Abstract
Cemento-ossifying fibroma (COF) is considered a benign osseous tumor. Herewith, we present a case of multiple central ossifying fibroma in a 35-yeaold woman. Intraorally, there was swelling in the left upper posterior teeth region and another diffused swelling in the fourth quadrant. Radiographs revealed the presence of well-defined mixed radiolucent–radiopaque area having thin radiolucent rim followed by thick sclerotic margin. No genetic correlation could be established. As bilateral COF is a rare entity, we present such a case with different radiographic appearance, using various radiographic techniques.
Keywords: Bilateral cemento-ossifying fibroma, case report, cemento-ossifying fibroma
Introduction
Ossifying fibroma was first reported in the jaw by Montgomery in 1927.[1] It is a bony tumor of the maxilla, possibly of odontogenic origin. Cemento-ossifying fibroma (COF) is considered a benign osseous tumor, very closely related to other lesions such as fibrous dysplasia, cementifying periapical dysplasia, or cemento-osseous florid dysplasia, however, creating its own entity in the 1992 WHO classification.[2] It is believed to be derived from the cells of the periodontal ligament. Consequently, one of its principal characteristics is the massive formation of cementum, cementoid substance, or calcified material in the interior of a predominantly fibrous.[3]
Case Report
A 35-year-old female patient reported to the department with a chief complaint of painless swelling on the left upper side of face since one and a half years. Initially, swelling was smaller in size, approximately 1 cm, and it grew slowly and has reached to its present size in last one and a half years. Patient was also suffering from difficulty in eating food.
Patient suffered from the same problem 15 years back in the left lower jaw region and was operated for the same problem. Half of the lower jaw of left side was removed. After fourteen and a half years, swelling reappeared in the left upper and right lower jaw regions.
Extraoral examination revealed that face of the patient was bilaterally asymmetrical with the diffused swelling roughly circular in shape measuring about 3 to 4 cm in its greatest dimension extending anteroposteriorly from the inner canthus of the eye to the angle of the mandible and superior-inferiorly from the infraorbital margin to the angle of the mouth. Color of the overlying skin was normal but had ill-defined borders [Figure 1].
Figure 1.
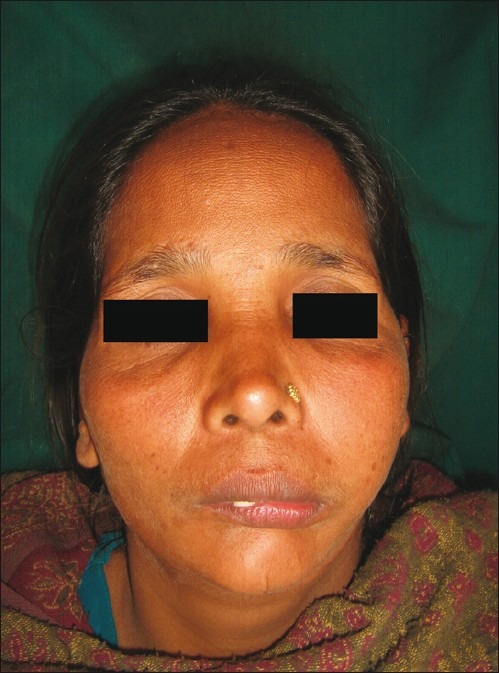
Extraoral photograph
On palpation, all inspectory findings were confirmed. Swelling was non-tender on palpation, hard in consistency, non-fluctuant, and had diffused borders, temperature of the overlying skin was not raised, TMJ was normal without any deviation, clicking sound, or tenderness. Submandibular lymph nodes on the left side were palpable, non-tender, firm in consistency, and mobile.
Intraoral examination showed solitary dome-shaped swelling measuring about 3 to 4 cm in its greatest dimension in the left upper posterior teeth region extending anteroposteriorly from the mesial surface of 24 to the retromolar area. Color of the overlying mucosa was normal. There was expansion of buccal and palatal cortical plates in relation to 24, 25, 26, 27, and 28. No vestibular obliteration was present in relation to 25, 26, 27, and 28 [Figure 2].
Figure 2.
