against HPV16 among women seronegative for HPV16 was 92.2%, approximately twice as high as that in seropositive women. The attack rate of persistent infection was lower in seropositive than seronegative women in the control arm, likely reflecting natural protection by serum antibodies and possibly other immune mechanisms (23) or reduced exposure a few years after initiation of sexual activity. The greater attack rate of persistent infection among seropositive than seronegative women in the vaccine arm may reflect high proportions of missed infections (possibly as the result of inadequate sampling of the genital tract, missed test results, or latent infections) in women who do not benefit from vaccination because they were infected before baseline. The absence of reduction in efficacy against HPV16 persistent infection among HPV18 seropositive women suggests that immune protection, rather than other correlates of sexual activity associated with antibody levels, explains the effect.
Similar efficacy against persistent infection with HPV16/18 in ATP analysis by age indicates that the vaccine is effective at protecting against new infections in unexposed women independent of age. The strong decrease in efficacy from 68% at ages 18 to 19 to 21% at ages 24 to 25 in the ITT cohort probably reflects that in the latter, there is a significantly larger fraction of women who have initiated sexual activity before vaccination and have been exposed to HPV. Rate reductions also clearly decrease with age and years since first intercourse, with the exception of virgins, who do not contribute time at risk until they start sexual activity. It should be noted, however, that the reduction in VE and rate reductions is only present in the group of women 24 to 25 years old. Interestingly, rate reductions tend to be greater among women with more partners (because their attack rate is greater) despite lower VE.
These results indicate that both susceptibility and rates of transmission are important parameters when the potential impact of prophylactic vaccines is assessed and have implications for vaccination efforts and screening policy. In the absence of a test to determine expected benefit of an individual woman, age appears to be clearly a criterion to consider for definition of target groups for vaccination. The decreases in estimates of VE seen with increasing age and time since sexual debut suggest that many infections that could eventually progress to cancer occur early and can only be prevented with adolescent vaccination.
The observation that vaccination did not substantially reduce the incidence of oncogenic infections has implications for screening programs. The positive predictive value of the tests will likely be reduced because many of the persistent infections by nonvaccine HPV types are unlikely to progress to significant lesions. The lack of reduction in infections with the lesser oncogenic types can lead to more diagnostic and therapeutic procedures than necessary in vaccinated cohorts.
Interestingly, we noted that VE against HPV16 and HPV18 tended to increase with time since vaccination, to 100% in the ATP cohort and to almost 95% in the ITT cohort, with a similar effect for the combined outcome of HPV31, 33, and 45 (to a maximum close to 60%). One possible explanation of increasing efficacy against persistent HPV16/18 infections with time since vaccination in the ATP cohort is waning influence of false-negative baseline HPV DNA results, for which efficacy is zero or low. This interpretation is supported by the reduced VE against HPV16 observed among women who were seropositive for anti-HPV16 antibodies. Similarly, the likely explanation in the ITT cohort is waning influence of baseline prevalent infections. In ITT, increased VE with time since vaccination reflects protection against new infections, but the impact of this protection in the out years needs to be interpreted in the context of the fact that exposure tends to be greatest early on after initiation of sexual activity, with reduced exposure typically observed with increasing age/time.
Most of the findings, including the stratified analyses, were similar when a 6-month HPV persistence end point was used, with the advantage that the number of end points was larger, indicating that 6-month persistence could serve as an adequate surrogate end point in HPV vaccine trials, particularly for the evaluation of HPV types that occur with lower frequency or have lower vaccine efficacies that require larger sample sizes to achieve statistical significance. In this study, for example, VE against HPV45 was not significant when the 12-month end point was used but was clearly so when the 6-month end point was used.
This analysis has some limitations and strengths. One of the limitations is that we had a relatively small sample size to accurately assess the lower efficacy of individual nonvaccine HPV types, as has been the case with other clinical trials of Cervarix (5). Those multicentric trials as well as those reported for Gardasil recruited smaller number of women in multiple research centers. In contrast, the Costa Rica HPV trial was conducted in a homogeneous population of young women at high risk of HPV infection. In this context, the results can be extrapolated to similar groups of women in areas of high HPV prevalence. It should be noted, however, that a high prevalence of HPV in young women is very common in most areas of the world, particularly those in which the vaccine is being considered to control the problem of cervical cancer. Differences in sexual practices, in particular the distribution of age at first intercourse in the population, should be taken into account when designing HPV vaccination programs.
