Abstract
Effects of early-life and middle-life conditions on exceptional longevity are explored in this study using two matched case-control studies. The first study compares 198 validated centenarians born in the United States in 1890-1893 to their shorter-lived siblings. Family histories of centenarians were reconstructed and exceptional longevity validated using early U.S. censuses, Social Security Administration Death Master File, state death indexes, online genealogies and other supplementary data resources. Siblings born to young mothers (<25 years) had significantly higher chances to live to 100 compared to siblings born to older mothers (odds ratio = 2.03, 95% CI = 1.33 - 3.11, P = 0.001) while paternal age and birth order were not associated with exceptional longevity. The second study explores whether people living to 100 and beyond are any different in physical characteristics at young age from their shorter-lived peers. A random representative sample of 240 men born in 1887 and survived to age 100 was selected from the US Social Security Administration database and linked to the US WWI civil draft registration cards collected in 1917 when these men were 30 years old. These validated centenarians were then compared to randomly selected controls matched by calendar year of birth, race and place of draft registration in 1917. It was found that ‘stout’ body build (being in the heaviest 15% of population) was negatively associated with survival to age 100 years. Farmer occupation and large number of children (4+) at age 30 increased the chances of exceptional longevity. Detailed description of dataset development, data cleaning procedure and validation of exceptional longevity is provided for both studies. These results demonstrate that matched case-control design is a useful approach in exploring effects of early-life conditions and middle-life characteristics on exceptional longevity.
Keywords: human longevity, maternal age, body build, farming background, fertility
Introduction
Studies of centenarians (persons living to age 100 and over) could be useful in identifying factors leading to long life and avoidance of fatal diseases. Even if some childhood or middle-life factors have a moderate protective effect on risk of death, this longevity advantage would result in accumulation of persons who experienced effects of these factors among long-lived individuals. Thus, study of centenarians may be a sensitive way to find genetic, familial, environmental, and life-course factors associated with lower mortality and better survival.
Most studies of centenarians in the United States are focused on either genetic (Barzilai and Shuldiner, 2001; Barzilai et al., 2006; Hadley et al., 2000; Puca et al., 2001) or psychological (Adkins et al., 1996; Hagberg et al., 2001; Jang et al., 2004; Martin et al., 2006) aspects of survival to advanced ages. On the other hand, several theoretical concepts suggest that early-life events and conditions may have significant long-lasting effect on survival to advanced ages (Barker, 1992; Fogel and Costa, 1997; Gavrilov and Gavrilova, 1991; Kuh and Ben-Shlomo, 1997). These ideas are supported by studies suggesting significant effects of early-life conditions on late-life mortality (Alter and Oris, 2005; Ben-Shlomo and Kuh, 2002; Bengtsson and Mineau, 2009; Costa, 2003; Elo and Preston, 1992; Gavrilov and Gavrilova, 2003; Hayward and Gorman, 2004; Smith et al., 2009a). The existence of correlations between early growth patterns and subsequent fitness is now established not only for human beings but for some other mammalian species as well (Lummaa and Clutton-Brock, 2002). Centenarian studies may be useful in finding factors and conditions operating early in life and affecting survival to advanced ages.
At the same time, studies of centenarians face significant difficulties in collecting reliable data, finding appropriate design, and methodology. Survival to age 100 is a rare event (only two men and 14 women out of 1000 from 1900 birth cohort survived to age 100), and therefore traditional methods of population-based sampling are difficult and not feasible for obtaining large samples of centenarians. The case-control design proved to be the most appropriate and cost-effective approach for studies of rare conditions (Breslow & Day 1980; Woodward 2005) and hence is extremely useful for centenarian studies. Breslow and Day (1980) suggested that the classic case-control design can be expanded in a variety of ways. One such expansion is a design suggested by Samuel H. Preston (Preston et al., 1998). According to this design a survival to advanced ages (rather than disease or death) is considered to be a case and relative survival probabilities are used instead of odds ratios.
Studies of effects of early-life conditions on exceptional longevity face additional difficulties from possible confounding due to between-family variation in childhood socio-economic conditions and parental genetic background. It was suggested that one possible solution to these challenges is to compare associations within sibships taking into account that socioeconomic and genetic background is similar for siblings from the same family (Gavrilov and Gavrilova, 2001; Smith et al., 2009b).
In this paper we present two examples of using matched case-control design to analyze effects of early-life and middle-life characteristics on survival to age 100. In the first study we make an attempt to find out why centenarians are different from their shorter-lived siblings. In the second study we compare centenarian characteristics recorded at young age to similar characteristics of their peers who did not manage to survive to this advanced age. For both studies we describe new historical data resources, which proved to be useful for centenarian studies of early-life effects as well as potential pitfalls and problems of handling these data.
Study 1: Why Centenarians are Different from their Shorter-lived Siblings
The within-family design may be useful for study of parental age at reproduction (and other characteristics that vary within the same family) on exceptional longevity. While detrimental effects of late reproduction on adverse reproductive outcomes and genetic diseases has been well documented (Bottini et al., 2001; Gavrilov and Gavrilova, 1997; Pellestor et al., 2005), less is known about the long-term effects of delayed parenting on the health and longevity of adult offspring. There is empirical evidence that the quality of female eggs in human beings rapidly declines with age (Bickel, 2005; Pellestor et al., 2005) and this deterioration starts rather early-before age 30 (Heffner, 2004). However, our earlier studies have not detected an association of maternal age with offspring mortality in historical populations of European aristocracy (Gavrilov and Gavrilova, 1997; Gavrilov and Gavrilova, 2000; Gavrilov and Gavrilova, 2001). Paternal age at reproduction was suggested to be the main factor determining human spontaneous mutation rate (Crow, 1997; Crow, 2000). A number of studies demonstrated that advanced paternal age may be detrimental for survival of adult offspring (Gavrilov and Gavrilova, 1997; Gavrilov and Gavrilova, 2000; Smith et al., 2009a) although other studies failed to show this effect (Hubbard et al., 2009; Robine et al., 2003).
We believe that the within-family design is a useful way to study parental age effects on longevity of the offspring. This paper presents the results of applying a within-family design to the study of parental age and birth order effects on exceptional longevity. For this purpose we collected family histories of 671 putative centenarians born in 1890-1899 in the United States and conducted thorough validation of their birth and death dates. Then for a subsample of 198 centenarians born in 1890-1893 an additional search for detailed information about all their siblings has been conducted. This procedure allowed us to create a sample of centenarians with information on lifespans of their siblings.
Design of the Study
This study explored the effects of early-life factors (birth order, paternal age, maternal age) on the likelihood of survival to advanced ages. Centenarians (cases) were compared to their “normal” shorter-lived siblings (controls) using a within-family approach.
The study applied a case-sibling design (see Figure 1), a variant of a matched case-control design in which siblings of cases (long-lived individuals) are used as controls (Woodward, 2005). This approach allows investigators to study within-family differences, not being confounded by between-family variation. Long-lived persons born in 1890-1893 were used as cases. Siblings born no more than 10 years apart from the cases were used as controls to maximize similarity in their family conditions and minimize the risk of lifespan being unknown for later born siblings, who were still alive by the time of data collection and hence could survive to age 100 in the future. Controls in our sample were born between 1881 and 1902 and only three persons born after 1899 had no information on their death dates and could potentially be alive and survive to age 100 by the time of our study. Taking into account that survival to age 108 is extremely rare it is safe to assume that these persons were dead by the time of our study and did not become centenarians. So we believe that centenarian status was correctly established for almost all siblings in our study.
Figure 1.
Description of Case-Sibling Design in this study
The main approach used in this study is based on comparison of children within rather than across families. Within a family, children are born to parents at different ages and this variation may be used to estimate the net effect of parental age more conclusively (Kalmijn and Kraaykamp, 2005).
Data Collection and Data Quality Control
Family histories (genealogies) proved to be a useful source of information for studies in historical demography (Adams and Kasakoff, 1984; Adams and Kasakoff, 1991; Anderton et al., 1984; Anderton et al., 1987; Bean et al., 1992; Kasakoff and Adams, 2000) and biodemography (Gavrilov and Gavrilova, 2001; Gavrilov et al., 2002; Kerber et al., 2001a). In this study data on long-lived individuals (survived to age 100 and beyond) were collected from computerized family histories available online (Rootsweb.com). Specifically, family data for 671 alleged centenarians born from 1890 through 1899 in the United States were extracted from publicly available computerized family histories of about 75 million individuals identified in the previous study (Gavrilova and Gavrilov, 1999).
Data quality control is an important part of all centenarian studies and in our case it included (1) preliminary quality control of computerized family histories (data consistency checks); (2) verification of centenarian's death date; (3) verification of birth dates for centenarians and their siblings (controls). All records (for centenarians and controls) were subjected to verification and quality control using several independent data sources. This study is primarily concerned with the possibility of incorrect dates reported in family histories. Previous studies demonstrated that age misreporting and age exaggeration in particular are more common among long-lived individuals (Elo et al., 1996; Hill et al., 2000; Jeune and Vaupel, 1999; Rosenwaike and Hill, 1996; Rosenwaike and Stone, 2003; Rosenwaike et al., 1998; Shrestha and Rosenwaike, 1996; Young et al., 2010). Therefore, the primary focus in our study was on the age verification for long-lived individuals. We followed the approach of age verification and data linkage developed by a team of demographers at the University of Pennsylvania (Elo et al., 1996; Hill et al., 2000; Preston et al., 1996; Rosenwaike and Hill, 1996; Rosenwaike and Stone, 2003; Rosenwaike et al., 1998). This approach involves death date verification using Social Security Administration Death Master File and birth date verification using early U.S. censuses. In order to validate the age of the centenarians, these records were linked to the Social Security Administration Death Master File records for death date validation and then to the records of the U.S. censuses for years 1900, 1910, and 1920 for birth date validation.