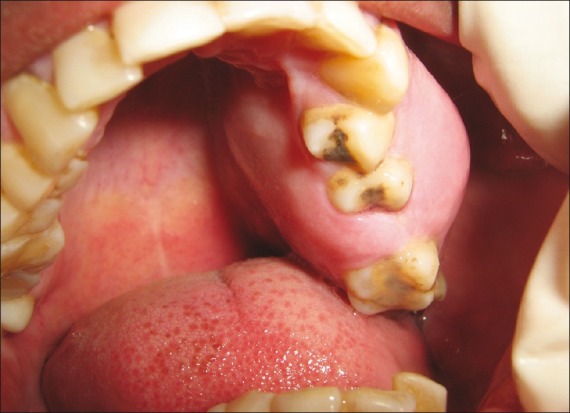
Intraoral swelling in the left maxillary alveolar ridge
Soft tissue examination for the lower arch revealed the presence of diffused swelling measuring about 1.5 to 2 cm in diameter in the fourth quadrant in relation to 44, 45, 46, and 47. Vestibular obliteration was present in relation to 43, 44, 45, 46, and 47 regions. Surface mucosa overlying the swelling was pale and stretched [Figure 3]. Left mandibular arch showed hemi- mandibulectomy [Figure 4].
Figure 3.
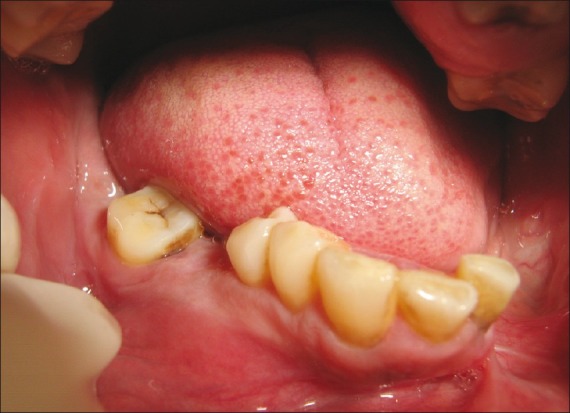
Intraoral swelling in the right mandibular alveolar ridge
Figure 4.
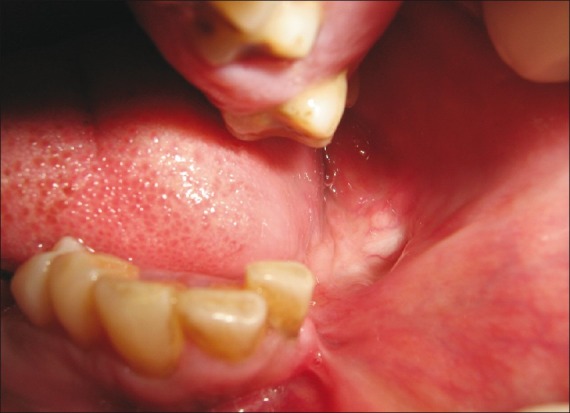
Hemimandibulectomy on the left side
On palpation, inspectory findings were confirmed. Swelling is non-tender on palpation, hard in consistency with well-defined borders. No vestibular tenderness and no pus discharge were evident. Cortex felt to be intact.
Hard tissue examination showed distally displaced teeth in relation to 26, 27, 28 and missing teeth in relation to 31 - 37 and 47. Other findings were attrition in relation to 41, 42, 43, and 47. The second quadrant teeth, i.e., 24-28, were non-tender on vertical percussion. Mobility was not detected in relation to any tooth. Generalized stains and calculus were present.
Considering the history and clinical examination, provisional diagnosis of Central Ossifying Fibroma of the left maxillary alveolar ridge and Residual cyst in relation to 46 was given.
Clinical Differential Diagnosis of Florid Cemento-Osseous Dysplasia, Central Odontogenic Fibroma , Osteoma, Paget's Disease, and Central Giant Cell Granuloma was considered.
Investigations such as the IOPAR of the left maxillary teeth region showed the presence of well-defined roughly spherical-shaped radiopacity with radiolucency at certain areas, having sharp radiolucent rim surrounded by sclerotic border standing mesially up to the root of 25 and distally up to the mesial root surface of 28. Lesion was applying pressure on the roots of 25, 26, 27, and 28 and had displaced the roots of 25 mesially and roots of 26, 27, and 28 distally. There was discontinuity of Lamina dura in relation to 25, 26, and 27 and vertical bone loss in relation to 24 and 25 [Figure 5].
