Abstract
Food restriction (FR) augments the behavioral and reinforcing effects of psychomotor stimulants such as cocaine or amphetamine; effects that may be related to the capacity of FR to increase plasma levels of ghrelin (GHR), a 28-amino acid orexigenenic peptide linked to activation of brain dopamine systems. The present study used wild-type (WT) mice or mutant mice sustaining knockout of either GHR (GHR(-/-)) or of the growth hormone secretagogue receptor (GHS-R(-/-)) and subjected to FR or not to evaluate the role of GHR and GHS-R in cocaine-stimulated locomotion. WT, GHR(-/-), and GHS-R(-/-) mice were either restricted to 60% of baseline caloric intake or allowed to free-feed (FF). Mice were treated with 0, 1.25, 2.5 and 5.0 mg/kg cocaine on separate test days (in random dose order) and forward locomotion was recorded on each drug day for 45 min after drug dosing. Food (and water) was available immediately after (but not during) each activity test. For FF mice, there was no interaction between cocaine and GHR status on locomotion. FR-WT mice treated with saline exhibited significant increases in anticipatory locomotion (relative to FF-WT mice), whereas FR-GHS-R(-/-) mice did not. Cocaine significantly increased locomotion in FR-GHR(-/-) and FR-GHS-R(-/-) mice to the levels noted in FR-WT mice. These results suggest that GHS-R activity, but not GHR activity, is required for FR to augment food-associated anticipatory locomotion, but do not support the contention that GHR pathways are required for the capacity of FR to augment the acute effect of cocaine on locomotion.
Keywords: Food intake, Ghrelin, Psychostimulants
INTRODUCTION
Food restriction (FR) can increase the rate of acquisition of learned responses for many reinforcers, including food as well as the psychostimulant drugs cocaine or amphetamine (Carr, 2002; Carroll, 1985; Carroll and Meisch, 1981). A link between caloric homeostasis and psychostimulant action in rats is further supported by studies in which FR augments the capacity of psychostimulants to enhance locomotion and to induce conditioned place preference (CPP) (Bell et al., 1997). FR also augments the rewarding effects of electrical stimulation of the lateral hypothalamus (LH) (Cabeza De Vaca et al., 1998; Fulton et al., 2000); a model system used to explore mechanisms that modulate reinforcement function in brain (Olds and Milner, 1954; Wise, 1996).
Prior studies suggest that signals related to the acute availability of metabolic fuels (e.g., glucose, free fatty acids) are unlikely to wholly account for FR-associated changes in psychostimulant action, inasmuch as short-term glucoprivation or lipoprivation does not alter LH self-stimulation in FR rats (Cabeza De Vaca et al., 1998; Carr, 2002; Fulton et al., 2000). Prolonged negative energy balance results in increased expression of NPY in the hypothalamus; however, administration of NPY does not alter LH self-stimulation (Cabeza De Vaca et al., 1998). Although FR can be viewed as a stressor, acute modulation of corticosterone availability does not reverse the capacity of FR to sensitize LH self-stimulation (Abrahamsen et al., 1995). These studies collectively suggest that FR may act through an as yet unidentified feeding-relevant system(s) to modulate psychostimulant reactivity.
Ghrelin (GHR) is a 28 amino acid peptide secreted peripherally from stomach and gut that functions as an orexigenic factor (Cummings et al., 2001; Hosoda et al., 2006; Kojima and Kangawa, 2005). Human plasma ghrelin levels are at a nadir after a meal and then peak prior to the next meal (Cummings et al., 2001). Plasma ghrelin levels increase during periods of FR, and decrease after eating (Toshinai et al., 2001). Ghrelin enhances food intake when administered either peripherally or centrally (Kojima and Kangawa, 2005; Murakami et al., 2002; Shimbara et al., 2004) and augments feeding-associated behaviors such as hoarding and foraging (Keen-Rhinehart and Bartness, 2005). Systemic ghrelin is passively transported across the blood–brain barrier (Banks et al., 2008; Banks et al., 2002; Diano et al., 2006) and ghrelin receptors have been located on brain dopamine neurons (Abizaid et al., 2006; Diano et al., 2006; Guan et al., 1997; Naleid et al., 2005).