Verification of death dates was accomplished through a linkage of family history data to the Social Security Administration Death Master File (DMF). This is a publicly available data source (available at the Rootsweb.com) that allows a search for individuals using various criteria: birth date, death date, first and last names, Social Security number and place of last residence. This resource covers deaths that occurred in the period 1937-2010 (Faig, 2001) and captures about 95% of deaths recorded by the National Death Index (Sesso et al., 2000). Many researchers suggest that the quality of Social Security Administration data for older persons is superior to vital statistics records because of stricter evidentiary requirements in application for Social Security Numbers or Medicare, whereas age reporting in death certificates is made by proxy informant (Faig, 2001; Kestenbaum, 1992; Kestenbaum and Ferguson, 2001; Rosenwaike and Stone, 2003). Definite match was established when information on first and last names (spouse last name for women), day, month and year of birth matched in DMF and family history (Sesso et al., 2000). In the case of disagreement in day, month or year of birth, the validity of match was verified on the basis of additional agreement between place of the last residence and place of death.
The lack of a match with the DMF could occur for a number of reasons: a misprint in genealogy, missing Social Security record (particularly if the person did not use Medicare benefits), difficulty in matching a person with a common name when the dates are not identical, etc. Also, DMF covers about 90 percent of all deaths for which death certificates are issued (see Faig, 2001) and about 92-96 percent of deaths for persons older than 65 years (Hill, Rosenwaike, 2001). Further work with non-matched cases using additional data sources (obituaries, state collections of death certificates) revealed that about half of non-matched cases are related to misprints in genealogies, and about 20 percent of non-matched cases have correct death dates (as confirmed by linkage to the state death indexes) although are not recorded in the DMF.
It should be noted that the linkage success rate to the DMF was substantially higher for persons born after 1889 - at 82 percent. This result is consistent with previous reports that quality and coverage of the DMF database was lower for persons born before 1890 (Faig, 2001). The 534 records for persons with confirmed centenarian status born after 1889 and matched to the DMF were used further in verification of centenarian birth dates through linkage to early censuses.
Verification of birth dates was accomplished through a linkage to the 1900 U.S. census data recorded when the person was a child (when age exaggeration is less common compared to claims of exceptional longevity made at old age). The preference was given to the 1900 census because it is more complete and detailed in regard to birth date verification (contains month and year of birth) compared to the 1910 and 1920 censuses. If person could not be found in 1900 census, then he/she was searched in 1910 census. In our study, the linkage of centenarian records to the early census data was facilitated by online availability of the entire indexed U.S. 1900, 1910 and 1920 censuses—a service provided by Genealogy.com and Ancestry.com. In this study we conducted a linkage of 534 centenarian records (for centenarians found in the DMF with confirmed centenarian status and born after 1889) to the early U.S. censuses. If individuals were not found in the 1900 Census, then attempts were made to locate them in the 1910, 1920 and 1930 censuses. In this study we obtained a good linkage success rate (91%) because of availability of powerful online indexes provided by the Ancestry.com service and supplemental information in family histories. These indexes allowed us to conduct search on the following variables: first, last names (including Soundex), state, county, township, birthplace, birth year (estimated from census), immigration year, relation to head-of-household. A definite match was established when information on parents and all children (name, order, age, place of birth) agreed in both census and family history (one-year disagreement in ages for some children was allowed). Possible matches agreed on the above mentioned components but allowed disagreement in one or two variables. Children residing outside the parental household (boarding school, hospital, etc.) were identified on the basis of their name, month, year, place of birth and proximity to parental household. For possible matches additional attempts were made to find information in supplementary sources (using state birth, death and marriage indexes, state censuses) and verify their validity. Information on birth dates, birth places and names of siblings available in genealogies produced unambiguous matches in overwhelming majority of cases. The success of large-scale linkage to early censuses accomplished in this study helped us to alleviate some initial concerns related to the possibilities of naming of children for deceased sibling, the lack of distinction between half- and full-sibs in some census records, the practice of placing children in other households due to family circumstances, and the difficulties of both name similarities and the frequent name changes associated with “Americanization” during the early 1900's.
The data consistency checks (an initial step of centenarian age validation) revealed a surprisingly small number of obvious data inconsistencies in computerized genealogies. In one case, the alleged centenarian had parents with incorrect birth dates (parents born later than the person himself). This case was dropped from the study. In another case, the centenarian's father was rather old (62 years) when the centenarian was born. This is not an impossible situation, so this case was left for further validation (this case was later confirmed through the DMF but not found in early censuses and therefore not included in the final analyses). All other records did not reveal obvious inconsistencies in event dates, so 990 records were left for further verification.
Of the 671 centenarians found in family histories, 551 (82 percent) were successfully linked to the Social Security Administration Death Master File (DMF). For 534 individuals (80%), their centenarian status was confirmed, i.e. they lived more than 100 years according to the DMF death date. For 17 persons death year reported in DMF was 1-2 years lower compared to genealogical record and they lived less than 100 years, so they were not included in the subsequent linkage to early censuses. Of the 534 records with validated death dates, 485 (91 percent) were successfully linked to early U.S. censuses. The success rate of linking records found in the DMF to early U.S. censuses was 91%, which was significantly higher than in the previous studies by other authors - 39-75% (Guest, 1987; Rosenwaike and Stone, 2003; Rosenwaike et al., 1998). The overall output of this age validation study was also acceptable, at 72%. Table 1 shows the results of the linkage to DMF and early censuses.
Table 1.
Results of linking genealogical records of centenarians first to the Social Security Administration Death Master File (DMF) and then to early U.S. censuses
| Steps of data verification | Number of records for centenarians born in 1890-99 |
||
|---|---|---|---|
| Males | Females | Both sexes | |
| Initial number of records | 160 (100%) | 511 (100%) | 671 (100%) |
| Found in DMF | 130 (81%) | 421 (82%) | 551 (82%) |
| Found in early censuses | 115 (72%) | 370 (72%) | 485 (72%) |
Because linked individuals may differ in a variety of ways from the total sample, it is important to test the data for non-match bias. Table 2 compares estimates for the linked sample with the non-linked group for selected characteristics available from the online family history records. To estimate the degree of non-match bias, two multivariate logit models predicting the linkage to (1) DMF and to (2) early censuses were conducted (Rosenwaike et al., 1998). There was no significant difference between individuals who were successfully linked to DMF or early censuses and non-linked ones regarding their birth year, birth order, sibship size, age at death, parental lifespan and gender. However the individuals linked to DMF were more likely to be ever married. Although marital status was not specifically analyzed in the studies of linkage to the SSA DMF (Rosenwaike et al., 1998), it was noted that non-married persons had significantly less accurate information recorded in death certificates. Death reporting to the SSA is likely to be worse for single individuals rather than individuals having many relatives. Study of linkage to early censuses revealed that being born in the Southern states is associated with significantly decreased likelihood of being linked. This phenomenon was observed by other authors (Rosenwaike and Stone, 2003; Rosenwaike et al., 1998) and most likely is caused by lower rates of 1900 Census enumeration for states located in the South (Rosenwaike and Stone, 2003).
Table 2.
Mean values and proportions (standard deviations) across matched and non-matched subgroups.
| Characteristic | Linkage to DMF | Linkage to 1900 Census | ||
|---|---|---|---|---|
| Matched (N=551) | Non-matched (N=120) | Matched (N=485) | Non-matched (N=49) | |
| Proportion of women | 0.764 (0.018) | 0.758 (0.039) | 0.763 (0.019) | 0.837 (0.053) |
| Age at death | 101.7 (1.2) | 99.6† (13.8) | 101.3 (1.2) | 101.0 (0.8) |
| Birth order | 3.6 (2.7) | 4.3 (3.2) | 3.6 (2.7) | 3.6 (2.8) |
| Sibship size | 7.1 (3.5) | 7.6 (3.5) | 7.1 (3.4) | 6.1 (3.8) |
| Proportion born in the Southern state | 0.347 (0.020) | 0.425 (0.045) | 0.328 (0.021)* | 0.489 (0.072)* |
| Proportion ever married | 0.907 (0.012)*** | 0.700 (0.042)*** | 0.907 (0.013) | 0.918 (0.040) |
| Number of children | 2.5 (2.8) | 2.2 (3.0) | 2.6 (2.9) | 2.3 (2.5) |
| Paternal lifespan | 73.9 (14.2) | 73.4 (16.4) | 74.2 (14.0) | 71.9 (16.8) |
| Maternal lifespan | 73.7 (17.3) | 75.2 (19.3) | 73.9 (17.2) | 70.4 (20.0) |
statistically significant difference between matched and non-matched sample, p<0.05
statistically significant difference between matched and non-matched sample, p<0.001
initial selection of centenarians was based on the difference between death and birth years, so that some centenarians did not survive a few months to their 100-year birthday.
Our study demonstrated that non-linkage bias is not observed for the majority of the analyzed characteristics. Better representation of married individuals in the SSA DMF and persons born in non-Southern states in the census records is most likely caused by better coverage of these individuals in the original data sources (DMF or censuses) rather than by the properties of the linkage procedure. Compared to other linkage studies, the percentages of non-linked records are rather low and they are lower than non-response rates in many population surveys (Groves, 2006).