Figure 5.
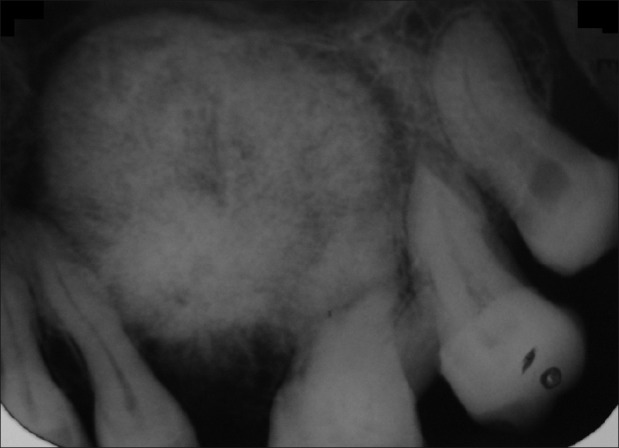
IOPAR showing well radiopacity surrounded by radiolucent rim followed by sclerotic border
IOPAR of the right mandibular teeth region revealed the presence of well-defined mixed radiolucent-radiopaque area extending mesially up to the distal root surface of 45, superiorly extending to the alveolar ridge, having thin radiolucent rim followed by thick sclerotic margin, with this margin thicker distally. There was discontinuity of lamina dura in relation to 45 and 47. IOPAR also showed the presence of radiolucent area around mesial surface of 47 involving the crown, and the root was suggestive of caries. Horizontal bone loss was evident in relation to 43-45 [Figure 6].
Figure 6.
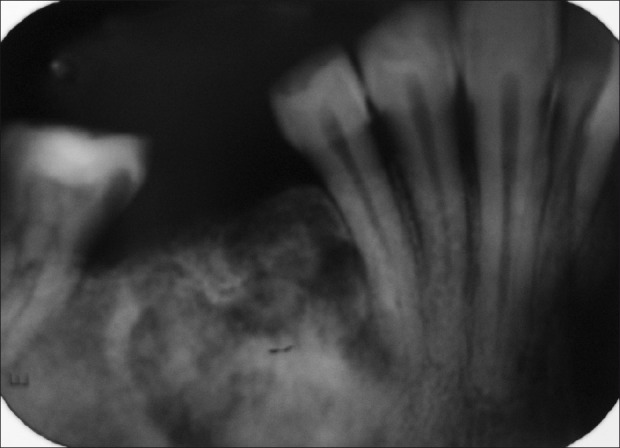
IOPAR showing mixed radiolucent-radiopaque region
Lateral cross-sectional Maxillary Occlusal Radiograph revealed the expansion of buccal and lingual cortical plate [Figure 7]. Mandibular cross-sectional radiograph showed the more uniform expansion of buccal and lingual cortical plate mimicking the cystic expansion [Figure 8].
Figure 7.
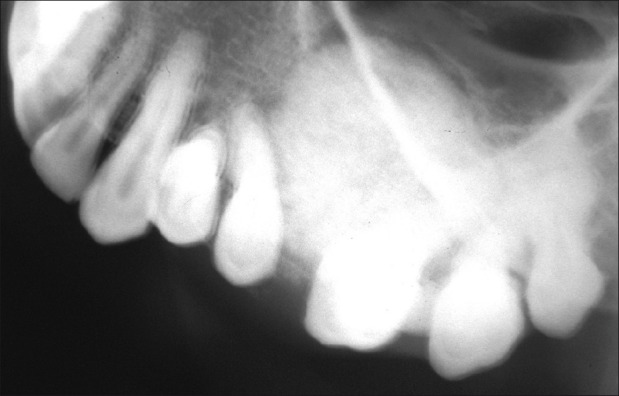
Maxillary occlusal radiograph showing expansion of buccal cortex
Figure 8.
