Abstract
An 11-year-old, 24-kg, intact female Siberian husky dog in anestrus had a 2-month history of polyuria and polydipsia. The dog had signs of mineralocorticoid excess such as hypertension and hypokalemia refractory to potassium supplementation. Abdominal ultrasound revealed an irregular mass in the left adrenal gland. The ACTH stimulation test for aldosterone concentration did not reveal hyperaldosteronism. Unilateral adrenalectomy was performed and histopathology identified adrenal cortical carcinoma. All clinical signs of mineralocorticoid excess ceased after surgery.
Résumé
Carcinome corticosurrénal unilatéral fonctionnel chez un chien. Une chienne Husky sibérien intacte et âgée de 11 ans, pesant 24 kg, en anœstrus, avait une anamnèse de 2 mois de polyurie et de polydipsie. La chienne présentait des signes d’excédent minéralocorticoïde, comme l’hypertension et l’hypokaliémie, réfractaire à la supplémentation en potassium. Une échographie abdominale a révélé une masse irrégulière dans la glande surrénale gauche. Le test de simulation d’ACTH pour la concentration d’aldostérone n’a pas révélé l’hyperaldostéronisme. Une adrénalectomie unilatérale a été réalisée et l’histopathologie a identifié un corticosurrénalome. Tous les signes cliniques d’excédent minéralocorticoïde ont cessé après la chirurgie.
(Traduit par Isabelle Vallières)
A drenocortical tumors are divided into adenomas and carcinomas. It is estimated that most of them (up to 80%) are malignant adenocarcinomas. These tumors may or may not be endocrinologically active (1,2). In most dogs, the hormone produced and secreted by these tumors is cortisol. Yet, in some cases adrenocortical tumors produce mineralocorticosteroids, mainly aldosterone, or, very rarely, its active precursor deoxycorticosterone (3,4). In this report we describe a dog with unilateral adrenocortical carcinoma and recumbency, hypertension, and hypokalemia as the major clinicopathological signs.
Case description
An 11-year-old, 24-kg, intact female Siberian husky dog in anestrus was presented to the Centre of Small Animal Health, Clinic Multiwet, with a 2-month history of polyuria, polydipsia, apathy, stiffness of the neck and limbs, reluctance to move, weight loss, pseudoanorexia resulting from reluctance to eating because of limb pain or weakness (the dog was fed by the owner in the prone position) and several diagnoses such as diabetes insipidus, renal failure, and liver damage. Diagnoses were based on polyuria and polydipsia, decrease in urine specific gravity (1.008), increase in urea concentration (20 mmol/L), and increased alanine aminotransferase activity (228 U/L). An electrocardiogram (ECG) obtained 2 wk earlier by the referring veterinarian did not reveal any abnormalities. The referring veterinarians had administered hydrochlorothiazide, and placed the dog on commercial renal diet and hepatic diet. Eventually, the referring veterinarians were unable to diagnose the disease, considered the prognosis to be unfavorable and, owing to progressive weakness and lack of response to the treatment, recommended euthanasia.
On presentation to the Centre of Small Animal Health, Clinic Multiwet, physical examination of the dog revealed recumbency, weakness, limb tremors, tactile and muscular hyperesthesia, normal or mildly increased spinal and cranial reflexes, tachycardia (180 beats/min), tachypnea (60 breaths/ min), and hypertension (systolic arterial pressure 190 mmHg). Temperature was within the reference interval (RI) (38.0°C). The mucous membranes and the skin were normal. The dog was not dehydrated.
Initial diagnostic tests included a complete blood (cell) count (CBC), serum biochemical profile, electrolyte analysis, urinalysis, chest and spine X-ray examination, and abdominal ultrasound examination. Results from the CBC, serum biochemical profile, and electrolyte analysis revealed thrombocytosis (629 × 109/L; RI: 200 to 580 × 109/L), increased alanine aminotransferase (ALT; 380 U/L, RI: 3 to 50 U/L), aspartate aminotransferase (AST; 470 U/L, RI: 1 to 37 U/L), and alkaline phosphatase (ALP; 230 U/L, RI: 20 to 155 U/L) activity, mildly increased blood urea concentration (22.9 mmol/L; RI: 7.1 to 16.1 mmol/L), and hypokalemia (2.5 mmol/L; RI: 4.1 to 5.4 mmol/L). Urinalysis revealed proteinuria (1.46 g/L), and a protein/creatinine ratio of 1.3. Urine specific gravity was 1.010. The X-ray examination revealed mild thoracic spondylosis. Abdominal ultrasound examination showed an irregular mass (3.6 × 2.8 cm) in the left adrenal gland. The right adrenal gland was normal (diameter 0.5 cm). The ultrasonography did not show any other abnormalities.