Changes in peripheral ghrelin levels occasioned by FR could result in changes in dopamine signaling in brain reinforcement systems. Consistent with this view, we reported that peripheral ghrelin (5 nmol, i.p.) administration (to non-food deprived rats) enhanced cocaine-induced (2.5, 5.0, 10.0 mg/kg) hyperlocomotion as compared to rats pretreated with saline (Wellman et al., 2005). In a subsequent study, we determined that systemically administered ghrelin (5 nmol/rat) can alter the rewarding properties of 0.3125 and 0.625 mg/kg cocaine, as indexed by a CPP procedure (Davis et al., 2007).
The aforementioned studies suggest that FR may act through a ghrelin-dependent pathway so as to augment the behavioral and reinforcing actions of cocaine. To further examine this hypothesis, the present study utilized mice sustaining ablation of either the ghrelin gene product (GHR-/-) or the ghrelin receptor (GHS-R-/-) (Sun et al., 2003; Sun et al., 2008) and wild-type (WT) mice in a study of the hyperlocomotor effects of cocaine. Half of the mice were allowed to free feed whereas the other half were food restricted to 60% of basal food intake levels. Forward locomotion in automated activity chambers was examined on four drug test days on which the mice were administered 0, 1.25, 2.5 or 5 mg/kg (i.p.) cocaine hydrochloride. Two non-drug test days were interposed between each drug test. The cocaine doses chosen for this study were based on our earlier CPP study in rats in which low doses of cocaine (0.3125-1.25 mg/kg) were shown to induce CPP when combined with systemic ghrelin (Davis et al., 2007). Our concern here was whether ablation of ghrelin or ghrelin receptors would blunt the capacity of FR to augment the hyper-locomotor actions of cocaine.
MATERIALS AND METHOD
Animals
Null mice were generated at Baylor College of Medicine by Dr. Roy G. Smith on a 129Sv and C57BL/6J background (Sun et al., 2003; Sun et al., 2008). To reduce the impact of genetic heterogeneity on metabolic phenotype, the GHR–/– and GHS-R–/– mice were backcrossed with C57BL/6J mice for 10 generations. To determine whether both null N10 mice types were congenic, the mice were analyzed for 110 microsatellite markers (Charles River Laboratory, Wilmington, MA). Besides the particular markers associated with gene deletion, all other markers were 100% identical to those characteristics of C57BL/6J mice, indicating that the N10 null mice are congenic (99.9% identical to C57BL/6J).
Following genotyping, the mice were shipped from Houston to College Station and maintained in quarantine for 30 days prior to transfer to the Psychology vivarium. The subjects were 53 mice (21 WT, 16 GHR–/–, and 16 GHS-R–/–) weighing 25-35 g at the start of the experiment. The mice were singly housed in polycarbonate rodent cages with wire flooring over paper pads. Each cage was also provided with compressed cotton bedding squares (Nestlets; Ancare). Food pellets were glued to plastic Petri dishes and hung from the side wall using copper wire loops (to allow for daily measures of food intake – see below). The colony room was maintained at 23.0±1° C at 70 % humidity under a 12h/12h illumination schedule (lights off at 1030 hr). The experimental procedures were approved by the Texas A&M University Laboratory Animal Care Committee.
Apparatus
The assessment of locomotion was made in a set of 8 automated optical beam activity monitors (Model RXYZCM-16; Accuscan Instruments, Columbus, OH, USA). Each monitor was housed within a 40 X 40 X 30.5 cm acrylic cage. To allow two mice to be run at one time, a set of acrylic dividers were placed in each cage that divided the chamber into four quadrants (of which two quadrants were used for the locomotion measures). Activity monitors and cages were located in a sound-proof room; extraneous noise was masked using a 40 dB [SPL] white noise generator within the test room. A multiplexor-analyzer monitored beam breaks from the optical beam activity monitors and tracked the simultaneous interruption of beams. The multiplexor-analyzer updated the position of each animal in the acrylic cage every 10 msec using a 100% real-time conversion system. Computerized integration of the data obtained from the monitor afforded the recording of general activity using total distance traveled scores (in cm) as the primary dependent measure (Sanberg et al., 1987).