At the next step, 198 records of centenarians born in 1890-1893 were extracted from our sample of validated centenarians born in 1890-1899 for additional reconstruction of sibship structure and lifespan of siblings in order to conduct within-family analyses. To this aim, complete family histories of selected 198 centenarians were reconstructed using the US Censuses, the US Social Security Administration data, genealogical records and other supplementary data resources. All birth dates of centenarian siblings were reconstructed using information available in computerized genealogies and early censuses. The procedure of death date verification using DMF is not feasible for validating death dates of shorter-lived siblings (used as controls), because data completeness of DMF is not very high for deaths occurred before the 1970s (systematic reporting of death dates in DMF started in the late 1970s – see Faig, 2001). State death indexes, cemetery records and obituaries cover longer periods of time. Taking into account that exact ages of death for controls are not particularly important for comparison (it is sufficient to assume that shorter-lived siblings did not survive to age 100) we relied on death date information recorded in family histories for siblings not found in external sources. This approach was used in the Utah Population Database study for individuals died before 1932 (Kerber et al., 2001b). As a result of family history reconstruction using computerized genealogies and early censuses data, each case (centenarian) had six control siblings on average when all reported siblings (N=1141) are taken into account (including those who died in childhood). The sibship size (seven siblings on average) in our centenarian families is higher than the average number of children in American families reported by the 1900 U.S. Census – 5.12 ± 0.01 (data obtained from the 5% sample of the U.S. 1900 census provided by the Integrated Public Use Microdata Series or IPUMS (Ruggles et al., 2010)). Somewhat larger sibship size in centenarian families compared to the general population can be explained by the fact that genealogies are more likely to be compiled for larger families and that longer-lived individuals in the United States were born more often in rural areas with higher fertility (Gavrilova and Gavrilov, 2007; Preston et al., 1998). As a result of family reconstitution efforts, death dates were identified for 930 siblings using DMF, state death indexes and online genealogies. In those cases where death dates for siblings were not available, their survival to adulthood was confirmed using information that they had spouse(s). There were 97 such additional cases. Birth order of all putative first-born centenarians was verified using available data from early censuses. To control for historical changes in living conditions, only data for siblings whose birth dates did not differ by more than ten years from centenarian birth date were used for further statistical analyses (763 cases).
Variables and Statistical Methods
In this study we analyzed family variables that vary within a family and are available in computerized genealogies: maternal and paternal age at reproduction and birth order. Maternal and paternal ages were available for all siblings because birth years of parents were available in both computerized genealogies and early censuses. Information about birth order was taken from genealogies and verified using early censuses (census information per se could not be used for birth order identification because of possible incompleteness of sibship in census records).
The statistical analyses of within-family effects were performed using a conditional multiple logistic regression model (fixed-effect model) to investigate the relationship between an outcome of being a case (long-lived person) and a set of prognostic factors (Breslow and Day, 1993; Hosmer and Lemeshow, 2001). The fixed-effects logit model can be written as (StataCorp, 2009):
and i = 1,2, ... n denotes the families (independent units) and t=1,2,... Ti denotes the children for the ith family; xit denotes vector of within-family covariates including maternal age and birth order. The likelihood to survive to advanced ages (to be in the long-lived group) is used as a dependent variable. Analyses were conducted using Stata statistical software, release 11 (StataCorp, 2009). The following variables were included in the model: birth order, paternal age, maternal age and sex (male or female).
Results and Discussion
We found that the odds to become a centenarian are 1.8 times higher for the first-born children compared to their later-born siblings (brothers and sisters) from exactly the same family (see Table 3). Next question explored was about the role of child mortality, which was very high a century ago, when the studied centenarians were born. So if the first-born children were more likely to survive to adult age then simply because of this selective child survival, the centenarians might become more prevalent among the first-born. To test this hypothesis we re-analyzed the data including only those siblings who survived to adulthood (were married or lived 20 years or more). It was found that even for adult persons the odds to live to 100 are almost twice higher for the fist-born persons (Table 3).
Table 3.
Odds ratios (95% confidence intervals) to become a centenarian as predicted by conditional logistic regression (fixed effects). Effects of parental age and birth order.
| Model† | N | First-born status | Born to young father (<25) | Born to young mother (<25) | Female sex |
|---|---|---|---|---|---|
| Model 1. All siblings | 950 | 1.77** (1.18-2.66) | 2.47*** (1.74-3.52) | ||
| Model 2. Siblings survived to adulthood†† | 797 | 1.95** (1.26-3.01) | 2.18*** (1.52-3.14) | ||
| Model 3: Siblings survived to adulthood | 797 | 1.76 (0.93-3.33) | 2.15*** (1.50-3.09) | ||
| Model 4: Siblings survived to adulthood | 797 | 1.88* (1.13-3.14) | 1.10 (0.52-2.32) | 2.18*** (1.52-3.13) | |
| Model 5: Siblings survived to adulthood | 797 | 1.52 (0.93-2.48) | 1.67* (1.01-2.76) | 2.16*** (1.50-3.11) | |
| Model 6: Siblings survived to adulthood | 797 | 2.02** (1.30-3.14) | 2.13*** (1.49-3.07) | ||
| Model 7: Siblings survived to adulthood; both parents survived to age 50 | 636 | 2.12** (1.27-3.54) | 2.23*** (1.49-3.36) | ||
| Model 8: Siblings survived to age 75 | 548 | 1.83* (1.12-2.98) | 2.15*** (1.43-3.21) |
p<0.05
p<0.01.
p<0.001
blank entry in the table means that variable was not included in the model.
adulthood is defined as survival to age 20 or being married when death date could not be identified.
Then we explored the role of paternal and maternal ages at reproduction as a potential explanation for the birth order effect using data for siblings survived to adulthood. We found that young (below 25) paternal age had no statistically significant association with odds of survival to age 100 (see Table 3, model 3). It turned out that the young father's age was far less important than the fist-born status itself in predicting the chances of exceptional longevity (see Table 3, model 4). Old paternal age (over 50) had no effect on survival to age 100 as well (data not shown). Thus, we did not find a significant association between paternal age and longevity in this within-family study.
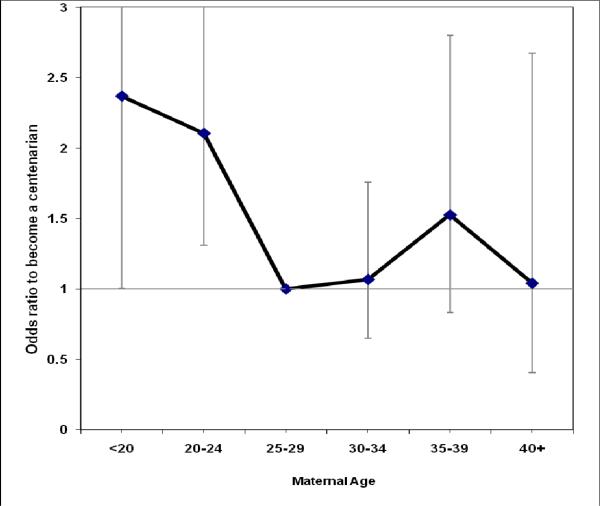
Finally, we included maternal age into analysis and it turned out that the young maternal age at childbirth was the most important predictor of exceptional survival, while the effect of the birth order itself has become statistically insignificant. These findings indicate that the beneficial effect of being first-born is driven mostly by the young maternal age at person's birth (being born to mother younger than 25 years) (Table 3, Model 5). Moreover, even at age 75 it is still important to be born to young mother in order to survive to 100 years, because the odds of exceptional survival are 1.8 times higher than for later-born siblings (Table 3, Model 8). Taking into account that birth order and parental age at reproduction may be a function of parental survival we repeated analyses for the offspring of parents who survived to age 50 and hence realized their reproductive potential. The results obtained for this sample (Table 3, model 7) are not different from the results obtained for the total sample of siblings survived to adult age (Table 3, model 6). It is interesting that the survival benefits of being born to young mother are observed only when the mother is younger than 25 years (Figure 2).
Figure 2.
Odds ratio to survive to 100 years as a function of maternal age at person's birth. Data for persons born to mother at 25-29 years are treated as a reference category. Based on logistic regression model, which includes five categories of maternal age and gender as covariates.
Thus, multivariate within-family analysis of the birth order effects on human longevity revealed that it is young age of mother that is responsible for apparent beneficial effects of first-born status on longevity. Within-family approach has great advantages over other methods, because it is free of confounding caused by between-family differences. However, it remains to be seen whether the observed effect could be reproduced in further studies.
The finding of beneficial effect of maternal age on offspring survival to age 100 in humans may have biological explanation. There is an empirical evidence that the quality of female eggs in human beings rapidly declines with age (Bickel, 2005; Pellestor et al., 2005) and this deterioration starts rather early—before age 30 (Heffner, 2004). Maternal age influences the biology of the mother-fetus relationship, with a negative effect on fetal development and predisposition to severe diseases such as type I diabetes (Gloria-Bottini et al., 2005).
Experiments on laboratory mice found that the offspring born to younger mothers live longer (Tarin et al., 2005). This study also demonstrated that the largest effect is observed at later life. Animal studies have also found that hormonal profiles in pregnant mice are different depending on maternal age (Wang and vom Saal, 2000). This may explain why adult offspring of adolescent and middle-aged mothers have lower body weight and delayed puberty and male offspring have smaller reproductive organs than those born to young adult mothers (Wang and vom Saal, 2000). Female offspring produce progeny whose birth weight depended on the age at pregnancy of their grandmothers, demonstrating a transgenerational effect of maternal age (Wang and vom Saal, 2000). Delayed motherhood in mice has also been demonstrated to have negative effects on behavioral traits of young adult offspring (Tarin et al., 2003). Data on the long-term effects of maternal age in human beings are scarce. One study showed that the lifespan of children decreased with increasing maternal age (Kemkes-Grottenthaler, 2004). Our earlier studies have not detected an association of maternal age with offspring mortality in historical populations of European aristocracy (Gavrilov and Gavrilova, 1997; Gavrilov and Gavrilova, 2000), but we believe that this might be due to some limitations in the tools to analyze the data (no control for within-family variation). These earlier between-family studies also found that the old age of father is related to lower lifespan of adult daughters (Gavrilov and Gavrilova, 2000; Gavrilov et al., 1997). Smith and colleagues also found detrimental effect of very old paternal age (70 and more) on mortality of daughters using between-family analysis of data from the Utah Population Database (Smith et al., 2009a). The authors admit that it is difficult at this moment to discriminate whether this paternal age effect is due to higher level of germ-line mutations in the sperm of old fathers or whether it is due to problems of having been reared by old parents (Smith et al., 2009a). Study by Smith and colleagues also showed very moderate negative effect of old maternal age (35+) on survival of sons (Smith et al., 2009a).