One of the strengths of this study is that it is a large trial in a stable community, which will allow long-term follow-up up of these cohorts. Moreover, the fact that the results of this trial are very similar to those obtained in the multicentric trials points to the generalizability of VE results. The fact that participation rates at enrollment were limited could also affect the external validity of the results but not the internal validity of the randomized trial. We used virological outcomes, which have some advantages because they are highly reproducible and do not present problems for causality assessment in the presence of multiple infections. However, the clinical significance of virological outcomes, particularly for nonvaccine types, is still under active debate.
In conclusion, the clear benefit of Cervarix against persistent HPV16/18 infections observed among unexposed women decreases with age and sexual experience. These findings, together with extensive data indicating that HPV is acquired early on after sexual debut (24, 25) and the possibility of natural immunity (23), suggest limited value, in general, for vaccination beyond a few years after adolescence in areas of high prevalence of HPV infection and high risk of cervical cancer. Efforts that focus vaccination on women before sexual debut may be most effective at reaching the most vulnerable groups.
Methods
Design, Subjects, and Procedures
The study was approved and supervised by the Institutional Review Boards of the Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA) in Costa Rica and the National Cancer Institute (NCI) in the United States. This analysis presents a double-blind randomized controlled trial of Cervarix among healthy women 18 to 25 years of age. Detailed methods have been published elsewhere (16). Potential participants from a census were invited (June 2004 to December 2005). After the participants provided informed consent, an interview, medical history, physical examination, and pregnancy test were conducted. For eligibility, women had to be healthy, not pregnant, not breastfeeding, and using contraception during the vaccination period. Main exclusion criteria were chronic diseases, history of reactions to vaccines, and history of hepatitis A or vaccination against it. Women were recruited and randomized regardless of past sexual behavior, HPV status, or cytology.
A pelvic examination was performed on sexually experienced women. Exfoliated cells for cytology, HPV DNA, CT DNA, GC DNA, and other testing were collected with a Cervex-Brush (Rovers Medical Devices) by firmly rotating the brush 5 times 360 degrees around the cervical os. In women whose cervix exhibited extensive ectopy, the cervix brushing was also used on the ectocervix to insure sampling of the squamo-columnar junction. Blood was collected from all participants (16).
Randomization and Vaccines
Participants were randomized with equal chance to Cervarix or hepatitis A vaccine. Each dose of the HPV16/18 vaccine contained HPV16 and HPV18 L1 virus-like particle (20 µg of each) adjuvanted with 50 µg of 3-O-desacyl-4′-monophosphoryl lipid A and 0.5 mg of aluminum hydroxide. Each dose of the control hepatitis A vaccine contained 720 ELISA units of inactivated hepatitis A antigen and 0.5 mg of aluminum hydroxide. Both were formulated in 0.5-mL doses with identical packaging and appearance to assure blinding. Vaccination schedule consisted of 3 doses at 0, 1, and 6 months. Desirable windows for vaccination defined periods beyond which the corresponding dose was not administered (16). At 6 months, sexually active women self-collected vaginal cells for HPV testing, with results comparable with clinician-collected specimens (8).
Follow-Up
Each participant was scheduled for four annual follow-up examinations. Cytology was classified using the Bethesda system (26). Women with low-grade squamous intraepithelial lesion (LSIL) or HPV-positive atypical squamous cells of undetermined significance (ASC-US) were followed semiannually for safety until three normal results were obtained. A repeat LSIL, HPV-positive ASC-US, a single ASC-high-grade, high-grade squamous intraepithelial lesion–positive, or glandular abnormalities prompted colposcopy and treatment as needed. Unsatisfactory cytology was managed as LSIL.
Safety Monitoring
All participants were observed 30 to 60 minutes after vaccination. Adverse event and pregnancy information was actively collected during follow-up. An independent data and safety monitoring board has met regularly to examine unblinded adverse events (most recent meeting: February 2011) and repeatedly recommended continuation of the trial. The study is still blinded, and investigators had no access to unmasked data by arm; therefore, no safety data are presented in this report. However, the authors of two published reports (27, 28) on pregnancies and autoimmune conditions have included safety data from this study.
HPV DNA and Antibody Testing
Broad-spectrum PCR-based HPV DNA testing was performed at DDL Diagnostic Laboratory, on the basis of amplification and probe hybridization with use of the SPF10 HPV DNA enzyme immunoassay system followed by typing with the LiPA25 version 1 line detection system as described previously (29, 30). To ensure that HPV16 and HPV18 infections were not missed, all specimens positive for HPV DNA when SPF10 DNA enzyme immunoassay was used but negative for HPV16 or HPV18 by LiPA25 also were tested with type-specific primers/probes for the presence of HPV16 and HPV18 DNA (30,31). ELISA was used for the detection and quantification of IgG antibodies against HPV16 and 18 separately by GlaxoSmithKline as described (32).