Procedure
On the day prior to the start of testing, half of the animals in each genetic condition were randomly assigned to either FR or FF groups. This resulted in six treatment groups: FF-WT, FF-GHR–/–, FF-GHS-R–/–, FR-WT, FR-GHR–/–, and FR-GHS-R–/–. Mice in the FF groups continued to receive ad lib access to food, whereas mice in the FR groups were offered a ration of food each day equivalent to 60% of their daily food intakes starting at about 1200 hrs (just after the daily assessment of locomotion). In the initial phase of the project, animals were tested during 1 hr sessions each day for seven days, in squads of sixteen, two mice to a cage. With the room lights off, animals were placed in their respective test chambers for a 15-min baseline-recording period. On the last two days of this adaptation period (DAYS 6 and 7), each mouse received an i.p. injection of saline (10 ml/kg) at the end of the 15 min period. Each mouse was placed back in the chamber immediately following the injection for an additional 45 min period. On Days 8, 11, 14, and 17, all animals within each of the six groups received successive daily i.p. injections of 0, 1.25, 2.5, and 5.0 mg/kg of cocaine hydrochloride. Cocaine was a generous gift from Kevin Gormley of the Basic Research Division of NIDA. Cocaine doses were computed as the salt and were delivered in 10 ml/kg body weight. Each mouse received each drug dose once and drug doses were given in random order. On the two days in between each drug dose (Days 9-10, 12-13, 15-16), no injections were given and tests were conducted as above. Neither food nor water was available in the activity chambers.
Data Analyses
The overall design of the present study is a split-plot factorial with between-group factors of GHR status (WT, GHR–/– and GHS-R–/–) and deprivation condition (FF or FR) and a within-group factor of cocaine dose (0, 1.25, 2.5 and 5.0 mg/kg). The dependent measures were total distance traveled scores (cm) and stereotypy counts for each 45 min test session. Because the treatment means and variances of these measures were proportional, these values were subjected to a square-root transformation (Kirk, 1982). Additionally, because preliminary data analyses indicated that GHR status interacted with FR condition after vehicle injection (see below), analyses of covariance were computed using baseline locomotion scores after vehicle injection as the covariate. Statistical significance was deemed to be p < 0.05 and the Bonferroni procedure was used to examine mean group differences.
RESULTS
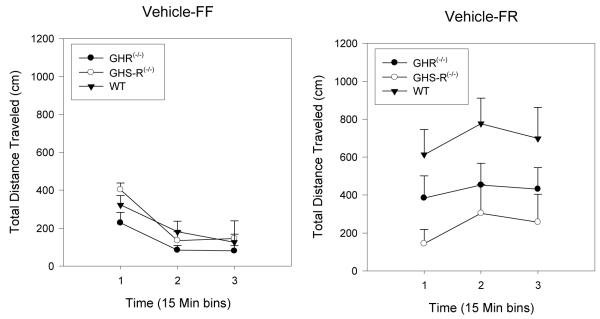
There were no significant baseline differences among the FF groups after vehicle injection (Figure 1, left panel). In the FR condition (Figure 1, right panel), WT mice exhibited the expected significant increase in basal locomotion after vehicle, whereas GHS-R-/- mice did not. GHR(-/-) mice subjected to FR exhibited an intermediate increase in locomotion, relative to the WT and GHS-R(-/-) groups. ANOVA of these data revealed a significant effect of deprivation condition (F(1, 39) = 16.5, p < 0.0001), of GHR status (F(2, 39) = 16.5, p < 0.013), and a significant interaction between deprivation condition and GHR status (F(2, 39) = 4.6, p < 0.017). These basal differences were the basis for computing subsequent data analyses using locomotion after vehicle injection as the covariate.
Figure 1.
Mean group (+ SEM) total distance traveled scores (cm) over three successive 15 min periods after vehicle injection in WT, GHR(-/-) and GHS-R(-/-) mice subjected to either FF (panel A) or FR (panel B) conditions. The bar above each symbol reflects the standard error of the mean for that value.