The fact that lifespan of offspring depends on mother's age at their birth even in laboratory animals indicates that some fundamental biological mechanisms may be involved. Such possible epigenetic mechanisms as changes in genomic imprinting in oocytes of aging females may be a plausible hypothesis (Comings and Macmurray, 2001; Comings and MacMurray, 2006). Another plausible biological hypothesis is the “telomere theory of reproductive senescence” in females (Keefe et al., 2005), which posits that eggs ovulating from older females have shorter telomeres because of late exit from the oogonial “production line” (Polani and Crolla, 1991) during fetal life, with incomplete restoration by telomerase (Keefe et al., 2005). Telomeres are DNA repeats which cap and protect chromosome ends, so that longer telomeres in eggs of younger females may be beneficial for offspring lifespan. However, in human beings some additional sociobehavioral mechanisms may be also involved, on top of more general biological mechanisms. For example, it may be suggested that maternal-age effects in human beings are mediated through the length of ‘mothering’ (duration of maternal care and supervision). Children born to young mothers (below 25) are exposed to maternal care for longer time, on average. It was shown that maternal support is not distributed evenly within a sibship and that daughters often get a preference (Suitor et al., 2006). The mothering hypothesis also implies that there should be a close geographical proximity between the mother and her grown child to allow a long-term maternal care and influence to manifest itself through personal interactions. For example, Smith and colleagues found that “mothers who are not physically proximate to their adult offspring elevate the risks of mortality of the offspring” (Smith et al., 2005). Another possible sociobehavioral explanation of the observed maternal age effects is that in human beings they are mediated through the size of social support group (numbers of younger siblings) that could provide care and support in later life. More research is needed to disentangle the role of biological and social mechanisms in maternal age effects on human longevity.
Study 2: Midlife Physical Characteristics and Longevity
Incorporation of physical characteristics into demographic analysis of mortality widens a scope of explanatory variables in biodemographic research on health outcomes (Crimmins and Seeman, 2000). For example, the early studies by Barker and colleagues linked low birth weight to increased mortality from cardiovascular diseases later in life (Barker, 1992; Barker et al., 1993). Later research demonstrated that the relationships between birth weight, adult age adiposity and late-life diseases are complex and both poor growth during fetal life and infancy on one hand, and rapid catch-up growth and childhood weight gain on the other hand, contribute to subsequent disease risk (Wells, 2007). Unfortunately detailed birth weight information is not yet available for historical populations and centenarian cohorts. At the same time, an individual's height at young adult age seems to be a good indicator of person's nutritional and infectious disease history at least in historical data (Alter, 2004; Alter et al., 2004; Elo and Preston, 1992). Most studies, starting with Waaler's pioneer work, found a negative relationship between body height and mortality later in life (Elo and Preston, 1992; Waaler, 1984). A study of Union Army veterans found that the relationship between height and subsequent mortality was negative (Costa, 1993b; Costa and Lahey, 2005; Fogel and Costa, 1997), findings similar to a study of modern Norwegian males (Costa, 1993a; Costa, 1993b; Costa and Lahey, 2005; Fogel and Costa, 1997). Infectious diseases (and diarrhoeal diseases in particular) can result in growth retardation leading to shorter adult height. For example, conscripts from high-mortality districts of antebellum New York were shorter than those from healthier districts (Haines et al., 2003).
Adult body height is affected by both environmental (early-life nutrition and exposure to infections) and genetic factors. It was found that familial resemblance in height was suppressed in the past possibly because of early environmental effects (Lauderdale and Rathouz, 1999). It was suggested that population of the United States at the end of the 19th century had relatively good nutritional status but very high burden of infections (Preston and Haines, 1991). Thus, we may hypothesize that low height of males born in the end of the 19th century may be related to the infectious diseases during childhood. If the hypothesis of childhood infections as a possible cause of late-life chronic diseases is correct (Finch and Crimmins, 2004), we may expect that centenarians at young adult ages would be taller on the average than their peers who did not survive to advanced ages. According to this hypothesis “chronic inflammatory mechanisms drive much of the influence of early-life infections on later morbidity and mortality” (Finch and Crimmins 2004), and “height is also linked to infections and the inflammatory response [in childhood] ”, because “if infections occur during development, substantial energy is reallocated at the expense of growth, as required by the body for immune defense reactions and for repair” (Crimmins and Finch, 2006). Thus, one may expect that centenarians should be taller at their young adult ages, because, according to this hypothesis, they should have less childhood infections, which are detrimental both for body growth and subsequent longevity.
An alternative view was suggested by Samaras who believes that promoting rapid growth and maximum height attainment is not a desirable goal (Samaras, 2009; Samaras and Storms, 2002). His findings suggest that men with lower height and weight live on average longer than their taller and heavier peers (Samaras and Storms, 1992; Samaras et al., 2002). Animal studies of different strains and breeds within biological species of dogs, rats, and mice also showed that smaller animals live on average longer within a given species (Li et al., 1996; Michell, 1999; Miller et al., 2000; Patronek et al., 1997; Samaras et al., 2003). Biologists believe that rapid growth may be harmful and that somewhat delayed maturation would be beneficial for longevity and health (Rollo, 2002).
It is not clear what the body size of centenarians was during their young ages. Historical studies suggest that centenarians should be much taller than average due to better nutrition and avoidance of diseases early in life. On the other hand, biological data predict that centenarians should be shorter than average. There are reports that Japanese centenarians are shorter than persons of average lifespan (Chan et al., 1999; Chan et al., 1997a; Chan et al., 1997b), although these studies measured centenarians at old age, when their height had already decreased. Studies of centenarian body size measured at young adult age would help to resolve the existing controversy regarding the body size and longevity.
In this study we compare male centenarians when they were 30 years old to their shorter-lived peers using data from the US WWI Civilian Draft Registration Cards available now online through the Ancestry.com service. The uniqueness of this data source is that it contains information on physical characteristics of men subjected to registration including data on body height and body build. This allowed us to analyze the effects of physical characteristics at young age on longevity.
Study Design and Data
The study applied a matched case-control design where shorter-lived males matched with centenarian males by birth year, race and county of draft registration were used as controls (see Figure 3). This approach allowed us to eliminate effects of birth cohort, race and place of draft registration on survival. Using controls from the same geographical area (county) allowed us to mitigate a possible geographically-related subjectivity in height and build estimation.
Figure 3.
Matched case-control design of the study.
The development of the study sample was conducted in three stages. In the first stage, records of 240 males born in 1887 and survived to age 100 were randomly selected from the Social Security Administration Death Master File (DMF). We used the 1887 birth cohort in order to avoid possible effects of birth year “heaping” (rounding up birth years to end in 5 or 0). Also men born in 1887 reached age 30 in 1917, so their adult height has been attained by the time of draft registration. Taking into account that DMF covers 93-96 percent of deaths of persons aged 65+ (Hill and Rosenwaike, 2001), it was possible to apply a simple random sampling design for male centenarian data. The 1887 birth cohort may be considered practically extinct now, because it is highly unlikely that any man born in 1887 would live more than 120 years. Thus, we may expect that DMF contains records on almost all American centenarians born in 1887, which is another advantage of selecting 1887 birth cohort for this study. The DMF database contains about 2,500 death records of male centenarians born in 1887 and linking all of them to WWI civilian draft registration cards would require significant investment of time and effort. So in this study we used a sample of 240 (9.6%) randomly selected male centenarians born in 1887. In the second stage, the selected records were linked to the WWI civilian draft registration cards. In the third stage, each centenarian record has received matched control record randomly selected from the civilian draft registration records of persons of the same birth year, race and county of registration.
Brief Description of WWI Draft Registration Cards
In 1917 and 1918, approximately 24 million men born between 1873 and 1900 completed draft registration cards. Men already on active duty in the military were excluded from draft registration. Registration of eligible men has been determined to be close to 100%, which means that about 98% of adult men under age 46 living in the U.S. in 1917-18 completed registration cards (Banks, 2000). Instructions for filling in each question on the card were posted for all to read at each registration site, and the local newspapers sometimes printed copies of sample cards in the days prior to registration. In the vast majority of cases, volunteer staff at the local office filled in the information on the card, and the registrant then signed his name. More detailed description of this data source is available in a seven-volume set by Raymond H. Banks (Banks, 2000). Table 4 presents information available in the draft registration cards.
Table 4.
Information available from the WWI draft registration cards.
| Group | Description |
|---|---|
| Core demographic data | age, date/place of birth, race, citizenship |
| Geographical data | permanent home address |
| Working characteristics | occupation, employer's name |
| Family characteristics | Marital status, information about dependents (including children below age 12) |
| Physical characteristics | height (3 categories: tall, medium, or short), build (3 categories: slender, medium, or stout), eye color, hair color, baldness, disability |
The WWI civilian draft registration cards are available now online through the service provided by the Ancestry.com. Thus the linkage process was facilitated by availability of online indexes, actual digitized images of draft registration cards and the fact that the exact birth date (day, month, year) was available in both the WWI draft cards and the Social Security Administration Death Master File, in addition to person's names. This allowed us to obtain unambiguous matches in the majority of cases.
Model Specification and Statistical Methods
Variables
Physical characteristics
Draft registration cards reported three categories of body height (tall, medium, or short) and three categories of body build (slender, medium, or stout), which are the main variables of interest in this study. Additional physical characteristics include eye color and hair color (or baldness). In the case of inability to participate in military service due to disability, the type of disability is briefly described. However, cases of disability turned out to be very rare.