Statistical Analysis
Results presented are postlicensure analyses, conducted by an external group (Information Management Systems) under the direction of the investigators who remain masked to individuals’ randomizations. We defined persistence as detection of same-type HPV in samples collected at two visits, at least 10 months apart (minimum required for two consecutive annual visits), without intervening negatives. Similarly, 6-month persistence was calculated as detection of same-type HPV in samples collected at two visits, at least 4 months apart (minimum required for two consecutive semiannual visits). There were a total of 2,668 oncogenic infections with 10+ months between first detection and last detection, of which 496 (18.6%) are not counted as persistent because of intervening negatives.
We defined different cohorts for each end point of HPV infection. ATP cohorts include women who received 3 doses within protocol-defined windows, were protocol-compliant during vaccination, had no biopsy/treatment before the 6-month visit, and were HPV DNA-negative by PCR for the corresponding HPV type at enrollment and the 6-month visit (when receiving third dose; 2,635 women in the HPV vaccine arm and 2,677 in the control arm) (16). ITT cohorts include all randomized women, regardless of compliance or enrollment infection (3,727 in the HPV arm and 3,739 in the control arm). Balance by arm overall and within subgroups was evaluated by exact binomial test when the number of women was <30 and by the analogous normal approximation to the binomial test when the total was >30.
VE is the percentage reduction in end point related to vaccine administration, estimated as the complement of the ratio of the cumulative attack rates in the HPV and control arms. The attack rate is the percentage of women in the cohort who experience the end point. The confidence interval (CI) for VE is derived from the corresponding CI for the risk ratio. The exact conditional test was used for analyses of VE. The analytical unit for all analyses is the woman rather than the infection because our principal interest is to determine the proportion of women protected against persistent HPV infections with the potential to cause cancer in the woman.
We used the difference between the attack rates in the vaccinated and control arms to address the question of absolute impact of vaccination overall and in subgroups. The CI for the difference was calculated on the basis of the exact test.
The primary objective in our prespecified plan was to evaluate VE against 1-year persistent infection with HPV16 and/or HPV18 (HPV16/18). We evaluated cross-protection against HPV31/33/45, for individual oncogenic HPV types, and for all oncogenic types combined. Six-month persistent infection also was evaluated in secondary analyses. In addition, stratified VE was calculated by enrollment covariates (age, age at first intercourse, time since first intercourse, number of sexual partners, HPV DNA, and antibody status).
Oncogenic HPV types include HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 to 73 (68–73 cannot be differentiated with genotyping method used). PCR results from all visits of a participant were included in the analyses (annual, semiannual, and colposcopy).
More than 600 women received their 3 doses outside the strict ATP windows. Separate cohorts were defined to analyze efficacy in this subgroup by the use of similar criteria for ATP and ITT as described previously.
Our results are determined on the basis of an event-triggered statistical analysis plan (SAP) approved by the U.S. Food and Drug Administration. The SAP specifies a one-sided α-level of 0.001 for this “interim” analysis of persistent HPV16/18 infections. Results in this paper provide the most up-to-date available data from the latest data freeze of June 21, 2010. A previously published abstract for the International Papillomavirus Conference, held in July 2010, included analysis of persistent HPV16/18 infections from an earlier (January 1, 2010) data freeze; the P-value was <10−10 in the ATP cohort. For regulatory purposes, we consider that 2 freezes constitute 2 separate interim analyses, leaving 0.023 (= 0.025 − 0.001 × 2) as the one-sided α level when we perform our final analysis. Only the analysis of persistent HPV16/18 infections entails α spending according to the SAP. Other analyses are exploratory in the SAP and do not require adjustment.
SIGNIFICANCE.
In an independent trial of the bivalent ASO4-adjuvanted HPV16/18 vaccine (Cervarix) conducted among young women in Costa Rica, we confirmed the high efficacy against HPV16/18 persistent infection and partial cross-protection against HPV31/33/45. Furthermore, efficacy data suggest that the benefit of HPV vaccination is maximal when the vaccine is given to young women before they initiate sexual activity.