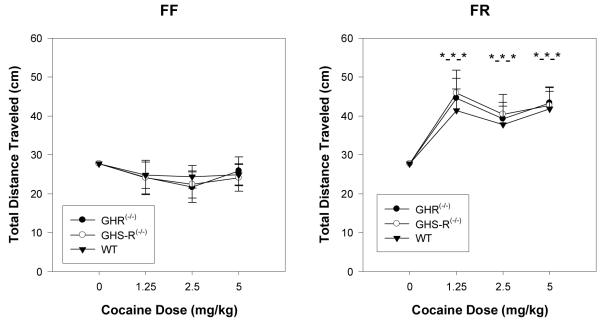
Figure 2 depicts the changes in locomotion noted in mice in the FF and FR conditions after injection of cocaine. Considering mice in the FF condition, cocaine administration, up to a dose of 5 mg/kg, did not significantly alter locomotion in any group. In contrast, cocaine administration significantly activated forward locomotion in mice maintained under the FR condition (F(3,114) = 8.57, p < 0.0001), but there was no significant effect of GHR status. The effect of cocaine on locomotion in the FR condition was not dose-dependent in that 1.25 mg/kg cocaine increased locomotion, relative to vehicle, but further increases to 2.5 and 5.0 mg/kg cocaine did not further increase locomotion.
Figure 2.
Total distance traveled scores (subjected to a square root transformation and covaried for locomotion after vehicle) after cocaine injection (0, 1.25, 2.5 and 5.0 mg/kg, i.p.) in WT, GHR(-/-) and GHS-R(-/-) mice subjected to either FF (left panel) or FR (right panel) conditions. The bar above each symbol reflects the standard error of the mean for that value. The triple stars (*-*-*) indicate that each cocaine condition for each GHR status group was significantly different from the respective baseline condition (p < 0.05).
Because GHR status significantly altered total distance traveled scores after vehicle treatment, the previous analysis used analysis of covariance to adjust for these initial differences. A problem, however, is that such an approach based on analysis of covariance can obscure real differences (“Lord Paradox”: cf: (Jamieson, 2004)). An additional analysis of variance was computed using percent change scores from vehicle baseline as the dependent measure. This analysis, like that computed using analysis of covariance, revealed no significant effect of GHR status on percent change from baseline scores (p < 0.067). This supplementary analysis suggests that the FR condition of the present study was sufficient to augment cocaine-induced hyperactivity to the same degree in each of the three GHR status groups. Recall that the predicted effect was that ablation of GHR or GHS-R was expected to blunt the augmentation of locomotion induced by cocaine in the FR condition.
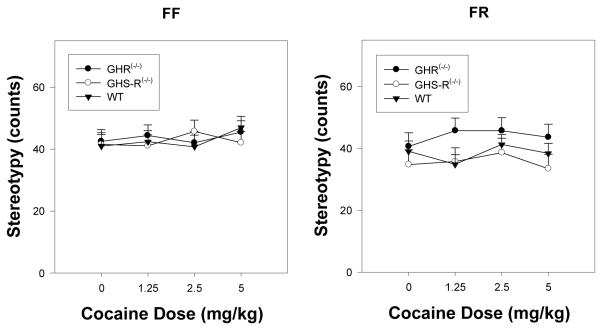
Figure 3 depicts changes in stereotypy counts in FF and FR groups as a function of GHR status and cocaine dose. There were no significant differences between the WT, GHR(-/-) and GHS-R (-/-) mice after vehicle treatment in either the FF or the FR conditions (p’s > at least 0.720). ANOVA of the stereotypy count data revealed a significant effect of deprivation condition (F(1,39) = 4.204, p < 0.047), with greater stereotypy counts noted in the FR condition relative to the FF condition. Although there was a trend (Figure 3B) for the GHR(-/-) mice to show greater stereotypy counts after cocaine, ANOVA of these data revealed no significant effects of either GHR status (p = 0.131) or an interaction of this factor with deprivation condition (p = 0.285) or cocaine dose (p = 0.986).