Race
Race was used as a basis for matching so this variable was not used in statistical analyses.
Place of birth
Place of birth reported in draft registration cards allowed us to identify foreign-born or native-born status of draft registration participants.
Marital status
Draft registration cards either recorded that the person is married or mentioned wife among the dependents.
Number of children
Draft registration cards recorded all proband's children below age 12. Taking into account that it seems rather unlikely for men to have children older than 12 at age 30 (corresponding to fatherhood before age 18 years), we may suggest that draft registration cards reported almost all existing children for men in our sample.
Occupation
Draft registration cards reported current occupation of registrants. We combined all the variety of occupations into five groups: farmers, white collar occupations (clerks, bankers, etc.), blue collar skilled occupations (repair mechanics, machinists), service occupations (grocers, barbers, salesmen) and unskilled occupations (laborers, kitchen hand, etc.).
Statistical methods
The statistical analyses were performed using a conditional multiple logistic regression model for matched case-control studies to investigate the relationship between an outcome of being a case (survival to age 100) and a set of predictor variables (Breslow and Day, 1993; Hosmer and Lemeshow, 2001). An important advantage of conditional logistic regression is its high statistical power (Woodward, 2005), which allows researchers to detect statistically significant effects even in samples with relatively small size.
When each matched set consists of a single control (1-1 matched study), the conditional likelihood is given by:
where xi1 and xi0 are vectors representing the prognostic factors for the case and control, respectively, of the ith matched set (Hosmer, Lemeshow, 2001). A subset of explanatory variables was pre-selected for possible inclusion in a multivariate model on the basis of their univariate analysis. Computations were conducted using Stata, release 11 (StataCorp, 2009).
Results and Discussion
Overall linkage rate to the draft registration card data was 72.5% (174 linked records). It should be noted that not all centenarians found in DMF could participate in the WWI draft registration. Study of additional data sources revealed that 2 persons in DMF sample served in a regular army during the draft registration, 7 persons had their SSNs issued after 1955 (suggesting late immigration) and in 6 cases we found misprints in SSA DMF (persons in fact were born in 1987 according to their death certificates). Elimination of these non-eligible cases increased the linkage success to 77.3%. Further analysis revealed very high proportion of persons with Eastern European, Italian and Spanish surnames among non-linked records (41%) compared to persons linked to the WWI draft registration records (only 9%). This suggests that many persons in the non-linkage group could immigrate to the United States after 1917. This suggestion was further confirmed by information about foreign-born status among draft registration controls. Table 5 describes demographic and socio-economic characteristics of centenarians (cases) and controls.
Table 5.
Characteristics of men born in 1887 and participating in the World War I civil draft registration (in the studied sample).
| Characteristic | Proportion (percent) | p-value for difference between cases and controls | |
|---|---|---|---|
| Centenarians (cases) N=171 | Controls N=171 | ||
| Race | |||
| white | 93.57 | 93.57 | - |
| black | 5.26 | 5.26 | - |
| other | 1.17 | 1.17 | - |
| Foreign born | 20.47 | 22.22 | 0.692 |
| Married | 68.42 | 63.74 | 0.361 |
| Had children | 52.63 | 42.11 | 0.051 |
| Farmer by occupation | 31.55 | 23.35 | 0.093 |
| Reported disability | 7.02 | 8.77 | 0.547 |
| Tall height | 28.07 | 34.50 | 0.200 |
| Stout build | 7.02 | 14.62 | 0.024 |
Note that the proportion of foreign-born individuals is similar in both cases and controls. Thus, we may conclude that the linkage success of centenarian cases to the WWI draft registration cards was not lower for foreign-born individuals compared to native-born persons. Proportions of foreign-born individuals in our sample are very close to the official data. For example, according to the 1920 U.S. census, proportion of foreign-born individuals in age group 20-44 is 17.7% (U.S. Department of Commerce, 1940), which is close to our estimates. Proportion of blacks in age group 20-44 was 9.8% according to the same census (U.S. Department of Commerce, 1940). Taking into account higher mortality of blacks compared to whites it is reasonable to expect decreasing proportion of blacks among centenarians (as it is the case in our sample, see Table 5). Comparison to official data suggests that the linkage of centenarian records to WWI draft registration cards was not subjected to significant biases regarding foreign-born status or race.
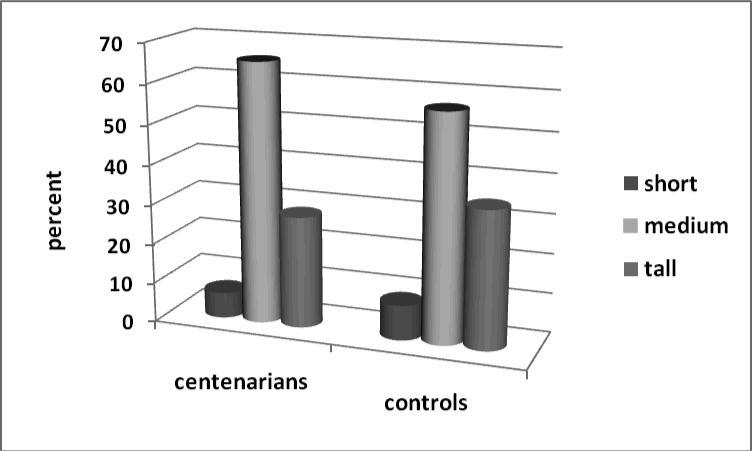
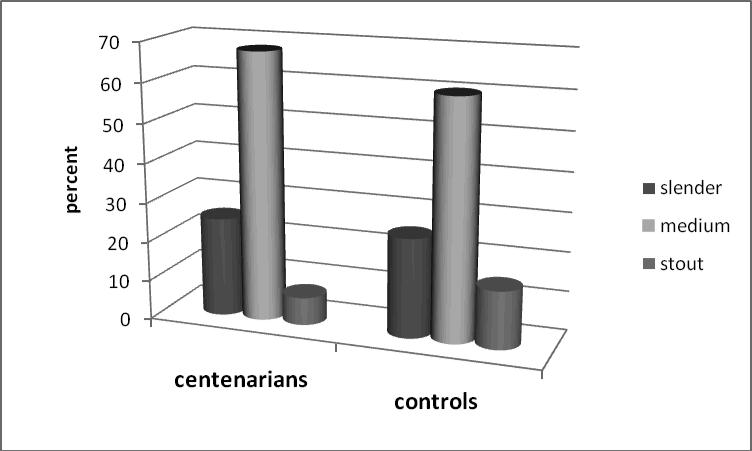
Table 5 also contains distribution of cases and controls according to their body height and body build. Note that the ‘tall’ category corresponds to the top 35 percent of the tallest men in control population. Stout body build corresponds to the top 15th percentile of the heaviest men in control population while this proportion is significantly lower in centenarian group (see Table 5). Figure 4 shows distribution of long-lived and control groups according to their height at age 30.
Figure 4.
Body height at age 30 and survival to age 100. Distribution of cases (future centenarians) and controls by the height category.
It is interesting to note that centenarians were not concentrated among the tallest men at age 30. In fact, most of them tend to be of medium height, although these differences were not statistically significant. Distribution of centenarians and controls by their body build at age 30 is presented in Figure 5. Only 7% of the future centenarians fell into the ‘stout’ category, compared to 15% of the control group. The difference in body build distributions between cases and controls was significant in univariate analyses (Table 5). Multivariate analyses using conditional logistic regression found that stout body build had a statistically significant association with lower survival rates to age 100 in all three models (see Table 6).
Figure 5.
Body build at age 30 and survival to age 100. Distribution of cases (future centenarians) and controls by the body build category.
Table 6.
Odds ratios (95% confidence intervals) of exceptional longevity (survival to age 100) for certain physical and socio-demographic characteristics of men at age 30. Multivariate conditional logistic regression.
| Characteristic | Model 1 | Model 2 | Model3 |
|---|---|---|---|
| Stout body build | reference | reference | reference |
| Slender or medium build | 2.62* (1.19 - 5.77) | 2.63* (1.17 - 5.89) | 2.63* (1.13 - 6.12) |
| Farmer by occupation vs other occupation | 2.00* (1.09 - 3.64) | 2.03* (1.09 - 3.78) | 2.20* (1.16 - 4.19) |
| Native born vs foreign born | 1.12 (0.63 - 1.99) | 1.13 (0.63 - 2.05) | |
| Married vs non-married | 0.76 (0.41 - 1.44) | 0.68 (0.35 - 1.34) | |
| No children | reference | reference | |
| 1-3 children | 1.62 (0.89 - 2.95) | 1.61 (0.87 - 2.98) | |
| 4+ children | 2.71* (0.99 - 7.39) | 2.59+ (0.92 - 7.28) | |
| Short height | reference | ||
| Medium or tall height | 1.35 (0.80 - 2.29) | ||
| Blue/grey eyes | 1.71† (0.99 - 2.95) | ||
| Light hair | 0.64 (0.31 - 1.32) | ||
| Disability | 0.68 (0.28 - 1.66) |
p ≤ 0.05
p=0.052
p=0.07
Thus, the study of height and build among men born in 1887 suggests that obesity at young adult age (30 years) is harmful for attaining exceptional longevity, while body height is far less important predictor of exceptional longevity. The finding that ‘stout’ body build predicts much lower survival rates to 100 years are generally consistent with the existing knowledge that high body mass index (BMI) and obesity are associated with increased mortality (Adams et al., 2006; Flegal et al., 2005; Flegal et al., 2007). Our findings also expand this knowledge further in three ways: 1) the detrimental effects of obesity may have an exceptionally long time range, that is obesity at young adult age (30 years) is still predictive for decreased chances of survival to age 100 years; 2) the significance of body build as a predictor of exceptional longevity is much higher than all other potentially important variables, such as body height, immigration status, marital status, and occupation (with exception that being a farmer is highly beneficial for attaining exceptional longevity); 3) contrary to expectations based on life extension of calorically restricted animals (Fontana et al., 2010), a ‘slender’ body build does not improve chances of survival to 100 years. It should be noted, that slender body build in the past could also be related to poor nutrition or infectious load (tuberculosis in particular) (Alter, 2004; Elo and Preston, 1992), but our data do not support the hypothesis of decreased chances of exceptional longevity for slender-built individuals.