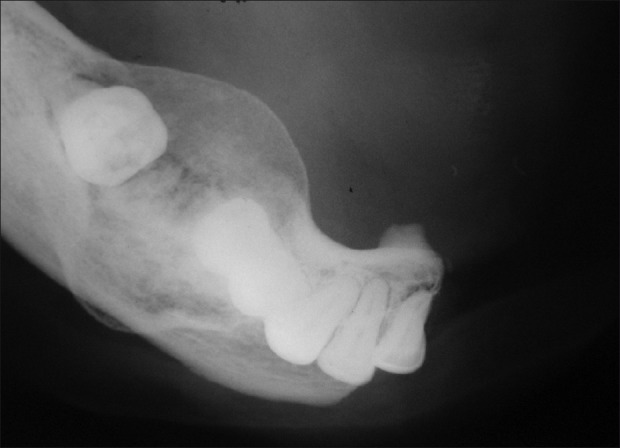
Mandibular occlusal radiograph showing expansion of both buccal and lingual cortex
OPG revealed the presence of well-defined roughly spherical-shaped radiopaque lesion with radiolucency at certain areas extending mesiodistally from the distal root surface of 25 to the mesial root surface of 28, having radiolucent rim surrounded by sclerotic border, which was thicker distally. Lesion had displaced floor of the maxillary sinus upward and was applying pressure over the roots of 25 - 28 and had displaced the roots of 25 mesially and roots of 26, 27, and 28 distally. Half of the mandibular jaw bone on the left side was missing. OPG also showed the presence of another mixed radiolucent–radiopaque lesion in relation to 43 - 47, extending mesially up to the distal root surface of 43 and distally up to the mesial surface of 47 having radiolucent margin followed by thick sclerotic border not involving the inferior alveolar canal [Figure 9].
Figure 9.
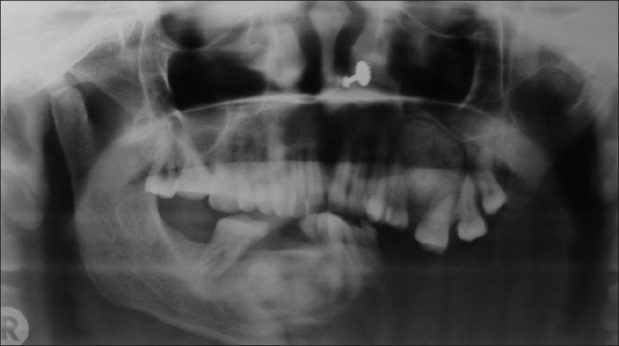
OPG showing well radiopacity surrounded by radiolucent rim followed by sclerotic border in the left maxillary alveolar ridge and mixed radiolucent-radiopaque region followed by radiolucent rim and by sclerotic border
PNS view was taken to see the involvement of sinuses which was not found, and was later confirmed by CT scan.
CT scan axial view showed hyper-attenuated mass measuring about 3 × 2 cm in diameter, involving the left maxillary alveolar ridge. There was no destruction of the cortex [Figure 10]. Coronal section showed the presence of iso-attenuated and hyper-attenuated mass roughly round in shape in relation to right mandibular alveolar ridge [Figure 11].
Figure 10.

Axial section of CT scan showing lesion in the maxillary alveolar ridge
Figure 11.
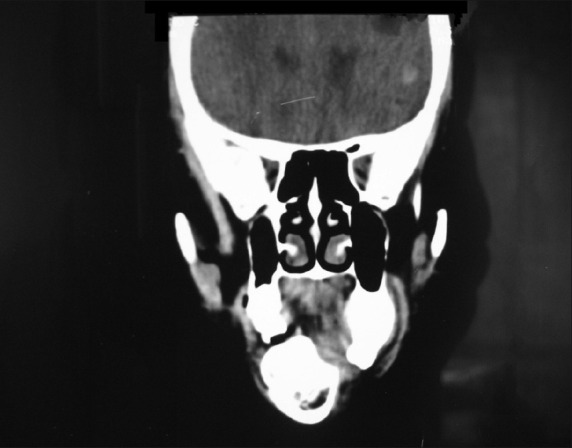
Coronal section of CT scan showing well-defined lesion in the right mandibular alveolar ridge
Radiographic Differential Diagnosis of Central ossifying fibroma, Florid cemento-osseous dysplasia, and Fibrous dysplasia was given.