Figure 3.
Mean group (+ SEM) stereotypy counts (subjected to a square root transformation) after cocaine injection (0, 1.25, 2.5 and 5.0 mg/kg, i.p.) in WT, GHR(-/-) and GHS-R(-/-) mice subjected to either FF (left panel) or FR (right panel) conditions. The bar above each symbol reflects the standard error of the mean for that value.
In the present study, mice in the FR condition were fed an amount of food per day equal to 60% of their baseline food intake. This food ration was given just after each daily locomotor test. This procedure thus resulted in the mice not only being restricted to 60% of basal intake but also resulted in the mice being tested in an acute deprivation condition. Observation of the mice suggested that the food ration was quickly consumed upon being offered in the home cage. Over the course of behavioral testing for mice in the FR condition, 2 WT mice, 2 GHR(-/-) mice, and 4 GHS-R(-/-) mice died. No mice in the FF condition died over the course of the experiment. Of the mice exposed to the FR condition, 3 deaths occurred during the first vehicle trial (one each from each GHR status group), two deaths occurred during the 3rd and 4th intertrials (one WT and one GHS-R mice) and three deaths occurred just after a drug trial (one GHS-R mouse after Drug trial 1; one GHR mouse after Drug trial 2 and one GHS-R mouse after Drug trial 3). To evaluate the possibility that different GHR status conditions were associated with different survival rates, the Gehan-Breslow statistic (Sigma Stat 3.0) was computed for the survival curves of this study; this statistic was not significant (p = 0.447).
DISCUSSION
The focus of the present study was to examine the impact of cocaine on locomotion in WT mice, GHR(-/-) mice and GHS-R(-/-) mice under conditions of free-feeding or food restriction. Our prior studies demonstrated that systemic injection of GHR was sufficient to augment the hyperlocomotion induced by cocaine in rats (Wellman et al., 2005) and to augment the CPP induced by cocaine in rats (Davis et al., 2007). Additionally, chronic daily injection of GHR in rats induced a degree of sensitization to a subsequent injection of cocaine (Wellman et al., 2008).
GHS-Rs are located on ventral tegmental area neurons (Abizaid, 2009; Abizaid et al., 2006; Diano et al., 2006; Guan et al., 1997; Naleid et al., 2005). Moreover, systemic GHR injection increases dopamine overflow within the shell region, but not the core region, of the nucleus accumbens (Quarta et al., 2009). These findings suggest that GHR and GHS-Rs are positioned so as to modulate reinforcement to addictive drugs that act on brain dopamine systems and provide partial support for the notion that FR may act through GHR-dependent pathways to augment cocaine reinforcement/reward.
At the doses of cocaine used in the present study (0, 1.25, 2.5 and 5.0 mg/kg), mice maintained under a free-feeding condition did not show elevated locomotion, whereas mice given cocaine under the FR condition did. The doses of cocaine chosen for the current study were quite low (1.25-5.0 mg/kg). Indeed, these doses were without effect in the FF mice. For the FR mice, however, these cocaine doses were sufficient to increase locomotion. The pattern of increase induced by these cocaine doses, however, was similar for WT, GHR(-/-) and GHS-R(-/-) mice. Our current results thus suggest that neither GHR nor GHS-R are required for the acute effects of cocaine under conditions of food restriction.
The present study, however did not assess whether GHR or GHS-R are critical for the development of sensitization to chronic administration of cocaine under conditions of food restriction. Moreover, this study did not examine the impact of GHR ablation on other measures of reward and reinforcement. A final conclusion in this matter awaits experiments in which GHR(-/-) and GHS-R(-/-) animals are tested for effects of FR on locomotor sensitization, acquisition of cocaine reinforcement (i.e. intravenous self-administration) or conditioned place preference to cocaine and other drugs of abuse (Bardo and Bevins, 2000). We note that Perello et al., (Perello et al., 2010) reported that the capacity of FR to augment the CPP induced by consumption of a high-fat diet is critically dependent on functional GHS-R activity. GHS-R(-/-) mice failed to show high-fat-induced CPP, as did mice pretreated with Compound 26, a GHS-R1a antagonist.