Our study also found that body height is not associated with survival to age 100 and centenarians tend to have medium height on average. Thus, our data do not support both hypotheses related to adult body height and longevity described earlier in this paper. It should be noted, however, that the lack of effect of body height on longevity found in our study may be related to small sample size and hence insufficient statistical power to reveal potential height-longevity effects. Also, it was shown that tall body height may have multidirectional associations with chronic diseases: positive association with cancer (Batty et al., 2009) and negative association with heart disease (Paajanen et al., 2010). Therefore, the final effect of tall body height on longevity may be weak and more complicated than that of obesity. Recent study of height and late-life mortality among Finnish men showed that persons who were taller at age seven lived longer than their shorter peers (Barker et al., 2011). However, a group of men with unexpectedly tall height at age seven had elevated mortality in middle age (Barker et al., 2011) that indicates detrimental effect of early compensatory growth on longevity.
Another finding of this study is a positive effect of being a farmer by occupation on survival to age 100. In addition to farming, several broad occupational groups were also studied: white collar occupations, blue collar skilled occupations, service occupations and unskilled occupations (laborers, kitchen hand, etc.). Neither of these occupational groups had a significant effect on attaining longevity in our study including wealthier white collar group. This result is consistent with our previous findings suggesting that children raised on farms (boys in particular) had higher chances to become centenarians (Gavrilova and Gavrilov, 2007). Similar results were obtained by other authors who studied childhood conditions and survival to advanced ages and also found much stronger effects of farm childhood on longevity for men than women (Hill et al., 2000; Preston et al., 1998; Stone, 2003). Preston and colleagues (Preston et al., 1998) suggested a hypothesis that farm childhood effect on longevity is stronger for men compared to women because men raised on farms become farmers by occupation and continue to live on farms in healthier environments. Our findings presented here are consistent with this hypothesis.
Being married by age 30 had no statistically significant effect on survival to age 100. However the number of children at age 30 demonstrated positive effects on chances of exceptional longevity (see Table 6). It is interesting to note that large initial number of children born by age 30 increases man's chances to attain exceptional longevity by a factor 2.6 – 2.7 (Table 6). Positive association of the number of children with longevity found in our study seems to be different from the predictions of some evolutionary theories of aging (disposable soma theory). According to the disposable soma theory, “there may be a trade-off between reproductive success and longevity, because resources invested in longevity assurance may be at expense of reproduction” and this mechanism operates for both males and females (Westendorp and Kirkwood, 1998). On the other hand, our finding of positive relation between reproductive success and longevity may have reasonable explanations, both of social and biological nature. First, a large number of children being born earlier in life may provide a necessary caregiving and material support for parent at his older ages. Second, high fertility at young age may be a marker of man's overall good health. Further studies of centenarians including studies of genealogical data may shed more light on the mechanisms of this interesting phenomenon.
A number of limitations of the data need to be considered in evaluating the results related to body build and height characteristics. Although draft registration cards contain valuable information on individual physical markers, this resource is not free of limitations. The main difficulty we faced here was using height and build data measured in a categorical rather than continuous scale - in three broad categories, which are less precise than measures provided in specialized health surveys like NHANES. During the WWI draft registration, local staff was asked to classify individual men as to height and weight. The three categories provided were rather vague, and occasionally the staff wrote in actual weight and height instead. In addition to this, some errors in reporting physical characteristics were also mentioned (Banks, 2000). Nevertheless, the data were measured by the volunteer staff in the registration office at the time when centenarians were young adults and hence are not subjected to self-report and recall bias. Also, using county-matched controls helps to avoid possible regional differences in defining “tallness” or “shortness.” This study provided the first estimates of height and build for U.S. centenarians at young ages, which may be helpful to test alternative hypotheses on early growth and longevity (Costa and Lahey, 2005; Fogel, 2003; Miller et al., 2002; Willcox et al., 2006). The results of this study also demonstrate the usefulness of the US WWI draft registration cards as a new promising source of information for finding factors associated with lower mortality and better survival.
Conclusions
We presented results of two studies demonstrating that applying matched case-control design is a useful way to study effects of early-life conditions on exceptional longevity. In the first study a sample of centenarians was drawn from a large set of computerized family histories available online. Linkage of records obtained from online genealogies to official resources provided an external validity for birth dates of centenarians and their siblings as well as death dates for long-lived individuals. Within-family comparison of centenarians to their shorter-lived siblings gives an opportunity to control for unobserved common conditions early in life and common genetic background. The study demonstrated that young age of mother at birth proved to be the best predictor of exceptional longevity in this data sample when compared to birth order or paternal age. Young age of mother has long-lasting effect on survival to age 100 and is observed even after age 70. This result suggests that delayed childbearing in contemporary populations may potentially have adverse effects on the health of future generations.
In the second study we applied matched case-control design to analyze the effects of physical characteristics at young adult age on survival to age 100. Using a random sample of U.S. centenarians born in the same year it was possible to conduct a linkage of these records to WWI civil draft registration cards collected in 1917-1918, which had information on physical characteristics of registrants. Matched case-control design allowed us to control for birth cohort, race and contextual variables on longevity. The study shows that being overweight at young age may significantly decrease chances of living to 100. It also supported previous findings that being raised on a farm improves survival to advanced ages (Gavrilova and Gavrilov, 2007; Preston et al., 1998).
The results obtained in these studies demonstrate that factors operating early in life are important components in determining exceptional longevity. While genetic factors undoubtedly play an important role in survival to advanced ages, there are also a number of other factors associated with living to age 100 and beyond. Adding new historical data resources into arsenal of centenarian studies will bring better understanding of the role of early-life conditions in exceptional longevity.
Acknowledgments
This study was supported by the U.S. National Institute on Aging (grant R01 AG028620). We are most grateful to two anonymous reviewers for their constructive criticism and useful suggestions. Earlier results of this study were presented and discussed at the 2006 and 2008 annual meetings of the Population Association of America, the 2008 annual meeting of the Gerontological Society of America, the 2008 Society of Actuaries international symposium “Living to 100 and Beyond” and the 2007 and 2009 annual meetings of the International Network on Health Expectancy and the Disability Process (REVES).
References
- Adams JW, Kasakoff AB. Migration and the Family in Colonial New-England - the View from Genealogies. J Fam Hist. 1984;9:24–43. [Google Scholar]
- Adams JW, Kasakoff AB. Estimates of Census Underenumeration Based on Genealogies. Soc Sci Hist. 1991;15:527–543. [Google Scholar]
- Adams K, Schatzkin A, Harris T, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann M. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- Adkins G, Martin P, Poon LW. Personality traits and states as predictors of subjective well-being in centenarians, octogenarians, and sexagenarians. Psychol Aging. 1996;11:408–416. doi: 10.1037//0882-7974.11.3.408. [DOI] [PubMed] [Google Scholar]
- Alter G. Height, frailty, and the standard of living: Modelling the effects of diet and disease on declining mortality and increasing height. Pop Stud-J Demog. 2004;58:265–279. doi: 10.1080/0032472042000272339. [DOI] [PubMed] [Google Scholar]
- Alter G, Oris M. Childhood conditions, migration, and mortality: Migrants and natives in 19th-century cities. Soc Biol. 2005;52:178–191. doi: 10.1080/19485565.2005.9989108. [DOI] [PubMed] [Google Scholar]
- Alter G, Neven M, Oris M. Stature in transition - A micro-level study from nineteenth-century Belgium. Soc Sci Hist. 2004;28:231–247. [Google Scholar]
- Anderton DL, Bean LL, Willigan JD, Mineau GP. Adoption of Fertility Limitation in an American-Frontier Population - an Analysis and Simulation of Socio-Religious Subgroups. Soc Biol. 1984;31:140–159. doi: 10.1080/19485565.1984.9988569. [DOI] [PubMed] [Google Scholar]
- Anderton DL, Tsuya NO, Bean LL, Mineau GP. Intergenerational Transmission of Relative Fertility and Life Course Patterns. Demography. 1987;24:467–480. [PubMed] [Google Scholar]
- Banks R. World War I Civilian Draft Registrations. [database on-line] Ancestry.com; Provo, UT: 2000. [Google Scholar]