Supplementary Material
Acknowledgments
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project, including Bernardo Blanco and his team (census); Ricardo Cerdas and Ana Hernández (blood processing); José Miguel González, Osman López, Johnny Matamoros, Manuel Sánchez, Rafael Thompson, and Jorge Umaña (field activity coordinators); Su Yen Araya, Hazel Alvarez, Hayleen Campos, Muriel Grijalba, Ana Cristina Monge, Ana Peraza, Diana Robles, María Fernanda Sáenz, Dorita Vargas, and Jessica Vindas (clinic coordinators); Paola Alvarez, Dinia Angulo, Ana Live Arias, Betzaida Barrantes, Andrea Bolaños, Diego Bonilla, Marianela Bonilla, Mary José Calvo, Loretto Carvajal, Jessenia Chinchilla, Blanca Cruz, Marianela Herrera, Andrea Interiano, Fabiola Jiménez, Erick Lagos, Viviana Loría, Andrea Messeguer, Rebeca Ocampo, Ana Cristina Ocampo, Silvia Padilla, Angie Ramírez, Daniela Romero, Byron Romero, Yessenia Ruiz, Daniela Ruiz, Genie Saborío, Sofía Soto, Malena Salas, Adriana Torrez, Natalia Ugalde, Adriana Vallejos, Yesenia Vásquez, Maricela Villegas (clinicians); Marta Alvarado, Ana Cristina Arroyo, Gloriana Barrientos, Diana Díaz, Marlen Jara, Maureen Matarrita, María Ester Molina, Elida Ordóñez, Adriana Ramírez, Gina Sánchez, and Sihara Villegas (nurses); Arianne Castrillo and Vivian López (education and outreach effort coordinators); Karla Coronado (appointment coordinator); Ricardo Alfaro (quality control coordinator); Yenory Estrada (pharmacist); Charles Sánchez and Livia Romero (document center coordinators); Cristian Montero (quality assurance, regulatory); and Carlos Avila and Eric Alpízar (IT coordinators). Special recognition is also extended to Sofía Elizondo, Executive Director of Fundación INCIENSA, and her staff for their administrative support. In the United States we would like to extend our appreciation to the team from Information Management Services, responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Julie Buckland and Laurie Rich. We acknowledge the contributions made by individuals at Westat, Inc., who provided project development and/or monitoring support, including Maribel Gomez, Kirk Midkiff, Isabel Trejos, and Susan Truitt. We acknowledge the assistance provided by Carla Chorley, Troy Moore, Kathi Shea, Mindy Collins, and Heather Siefers in the establishment of a specimen and vaccine repository for our trial and in their continued assistance with the handling and shipment of specimens. From GSK Biologicals, we would like to acknowledge the contributions of Gary Dubin, Anne Schuind, Kelechi Lawrence, Darrick Fu, and Bruce Innis for their contribution to discussions regarding trial conduct and Francis Dessy and Catherine Bougelet for HPV16/18 antibody testing. We would like to thank members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Wasima Rida, Henriette Raventós, Luis Rosero-Bixby, and Sarah Thomas).
Appendix
Names and Affiliations of Investigators in the Costa Rica Vaccine Trial Group
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (Cytopathologist)
Manuel Barrantes (Field Supervisor)
M. Concepción Bratti (Co-Investigator)
Fernando Cárdenas (General Field Supervisor)
Bernal Cortés (Specimen and Repository Manager)
Albert Espinoza (Head, Coding and Data Entry)
Paula González (Co-Investigator)
Diego Guillén (Pathologist)
Rolando Herrero (Co-Principal Investigator)
Silvia E. Jiménez (Trial Coordinator)
Jorge Morales (Colposcopist)
Lidia Ana Morera (Head Study Nurse)
Elmer Pérez (Field Supervisor)
Carolina Porras (Co-Investigator)
Ana Cecilia Rodríguez (Co-Investigator)
Libia Rivas (Clinician’s Coordinator)
Luis Villegas (Colposcopist)
University of Costa Rica, San José, Costa Rica
Ivannia Atmella (Microbiologist, Immunology Laboratory)
José Bonilla (Head, HPV Immunology Laboratory)
Enrique Freer (Director, HPV Diagnostics Laboratory)
Alfonso García-Piñeres (Immunologist)
Margarita Ramírez (Microbiologist, Immunology Laboratory)
Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory)
U.S. National Cancer Institute, Bethesda, Maryland
Allan Hildesheim (Co-Principal Investigator and NCI Co-Project Officer)
Aimee R. Kreimer (Investigator)
Douglas R. Lowy (HPV Virologist)
Nora Macklin (Trial Coordinator)
Mark Schiffman (Medical Monitor and NCI Co-Project Officer)
John T. Schiller (HPV Virologist)
Mark Sherman (QC Pathologist)
Diane Solomon (Medical Monitor and QC Pathologist)
Sholom Wacholder (Statistician)
SAIC, NCI-Frederick, Frederick, Maryland
Ligia Pinto (Head, HPV Immunology Laboratory)
Troy Kemp (Scientist, HPV Immunology Laboratory)
Women’s and Infants’ Hospital, Providence, Rhode Island
Claire Eklund (QC Cytology)
Martha Hutchinson (QC Cytology)
Georgetown University, Washington, DC
Mary Sidawy (Histopathologist)
DDL Diagnostic Laboratory, Voorburg, The Netherlands
Wim Quint (Virologist, HPV DNA Testing)
Leen-Jan van Doorn (HPV DNA Testing)
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://www.cancerdiscovery.aacrjournals.org).