Microscopic section showed the presence of fibro-cellular connective tissue with calcified material. Fibro-cellular connective tissue is composed of thick bundles of collagen fibers and large plump, proliferating fibroblast with eosinophilic cytoplasm and dark nucleus. The calcified material is composed of few irregular trabeculae of bone and numerous spherical-shaped cementum-like materials [Figure 12]. These findings are suggestive of cementifying/ossifying fibroma.
Figure 12.
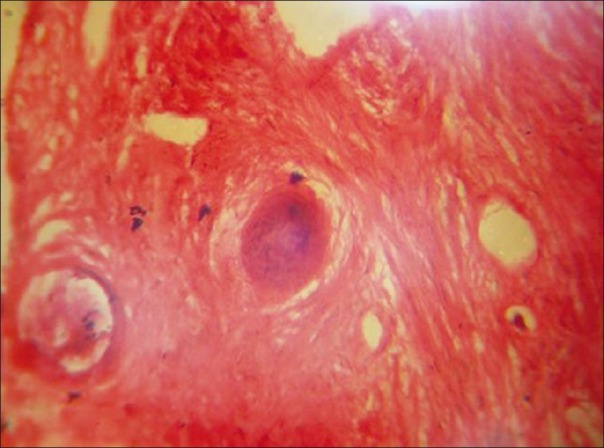
Histopathological picture
Considering the history clinical examination, radiographic investigation, and histopathological finding, final diagnosis of central cementifying/ossifying fibroma of left maxillary and right mandibular ridge and chronic generalized gingivitis was given.
Discussion
The term benign fibro-osseous lesion has been used in the literature to describe a spectrum of lesions ranging from fibrous dysplasia to ossifying fibroma, including cementifying or COF, psammomatoid ossifying fibroma, psammo-osteoid fibroma, juvenile or young ossifying fibroma, and juvenile active ossifying fibroma.[4] Ossifying fibroma is a rare, expansile, benign tumor that predominantly involves the maxillary (approximately 10–20% of cases) and mandibular (approximately 75%) bone.[1] In rare cases, the tumor may involve the nasal cavity and long bones. It is a bony tumor of the maxillas of possible odontogenic origin. It is believed to be derived from the cells of the periodontal ligament.[5] Consequently, one of its principal characteristics is the massive formation of cementum, cementoid substance, or calcified material in the interior of a predominantly fibrous.[3]
In 1872, Menzel gave the first description of variant of ossifying fibroma, called COF.[6] Ossifying fibroma was first reported in the jaw by Montgomery in 1927.[1] In 1968, Hamner and colleagues[7] proposed that ossifying fibroma, cement-ossifying fibroma, and ossifying fibroma are the histological variants originating from the periodontal ligament, although WHO designates the cementifying fibroma as odontogenic and the ossifying fibroma as nonodontogenic in origin and suggests that they are separate entities. Finally, it was concluded that separation of the three conditions is arbitrary because the clinical, radiologic, and prognostic features of the lesions are identical.
COF has been defined by WHO as a demarcated, or rarely encapsulated, neoplasm consisting of fibrous tissue containing varying amount of mineralized material resembling bone or cementum.[2]
Its synonyms are cementifying fibroma, COF, fibro-osteoma, osteofibroma, benign fibro-osseous lesion of periodontal ligament origin, and benign periodontoma.[8]
Etiopathogenesis may be traumatic or developmental.[3,9] It is generally thought to arise from PDL.[10] The connective tissue of the periodontal membrane harbors the potential for elaboration of both bone and cementum. Bernier and Thompson[10] speculated that infection with resulting inflammation and fibrosis of the periapical area might stimulate the periodontal membrane. After trauma, such as tooth extraction, the remaining periodontal tissue that is attached to the wall of the alveolus may serve as the origin of COF.
The fact that this tumor is most common in the jaws is related to the vast amount of mesenchymal cellular induction into bone (lamina dura) and cementum in odontogenesis; therefore, the probability of induction error or genetic alteration leading to a neoplasm is greater.[9]
Clinically, it is most commonly seen in third and fourth decade of life (56%) with the average age of 36 years. There is striking predilection feminine sex with a ratio of 5 : 1.[7,12] The mandibular premolar–molar area is the most common site.[9,12] The findings of the current case show all the features discussed above.