- Barker DJP. The Fetal and Infant Origins of Adult Disease. BMJ Books; London: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Kajantie E, Osmond C, Thornburg KL, Eriksson JG. How Boys Grow Determines How Long They Live. Am J Hum Biol. 2011;23:412–416. doi: 10.1002/ajhb.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular-disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Shuldiner AR. Searching for human longevity genes: the future history of gerontology in the post-genomic era. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2001;56:M83–7. doi: 10.1093/gerona/56.2.m83. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Derby CA, Bauman JM, Lipton RB. A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology. 2006;67:2170–2175. doi: 10.1212/01.wnl.0000249116.50854.65. [DOI] [PubMed] [Google Scholar]
- Batty GD, Shipley MJ, Gunnell D, Huxley R, Kivimaki M, Woodward M, Lee CMY, Smith GD. Height, wealth, and health: An overview with new data from three longitudinal studies. Econ. Hum. Biol. 2009;7:137–152. doi: 10.1016/j.ehb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Bean LL, Mineau GP, Anderton DL. High-Risk Childbearing - Fertility and Infant-Mortality on the American Frontier. Soc Sci Hist. 1992;16:337–363. [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- Bengtsson T, Mineau GR. Early-life effects on socio-economic performance and mortality in later life: A full life-course approach using contemporary and historical sources Introduction. Social Science & Medicine. 2009;68:1561–1564. doi: 10.1016/j.socscimed.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Bickel SE. Aging (not so) gracefully. Nature Genetics. 2005;37:1303–4. doi: 10.1038/ng1205-1303. [comment] [DOI] [PubMed] [Google Scholar]
- Bottini E, Meloni GF, MacMurray J, Ammendola M, Meloni T, Gloria-Bottini F. Maternal age and traits of offspring in humans. Placenta. 2001;22:787–789. doi: 10.1053/plac.2001.0711. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol.1. The Analysis of Case-Control Studies. Oxford University Press; Oxford: 1993. [Google Scholar]
- Chan PC, Suzuki M, Yamamoto S. A comparison of anthropometry, biochemical variables and plasma amino acids among centenarians, elderly and young subjects. J Am Coll Nutr. 1999;18:358–365. doi: 10.1080/07315724.1999.10718876. [DOI] [PubMed] [Google Scholar]
- Chan YC, Suzuki M, Yamamoto S. Dietary, anthropometric, hematological and biochemical assessment of the nutritional status of centenarians and elderly people in Okinawa, Japan. J Am Coll Nutr. 1997a;16:229–235. doi: 10.1080/07315724.1997.10718679. [DOI] [PubMed] [Google Scholar]
- Chan YC, Suzuki M, Yamamoto S. Nutritional status of centenarians assessed by activity and anthropometric, hematological and biochemical characteristics. J Nutr Sci Vitaminol. 1997b;43:73–81. doi: 10.3177/jnsv.43.73. [DOI] [PubMed] [Google Scholar]
- Comings DE, Macmurray J. Maternal age as a confounding variable in association studies. American Journal of Medical Genetics. 2001;105:564–564. [Google Scholar]
- Comings DE, MacMurray JP. Maternal age at the birth of the first child as an epistatic factor in polygenic disorders. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2006;141:1–6. doi: 10.1002/ajmg.b.30026. [DOI] [PubMed] [Google Scholar]
- Costa DL. Height, Wealth, and Disease among the Native-Born in the Rural, Antebellum North. Soc Sci Hist. 1993a;17:355–383. [Google Scholar]
- Costa DL. Height, Weight, Wartime Stress, and Older Age Mortality - Evidence from the Union Army Records. Explor Econ Hist. 1993b;30:424–449. [Google Scholar]
- Costa DL. Understanding mid-life and older age mortality declines: evidence from Union Army veterans. J Econometrics. 2003;112:175–192. [Google Scholar]
- Costa DL, Lahey J. Becoming oldest old: evidence from historical U.S. data. Genus. 2005;61:125–161. [Google Scholar]
- Crimmins E, Finch C. Infection, inflammation, height, and longevity. Proc. Natl. Acad. Sci. U S A. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Seeman T. Integrating biology into demographic research on health and aging (With a focus on the MacArthur Study of Successful Aging) In: Finch CE, et al., editors. Cells and Surveys. National Academy Press; Washington, DC: 2000. pp. 9–41. [Google Scholar]
- Crow JF. The high spontaneous mutation rate: Is it a health risk? P Natl Acad Sci USA. 1997;94:8380–8386. doi: 10.1073/pnas.94.16.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF. The origins patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- Elo IT, Preston SH. Effects of early-life condition on adult mortality: A review. Popul Index. 1992;58:186–222. [PubMed] [Google Scholar]
- Elo IT, Preston SH, Rosenwaike I, Hill M, Cheney TP. Consistency of age reporting on death certificates and social security records among elderly African Americans. Soc Sci Res. 1996;25:292–307. [Google Scholar]
- Faig K. Reported deaths of centenarians and near-centenarians in the U.S. Social Security Administration's Death Master File, The Society of Actuaries “Living to 100 and Beyond International Symposium”. Society of Actuaries, Shaumburg. 2001 [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human lifespans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Flegal K, Graubard B, Williamson D, Gail M. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Flegal K, Graubard B, Williamson D, Gail M. Impact of Smoking and Preexisting Illness on Estimates of the Fractions of Deaths Associated with Underweight, Overweight, and Obesity in the US Population. Am J Epidemiol. 2007;166:975–82. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- Fogel RW. Secular trends in physiological capital - implications for equity in health care. Perspect Biol Med. 2003;46:S24–S38. [PubMed] [Google Scholar]
- Fogel RW, Costa DL. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography. 1997;34:49–66. [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending Healthy Life Span-From Yeast to Humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. The Biology of Life Span: A Quantitative Approach. Harwood Academic Publisher; New York: 1991. [Google Scholar]
- Gavrilov LA, Gavrilova NS. Parental age at conception and offspring longevity. Reviews in Clinical Gerontology. 1997;7:5–12. [Google Scholar]
- Gavrilov LA, Gavrilova NS. Human longevity and parental age at conception. In: Robine J-M, et al., editors. Sex and Longevity: Sexuality, Gender, Reproduction, Parenthood. Springer-Verlag; Berlin, Heidelberg: 2000. pp. 7–31. [Google Scholar]
- Gavrilov LA, Gavrilova NS. Biodemographic study of familial determinants of human longevity. Population: An English Selection. 2001;13:197–222. [Google Scholar]
- Gavrilov LA, Gavrilova NS. Early-life factors modulating lifespan. In: Rattan SIS, editor. Modulating Aging and Longevity. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2003. pp. 27–50. [Google Scholar]
- Gavrilov LA, Gavrilova NS, Olshansky SJ, Carnes BA. Genealogical data and the biodemography of human longevity. Soc Biol. 2002;49:160–173. doi: 10.1080/19485565.2002.9989056. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS, Kroutko VN, Evdokushkina GN, Semyonova VG, Gavrilova AL, Lapshin EV, Evdokushkina NN, Kushnareva YE. Mutation load and human longevity. Mutat Res-Fund Mol M. 1997;377:61–62. doi: 10.1016/s0027-5107(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Gavrilova NS, Gavrilov LA. Data resources for biodemographic studies on familial clustering of human longevity. Demographic Research. 1999;1:1–48. [Google Scholar]
- Gavrilova NS, Gavrilov LA. Search for Predictors of Exceptional Human Longevity: Using Computerized Genealogies and Internet Resources for Human Longevity Studies. North American Actuarial Journal. 2007;11:49–67. [Google Scholar]
- Gloria-Bottini F, Cosmi E, Nicotra M, Cosmi EV, Bottini E. Is delayed childbearing changing gene frequencies in western populations? Hum Biol. 2005;77:433–441. doi: 10.1353/hub.2005.0062. [DOI] [PubMed] [Google Scholar]
- Groves RM. Nonresponse rates and nonresponse bias in household surveys. Public Opinion Quarterly. 2006;70:646–675. [Google Scholar]
- Guest M. Notes from the National Panel Study: Linkage and migration in the late nineteenth century. Hist Method. 1987;20:63–77. [Google Scholar]
- Hadley EC, Rossi WK, Albert S, Bailey-Wilson J, Baron J, Cawthon R, Christian JC, Corder EH, Franceschi C, Kestenbaum B, Kruglyak L, Lauderdale DS, Lubitz J, Martin GM, McClearn GE, McGue M, Miles T, Mineau G, Ouellett G, Pedersen NL, Preston SH, Page WF, Province M, Schachter F, Schork NJ, Vaupel JW, Vijg J, Wallace R, Wang E, Wijsman EM, Wor NAGE. Genetic epidemiologic studies on age-specified traits. Am J Epidemiol. 2000;152:1003–1008. doi: 10.1093/aje/152.11.1003. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Alfredson BB, Poon LW, Homma A. Cognitive functioning in centenarians: A coordinated analysis of results from three countries. J Gerontol B-Psychol. 2001;56:P141–P151. doi: 10.1093/geronb/56.3.p141. [DOI] [PubMed] [Google Scholar]
- Haines MR, Craig LA, Weiss T. The short and the dead: Nutrition, mortality, and the “antebellum puzzle” in the United States. J Econ Hist. 2003;63:382–413. [Google Scholar]
- Hayward MD, Gorman BK. The long arm of childhood: The influence of early-life social conditions on men's mortality. Demography. 2004;41:87–107. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- Heffner LJ. Advanced maternal age--how old is too old? New Engl J Med. 2004;351:1927–9. doi: 10.1056/NEJMp048087. [DOI] [PubMed] [Google Scholar]
- Hill ME, Rosenwaike I. The Social Security Administration's Death Master File: The completeness of death reporting at older ages. Soc Secur Bull. 2001;64:45–51. [PubMed] [Google Scholar]
- Hill ME, Preston SH, Rosenwaike I, Dunagan JF. Childhood conditions predicting survival to advanced age among white Americans.. 2000 Annual meeting of the Population Association of America; Los Angeles. 2000. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley & Sons; New York: 2001. [Google Scholar]
- Hubbard RE, Andrew MK, Rockwood K. Effect of parental age at birth on the accumulation of deficits, frailty and survival in older adults. Age Ageing. 2009;38:380–385. doi: 10.1093/ageing/afp035. [DOI] [PubMed] [Google Scholar]