Although our laboratory has focused on a potential role of ghrelin as mediating the capacity of FR to augment drug induced reward, an alternative view is that ghrelin can modulate drug reinforcement, independent of food restriction. Indeed, a number of recent studies suggest that antagonism of central ghrelin signaling can diminish reward associated with drugs of abuse. In particular Jerlhag’s group reported that acute administration of ethanol increased locomotion in WT mice but not in GHS-R mice. Moreover, GHS-R(-/- ) mice failed to show alcohol-induced CPP as did mice pretreated with the GHS-R1a antagonist JMV2959. In spite of the reported impact of GHR ablation on the capacity of ethanol to induce locomotion and support CPP, this group noted no difference in oral alcohol intake between WT, GHR(-/-) and GHS-R(-/-) mice, suggesting no effect of ghrelin gene status on alcohol consumption per se (Jerlhag et al., 2009).
A further consideration of the present results is that GHS-R(-/-) mice were relatively intolerant of a 40% restriction in daily food intake, with 4 GHS-R(-/-) mice dying during the course of behavioral testing relative to 2 mice each in the WT and GHR(-/-) conditions. This outcome is similar to the report of mortality noted by Blum and coworkers (Blum et al., 2009) in GHS-R(-/-) mice subjected to a 4 hour per day restricted feeding schedule. In the Blum study, the WT mice were able to increase their food intake to nearly control levels whereas GHS-R did not. In the present study, food restriction was based on a percentage of the total weight of food being made available to each mouse on a daily basis. In the Perello et al., (Perello et al., 2010) study, WT and GHS-R offered a 4 hour food access period were able to consume 70% of their normal calories and no mortality was noted for mice in the GHS-R condition relative to WT mice. The exact reasons for the differential mortality between the present study and those of Blum et al (Blum et al., 2009) and Perello et al., (Perello et al., 2010) are unclear. The potential intolerance of GHS-R(-/-) mice to food restriction may represent a difficulty for studies probing the mechanisms by which FR alters drug reinforcement.
In the present study, ghrelin null mice failed to increase their locomotion after vehicle administration in response to the FR condition. Prior studies indicate that FR or starvation can decrease or increase daily locomotion (e.g. behavioral arousal), depending on the magnitude of the restriction and the duration of the FR episode (Campbell and Baez, 1974; Moorcroft et al., 1971; Wang et al., 2006). When activity increases during food restriction, the increase can be entrained to a limited access feeding period, increasing sharply just prior to the onset of eating (e.g. anticipatory locomotion: (Blum et al., 2009; LeSauter et al., 2009)). Blum reported that ablation of the GHS-R in mice attenuated the increase in anticipatory locomotion noted just prior to start of a scheduled 4 hour meal. The present study in effect represents an assessment of anticipatory locomotion in that mice in the FR condition were offered 60% of their daily calories just after the assessment of locomotion. GHS-R(-/-) mice did not exhibit a significant enhancement of anticipatory locomotion in response to FR, whereas the GHR(-/-) mice exhibited a blunted increase in anticipatory locomotion, relative to WT mice. The present study confirms and extends the observation of Blum (Blum et al., 2009) and further suggests that the GHS-R is required for the expression of anticipatory locomotion in response to FR. A similar reduction in anticipatory locomotion was noted in mice for which the pre-proghrelin gene was ablated (Szentirmai et al., 2009). Conversely, LeSauter (LeSauter et al., 2009) reported that systemic injection of ghrelin was sufficient to elevate anticipatory activity in food satiated mice. The present results support the view that ghrelin pathways involving the ghrelin receptor are required for anticipatory locomotion to FR and that ablation of ghrelin attenuates anticipatory locomotion. This pattern would seem to suggest that factors other than ghrelin can modulate the GHS-R to increase anticipatory locomotion.
ACKNOWLEDGEMENTS
Portions of this manuscript were presented at the 2009 meeting of the Society for Neuroscience. This project was supported by NIDA 1R01DA013188B to PJW and R01 AG019230 to RGS.