COF manifest it as slow-growing, asymptomatic,[12] intraosseous masses,[3] mostly detected incidentally during a routine radiographic survey.[2,13] Larger lesion grow more rapidly and extensive and could even provoke a mandibular fracture.[13] Infrequently, it may involve the jaws bilaterally or multiple quadrants.[10] Hamner and colleagues[7] found multiple lesions in some of their patients. In a series reviewed by Hauser et al,[14] 20% of the cases showed facial asymmetry, other findings included pain in four patients and numbness in two patients. Displacement of teeth is also seen in some cases[8] (as in the current case).
Radiographically, it has a striking predilection for the mandible ranging from 70% to 89%. Lesion appears limited to tooth-bearing area with an intimate relationship to the root of the teeth or periapical region. A few have extended to angle-ramus area or encroached on the maxillary sinus. Eversole and colleagues reported the following location: molar region, 52%; premolar area, 25%; incisor area, 13%; and cuspid region, 11%.[8] Radiographically, the COF presents as a well-defined unilocular or multilocular lesion with smooth contours. The maturity of the lesion determines the degree of radiopacity; the immature lesion may present as completely radiolucent, whereas the mature lesion may appear completely radiopaque.[12]
Lesion shows mixed radiolucent-radiopaque appearance. Hauser et al.[14] reported that 26% were lytic type, 63% were lytic with radiopaque foci, and 12% consisted of a diffuse, homogenous appearance that was mildly radiopaque (ground glass appearance).[4] Marginal area of the mass is radiolucent with radiopaque foci. A sclerotic rim sometimes is present within the host bone at the margin; it may be smooth and delicate or it may be slightly irregular, more diffuse, and of varying thickness up to approximately 3 mm. In some cases, lesion may be “punched out” with no sclerosis at the margin. Smaller lesions may be less well defined.[1] The expanded cortex is very thin and may seem to disappear on plain radiographs. Diameter of the lesion ranges from 1 to 7 cm. Divergence of adjacent roots (17%) and root resorption (11%) may also be seen. Displacement of developing teeth may also be seen.[13] In the current case, migration of root tips was observed.
Typical ossifying fibroma grows in centrifugal fashion producing ball-like circular lesion. Lesion enlarges equally in all direction producing expansion of buccal and lingual cortical plate and most notably the inferior cortex. Expanded inferior cortex is parallel to margin of tumor mass above. Inferior bowing of the lower border of the mandible is almost a constant feature in larger lesions.[15,16]
Pathologic examination of the central COF shows a proliferation of irregularly shaped calcifications within a hypercellular fibrous connective tissue stroma. The calcifications are extremely variable in appearance and represent various stages of bone and cementum deposition.[17,18] Histologic differentiation between osteoid and cementum is difficult. In some cases, most of the calcified fragments are immature cementum, with basophilic coloration on hematoxylin and eosin-stained sections. These tumors have been named central cementifying fibroma. In other cases, the calcified fragments are osteoid, with typical eosinophilic coloration on hematoxylin and eosin-stained sections. These tumors have been named central ossifying fibromas.[11]
Conclusion
Central ossifying fibromas are slow-growing, ovoid or round-shaped, and well-demarcated bone tumors. They may appear at any age, especially in white adult female patients, usually in the mandible. Radiographically, most of them are very well-circumscribed mixed lesions. Expansion of the inferior border of the mandible and well-defined radiographic borders seem to be the most common manifestations of central ossifying fibroma. No resorption of the roots is usually present. Regarding the histology, trabecular structures are more common than cementicle-like masses. Sometimes, a fibrous capsule is noted.