- Jang YR, Poon LW, Martin P. Individual differences in the effects of disease and disability on depressive symptoms: The role of age and subjective health. Int J Aging Hum Dev. 2004;59:125–137. doi: 10.2190/RT1W-2HD7-KG5X-K1FB. [DOI] [PubMed] [Google Scholar]
- Jeune B, Vaupel J. Validation of Exceptional Longevity. Odense University Publisher; Odense: 1999. [Google Scholar]
- Kalmijn M, Kraaykamp G. Late or later? A sibling analysis of the effect of maternal age on children's schooling. Social Science Research. 2005;34:634–650. [Google Scholar]
- Kasakoff AB, Adams JW. The effects of migration, place, and occupation on adult mortality in the American North, 1740-1880. Hist Method. 2000;33:115–130. doi: 10.1080/01615440009598954. [DOI] [PubMed] [Google Scholar]
- Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, Agarwal S, Blasco MA. Telomere length predicts embryo fragmentation after in vitro fertilization in women - Toward a telomere theory of reproductive aging in women. American Journal of Obstetrics and Gynecology. 2005;192:1256–1260. doi: 10.1016/j.ajog.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Kemkes-Grottenthaler A. Parental effects on off spring longevity - evidence from 17th to 19th century reproductive histories. Ann Hum Biol. 2004;31:139–158. doi: 10.1080/03014460410001663407. [DOI] [PubMed] [Google Scholar]
- Kerber RA, O'Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. Journal of Gerontology. 2001a;56A:B130–B139. doi: 10.1093/gerona/56.3.b130. [DOI] [PubMed] [Google Scholar]
- Kerber RA, O'Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2001b;56:B130–9. doi: 10.1093/gerona/56.3.b130. [DOI] [PubMed] [Google Scholar]
- Kestenbaum B. A Description of the Extreme Aged Population Based on Improved Medicare Enrollment Data. Demography. 1992;29:565–580. [PubMed] [Google Scholar]
- Kestenbaum B, Ferguson BR. Mortality of the extreme aged in the United States in the 1990s, based on improved Medicare data. The Society of Actuaries “Living to 100 and Beyond International Symposium”; Orlando, FL. 2001. [Google Scholar]
- Kuh D, Ben-Shlomo B. A Life Course Approach to Chronic Disease Epidemiology. Oxford University Press; Oxford: 1997. [Google Scholar]
- Lauderdale DS, Rathouz PJ. Evidence of environmental suppression of familial resemblance: height among US Civil War brothers. Ann Hum Biol. 1999;26:413–426. doi: 10.1080/030144699282543. [DOI] [PubMed] [Google Scholar]
- Li Y, Deeb B, Pendergrass W, Wolf N. Cellular proliferative capacity and life span in small and large dogs. J Gerontol a-Biol. 1996;51:B403–B408. doi: 10.1093/gerona/51a.6.b403. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol Evol. 2002;17:141–147. [Google Scholar]
- Martin P, da Rosa G, Siegler IC. Personality and longevity: findings from the Georgia Centenarian Study. Age. 2006;28:343–352. doi: 10.1007/s11357-006-9022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell AR. Longevity of British breeds of dog and its relationships with sex, size, cardiovascular variables and disease. Veterinary Record. 1999;145:625–629. doi: 10.1136/vr.145.22.625. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chrisp C, Atchley W. Differential longevity in mouse stocks selected for early life growth trajectory. J Gerontol a-Biol. 2000;55:B455–B461. doi: 10.1093/gerona/55.9.b455. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Paajanen TA, Oksala NKJ, Kuukasjarvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur. Heart J. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: Implications for gerontology research. J Gerontol a-Biol. 1997;52:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenetic & Genome Research. 2005;111:206–12. doi: 10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- Polani PE, Crolla JA. A Test of the Production Line Hypothesis of Mammalian Oogenesis. Hum Genet. 1991;88:64–70. doi: 10.1007/BF00204931. [DOI] [PubMed] [Google Scholar]
- Preston SH, Haines MR. Child mortality in late nineteenth-century America. Princeton University Press; Princeton, NJ: 1991. Fatal years. [Google Scholar]
- Preston SH, Hill ME, Drevenstedt GL. Childhood conditions that predict survival to advanced ages among African-Americans. Social Science & Medicine. 1998;47:1231–1246. doi: 10.1016/s0277-9536(98)00180-4. [DOI] [PubMed] [Google Scholar]
- Preston SH, Elo IT, Rosenwaike I, Hill M. African-American mortality at older ages: Results of a matching study. Demography. 1996;33:193–209. [PubMed] [Google Scholar]
- Puca AA, Daly MJ, Brewster SJ, Matise TC, Barrett J, Shea-Drinkwater M, Kang S, Joyce E, Nicoli J, Benson E, Kunkel LM, Perls T. A genome-wide scan for linkage to human exceptional longevity identifies a locus on chromosome 4. P Natl Acad Sci USA. 2001;98:10505–10508. doi: 10.1073/pnas.181337598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine JM, Cournil A, Henon N, Allard M. Have centenarians had younger parents than the others? Exp Gerontol. 2003;38:361–365. doi: 10.1016/s0531-5565(02)00245-0. [DOI] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts the life span of mammals. Evolution & Development. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Rosenwaike I, Hill ME. The accuracy of age reporting among elderly African Americans - Evidence of a birth registration effect. Res Aging. 1996;18:310–324. [Google Scholar]
- Rosenwaike I, Stone LF. Verification of the ages of supercentenarians in the United States: Results of a matching study. Demography. 2003;40:727–739. doi: 10.1353/dem.2003.0038. [DOI] [PubMed] [Google Scholar]
- Rosenwaike I, Hill ME, Preston SH, Elo IT. Linking death certificates to early census records - The African American matched records sample (American genealogy). Hist Method. 1998;31:65–74. [Google Scholar]
- Ruggles S, Alexander JT, Genadek K, Goeken R, Shroeder MB, Sobek M. Integrated Public Use Microdata Series (IPUMS): Version 5.0 [Machine-readable database] University of Minnesota; Minneapolis, MN: 2010. [Google Scholar]
- Samaras TT. Should we be concerned over increasing body height and weight? Exp Gerontol. 2009;44:83–92. doi: 10.1016/j.exger.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Samaras TT, Storms LH. Impact of Height and Weight on Life-Span. B World Health Organ. 1992;70:259–267. [PMC free article] [PubMed] [Google Scholar]
- Samaras TT, Storms LH. Secular growth and its harmful ramifications. Med Hypotheses. 2002;58:93–112. doi: 10.1054/mehy.2001.1463. [DOI] [PubMed] [Google Scholar]
- Samaras TT, Storms LH, Elrick H. Longevity, mortality and body weight. Ageing Res Rev. 2002;1:673–691. doi: 10.1016/s1568-1637(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Samaras TT, Elrick H, Storms LH. Is height related to longevity? Life Sci. 2003;72:1781–1802. doi: 10.1016/s0024-3205(02)02503-1. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Paffenbarger RS, Lee IM. Comparison of National Death Index and World Wide Web death searches. American Journal of Epidemiology. 2000;152:107–111. doi: 10.1093/aje/152.2.107. [DOI] [PubMed] [Google Scholar]
- Shrestha LB, Rosenwaike I. Can data from the decennial census measure trends in mobility limitation among the aged? Gerontologist. 1996;36:106–109. doi: 10.1093/geront/36.1.106. [DOI] [PubMed] [Google Scholar]
- Smith KR, Garibotti G, Fraser A, Mineau GP. Adult mortality and geographic proximity of parents in Utah in the 19th and 20th centuries. IUSSP Symposium on “Kinship and Demographic Behavior”; Salt Lake City, Utah. 2005. [Google Scholar]
- Smith KR, Mineau GR, Garibotti G, Kerber R. Effects of childhood and middle-adulthood family conditions on later-life mortality: Evidence from the Utah Population Database, 1850-2002. Social Science & Medicine. 2009a;68:1649–1658. doi: 10.1016/j.socscimed.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Gagnon A, Cawthon RM, Mineau GP, Mazan R, Desjardins B. Familial Aggregation of Survival and Late Female Reproduction. J Gerontol a-Biol. 2009b;64:740–744. doi: 10.1093/gerona/glp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . Stata Statistical Software: Release 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- Stone L. Early-life conditions and survival to age 110 in the U.S.. workshop “Early Life Conditions and Longevity. Reconstructive Lives from Cradle to Grave.”; Geneva. 2003. [Google Scholar]
- Suitor JJ, Pillemer K, Sechrist J. Within-family differences in mothers’ support to adult children. J Gerontol B-Psychol. 2006;61:S10–S17. doi: 10.1093/geronb/61.1.s10. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V, Manzanedo C, Minarro J, Hermenegildo C, Cano A. Long-term effects of delayed motherhood in mice on postnatal development and behavioural traits of offspring. Human Reproduction. 2003;18:1580–1587. doi: 10.1093/humrep/deg349. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V, Rausell F, Navarro S, Hermenegildo C, Cano A. Delayed motherhood decreases life expectancy of mouse offspring. Biology of Reproduction. 2005;72:1336–43. doi: 10.1095/biolreprod.104.038919. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Commerce . Statistical Abstract of the United States 1939. United States Government Printing Office; Washington, DC: 1940. [Google Scholar]
- Waaler HT. Height, weight and mortality. Acta Medica Scandinavica. 1984;(679) doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- Wang MH, vom Saal FS. Maternal age and traits in offspring - The timing of a mouse's first litter influences the development of her pups. Nature. 2000;407:469–470. doi: 10.1038/35035156. [DOI] [PubMed] [Google Scholar]
- Wells JCK. The programming effects of early growth. Early Human Development. 2007;83:743–748. doi: 10.1016/j.earlhumdev.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Westendorp RGJ, Kirkwood TBL. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7:173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- Woodward M. Study Design and Data Analysis. Chapman & Hall/CRC; Boca Raton, FL: 2005. Epidemiology. [Google Scholar]
- Young RD, Desjardins B, McLaughlin K, Poulain M, Perls TT. Typologies of extreme longevity myths. Curr Gerontol Geriatr Res. 2010;2010:423087. doi: 10.1155/2010/423087. [DOI] [PMC free article] [PubMed] [Google Scholar]