Disclosure of Potential Conflicts of Interest
The Costa Rican Vaccine Trial is a longstanding collaboration between investigators in Costa Rica and NCI. The trial is sponsored and funded by NCI (N01-CP-11005) with support from the NIH Office of Research on Women's Health and conducted in agreement with the Ministry of Health of Costa Rica. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Vaccine was provided for our trial by GSK Biologicals, under a Clinical Trials Agreement with NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. D.R. Lowy and J.T. Schiller are named inventors on U.S. government-owned HPV vaccine patents that are licensed to GSK and Merck, and so are entitled to limited royalties as specified by federal law. None of the other coauthors have any potential conflicts of interest to report.
REFERENCES
- 1.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011 Apr 6; doi: 10.1093/annonc/mdr015. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 4.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 5.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 6.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 7.Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis. 2009;199:919–922. doi: 10.1086/597308. [DOI] [PubMed] [Google Scholar]
- 8.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. VRBPAC Background Document: Gardasil” HPV Quadrivalent Vaccine: May 18, 2006 VRBPAC Meeting. Available from: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4222B3.pdf.
- 10.Chang Y, Brewer NT, Rinas AC, Schmitt K, Smith JS. Evaluating the impact of human papillomavirus vaccines. Vaccine. 2009;27:4355–4362. doi: 10.1016/j.vaccine.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle PE, Porras C, Quint WG, Rodriguez AC, Schiffman M, Gravitt PE, et al. Comparison of two PCR-based human papillomavirus genotyping methods. J Clin Microbiol. 2008;46:3437–3445. doi: 10.1128/JCM.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreon JD, Sherman ME, Guillen D, Solomon D, Herrero R, Jeronimo J, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007;26:441–446. doi: 10.1097/pgp.0b013e31805152ab. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins D. A review of cross-protection against oncogenic HPV by an HPV-16/18 AS04-adjuvanted cervical cancer vaccine: importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecol Oncol. 2008;110:S18–S25. doi: 10.1016/j.ygyno.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) National, state, and local area vaccination coverage among adolescents aged 13–17 years–United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018–1023. [PubMed] [Google Scholar]
- 16.Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 18.Castle PE, Wheeler CM, Solomon D, Schiffman M, Peyton CL. Interlaboratory reliability of Hybrid Capture 2. Am J Clin Pathol. 2004;122:238–245. doi: 10.1309/BA43-HMCA-J26V-WQH3. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Rodriguez AC, Burk RD, Herrero R, Wacholder S, Alfaro M, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. VRBPAC Briefing Document. CERVARIX. Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, Recombinant. Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document. 2009 Sep 9; Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee.
- 21.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–1589. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi AK, Katki HA, Hildesheim A, Rodriguez AC, Quint W, Schiffman M, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203:910–920. doi: 10.1093/infdis/jiq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Koutsky LA. Comparison of incident cervical and vulvar/vaginal human papillomavirus infections in newly sexually active young women. J Infect Dis. 2009;199:815–832. doi: 10.1086/597118. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez AC, Burk R, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. The natural history of human papillomavirus infection and cervical intraepithelial neoplasia among young women in the Guanacaste cohort shortly after initiation of sexual life. Sex Transm Dis. 2007;34:494–502. doi: 10.1097/01.olq.0000251241.03088.a0. [DOI] [PubMed] [Google Scholar]
- 26.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 27.Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ. 2010;340:c712. doi: 10.1136/bmj.c712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26:6630–6638. doi: 10.1016/j.vaccine.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 29.Kleter B, Van Doorn LJ, ter Schegeget J, Schrauwen L, van Krimpen K, Burger M, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153:1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleter B, Van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37:2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.