The differential diagnosis includes benign and malignant fibro-osseous lesions and odontogenic cysts and tumors. Histologically, it is usually not confused with other lesions, except fibrous dysplasia. Correlation of histology with radiographic studies and clinical presentation is needed for final diagnosis. Treatment may be surgical excision or radiotherapy (ineffective and contraindicated). Mandibular central COF shows a recurrence rate of 28%. Early diagnosis will circumvent the necessity of radical treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Montgomery AH. Ossifying fibroma of the jaw. Arch Surg. 1927;15:30–44. [Google Scholar]
- 2.Tamiolakis D, Thomaidis V, Tsamis I, Lambropoulou M, Alexiadis G, Seretis K, et al. Cementifying-ossifying fibroma of the maxilla: A case report. Internet J Dent Sci. 2005;2:2. [Google Scholar]
- 3.Sanchis JM, Peñarrocha M, Balaguer JM, Camacho F. Cemento-ossifying mandibular fibroma: A presentation of two cases and review of the literature. Med Oral. 2004;9:69–73. [PubMed] [Google Scholar]
- 4.Fırat Y, Fırat AK, Karakas HM. A case of frontal lobe abscess as a complication of frontal sinus ossifying fibroma. Dentomaxillofac Radiol. 2006;35:447–50. doi: 10.1259/dmfr/28037887. [DOI] [PubMed] [Google Scholar]
- 5.Brademann G, Werner JA, Janig U, Mehdorn HM, Rudert H. Cementoossifying of the premastoid region: Case report and review of the literature. J Larygol Otol. 1997;111:152–5. doi: 10.1017/s0022215100136709. [DOI] [PubMed] [Google Scholar]
- 6.Menzel A. Ein fail von osteofibroma des unterkiefers Lengenbecks. Arch Klin Chir. 1872;13:212. [Google Scholar]
- 7.Hamner JE, 3rd, Lightbody PM, Ketcham AS, Swerdlow H. Cemento-ossifying fibroma of the maxilla. Oral Surg Oral Med Oral Pathol. 1968;26:579–87. doi: 10.1016/0030-4220(68)90341-1. [DOI] [PubMed] [Google Scholar]
- 8.Eversole LR, Merrell PW, Strub D. Radiographic characteristics of central ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1985;59:522–7. doi: 10.1016/0030-4220(85)90096-9. [DOI] [PubMed] [Google Scholar]
- 9.Jung SL, Choi KH, Park YH, Song HC, Kwon MS. Cemento-ossifying fibroma presenting as a mass of the parapharyngeal and masticator space. AJNR Am J Neuroradiol. 1999;20:1744–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Neville BW, Damm DD, Allen CM, Bouquot J. Oral and maxillofacial pathology. 2nd ed. Philadelphia: Saunders; 2009. [Google Scholar]
- 11.Bernier JL, Thompson HC. The histogenesis of the cementoma. Am J Orthod Oral Surg. 1946;32:543–55. doi: 10.1016/0096-6347(46)90078-6. [DOI] [PubMed] [Google Scholar]
- 12.Dalghous A, Alkhabuli JO. Cemento-ossifying fibroma occurring in an elderly patient. A case report and a review of literature. Libyan J Med. 2007;2:95–8. doi: 10.4176/061220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx RE, Stern D. Oral and Maxillofacial Pathology. ed 2nd. Illinois Quintessence Pub. Co; 2003. [Google Scholar]
- 14.Hauser MS, Freije S, Payne RW, Timen S. Bilateral ossifying fibroma of the maxillary sinus. Oral Surg Oral Med Oral Pathol. 1989;68:759–63. doi: 10.1016/0030-4220(89)90168-0. [DOI] [PubMed] [Google Scholar]
- 15.White SC, Pharoah MJ. Oral Radiology: Principles and interpretation. 5th ed. Maryland Heights, Missouri: Mosby; 2004. [Google Scholar]
- 16.Rosenberg A, Mokhtari H, Slootweg PJ. The natural course of an ossifying fibroma. A case report. Int J Oral Maxillofac Surg. 1999;28:454–6. [PubMed] [Google Scholar]
- 17.Mintz S, Velez I. Central ossifying fibroma: An analysis of 20 cases and review of the literature. Quintessence Int. 2007;38:221–7. [PubMed] [Google Scholar]
- 18.Su L, Weathers DR, Waldron CA. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifyng fibromas, A Pathologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:301–9. doi: 10.1016/s1079-2104(97)90348-6. [DOI] [PubMed] [Google Scholar]