Footnotes
AUTHORS CONTRIBUTION PSC, PJW, and RGS were responsible for the study concept and design. RAZ contributed to the breeding and genotyping of the mice. PSC, SB, JT and NH contributed to the acquisition of animal data. PSC and PJW assisted with data analysis and data interpretation. PSC and PJW drafted the manuscript. All authors critically reviewed content and approved the final version for publication.
Contributor Information
P. Shane Clifford, Behavioral Neuroscience Program, Department of Psychology, Texas A&M University, College Station, TX 77843-4235..
Sam Buckman, Behavioral Neuroscience Program, Department of Psychology, Texas A&M University, College Station, TX 77843-4235..
Jeff Thompson, Behavioral Neuroscience Program, Department of Psychology, Texas A&M University, College Station, TX 77843-4235..
Nigel Hart, Behavioral Neuroscience Program, Department of Psychology, Texas A&M University, College Station, TX 77843-4235..
Paul J. Wellman, Behavioral Neuroscience Program, Department of Psychology, Texas A&M University, College Station, TX 77843-4235.
REFERENCES
- Abizaid A. Ghrelin and dopamine: new insights on the peripheral regulation of appetite. J Neuroendocrinol. 2009;21:787–793. doi: 10.1111/j.1365-2826.2009.01896.x. [DOI] [PubMed] [Google Scholar]
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen GC, Berman Y, Carr KD. Curve-shift analysis of self-stimulation in food-restricted rats: relationship between daily meal, plasma corticosterone and reward sensitization. Brain Res. 1995;695:186–194. doi: 10.1016/0006-8993(95)00764-h. [DOI] [PubMed] [Google Scholar]
- Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29:2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vaca S Cabeza, Holiman S, Carr KD. A search for the metabolic signal that sensitizes lateral hypothalamic self-stimulation in food-restricted rats. Physiol Behav. 1998;64:251–260. doi: 10.1016/s0031-9384(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Baez LA. Dissociation of arousal and regulatory behaviors following lesions of the lateral hypothalamus. J Comp Physiol Psychol. 1974;87:142–149. doi: 10.1037/h0036860. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology. 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept. 2007;140:148–152. doi: 10.1016/j.regpep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J Pharmacol Sci. 2006;100:398–410. doi: 10.1254/jphs.crj06002x. [DOI] [PubMed] [Google Scholar]
- Jamieson J. Analysis of covariance (ANCOVA) with difference scores. Int J Psychophysiol. 2004;52:277–283. doi: 10.1016/j.ijpsycho.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design. Wadsworth; Belmont, CA: 1982. [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorcroft WH, Lytle LD, Campbell BA. Ontogeny of starvation-induced behavioral arousal in the rat. J Comp Physiol Psychol. 1971;75:59–67. doi: 10.1037/h0030670. [DOI] [PubMed] [Google Scholar]
- Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int. 2009;54:89–94. doi: 10.1016/j.neuint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Zoloty SA, Willis R, Ticarich CD, Rhoads K, Nagy RP, Mitchell SG, Laforest AR, Jenks JA, Harkabus LJ, et al. Digiscan activity: automated measurement of thigmotactic and stereotypic behavior in rats. Pharmacol Biochem Behav. 1987;27:569–572. doi: 10.1016/0091-3057(87)90369-8. [DOI] [PubMed] [Google Scholar]
- Shimbara T, Mondal MS, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, Date Y, Nakazato M. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett. 2004;369:75–79. doi: 10.1016/j.neulet.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai E, Kapas L, Sun Y, Smith RG, Krueger JM. Restricted Feeding-Induced Sleep, Activity and Body Temperature Changes in Normal and Preproghrelin Deficient Mice. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol. 2006;68:223–251. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davis KW, Nation JR. Augmentation of cocaine hyperactivity in rats by systemic ghrelin. Regul Pept. 2005;125:151–154. doi: 10.1016/j.regpep.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Hollas CN, Elliott AE. Systemic ghrelin sensitizes cocaine-induced hyperlocomotion in rats. Regul Pept. 2008;146:33–37. doi: 10.1016/j.regpep.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]