Suspicion of primary hyperaldosteronism was based on the results of the clinical examination, electrolyte analysis, and abdominal ultrasound examination.
For initial treatment lactated Ringer’s solution (Solutio Ringeri Lactate Fresenius; Fresenius Kabi Polska Sp. z o, Warsaw, Poland), 30 mL/kg body weight (BW)/d with potassium supplementation (Kalium Chloratum WZF 15%; Warszawskie Zaklady Farmaceutyczne Polfa S.A., Warsaw, Poland), 0.15 mmol/kg BW/h, IV were administered. Concurrently the dog was given amlodipine (Amlozek; Adamed Sp. z o.o., Pienków, Poland), 0.1 mg/kg BW/d. The next day urinalysis with urine cortisol/creatinine ratio, electrolyte analysis, and ACTH stimulation test (Tetracosactide, Synacthéne 0.25 mg/mL; Sigma-Tau S.A.R.L., Paris, France), 0.25 mg IM were performed. Urinalysis revealed specific gravity 1.009, protein/creatinine ratio 1.3, and cortisol/creatinine ratio 6.8. Plasma baseline and ACTH-stimulated cortisol concentration both were normal (97 nmol/L; RI: 27.6 to 165.5 nmol/L; and 259 nmol/L; RI: 220 to 550 nmol/L, respectively). Plasma baseline aldosterone was surprisingly within the reference range (77.6 pmol/L; RI: 14 to 956 pmol/L) and after ACTH stimulation test amounted to 99.7 pmol/L. The hypokalemia (2.7 mmol/L) persisted. The dog was still recumbent and weak. Systolic arterial pressure decreased to 170 mmHg.
The potassium supplementation was increased to 0.2 mmol/kg BW/h because of the persistent hypokalemia, and treatment with lactated Ringer’s solution and amlodipine was continued.
On the third day of hospitalization the dog was still weak and recumbent. The systolic blood pressure was 160 mmHg. Hypokalemia (2.5 mmol/L) was still present despite the increased potassium supplementation. Then, spironolactone (Spironol; Gedeon Richter Polska Sp. z o.o., Grodzisk Mazowiecki, Poland), 2 mg/kg BW, q24h was administered, and potassium supplementation and treatment with lactated Ringer’s solution and amlodipine were continued.
After the next 2 d of therapy the dog was able to stand and eat. Systolic blood pressure decreased to 120 mmHg and plasma potassium concentration increased to 2.8 mmol/L. After 6 days of therapy with spironolactone and potassium supplementation normokalemia (4.2 mmol/L) was achieved.
Based on the results of the clinical examination, electrolyte analyses, abdominal ultrasound, ACTH stimulation test, and reaction to treatment, we suspected that there was a deoxycorticosterone excess. However, we were not able to determine the concentration of serum deoxycorticosterone because of lack of commercial tests for this mineralocorticoid by veterinary laboratories in Europe. Thus, we recommended unilateral adrenalectomy and histopathological examination of the mass in the adrenal gland.
Laparotomy was performed on the 8th day of hospitalization. A 3.5 × 4.5 cm well-defined adrenal left gland was identified. The tumor did not invade the vena cava. Unilateral adrenalectomy was performed. The left adrenal gland was excised and sent to the laboratory for histopathological examination. There were no visible nodules in the liver. Dexamethasone (Dexasone; ScanVet, Poland Sp. z o.o., Gniezno, Poland), 0.1 mg/kg BW was given intravenously just after the adrenalectomy (before the end of the surgery). After the surgery, dexamethasone was administered intramuscularly every 12 h at a dose of 0.1 mg/kg BW for the first 2 d, and at 0.05 mg/kg BW for the next 2 days. Concurrently, after surgery, lactated Ringer’s solution (30 mL/kg BW/d) with potassium supplementation (0.2 mmol/kg BW/h) was administered intravenously for 4 d. The dog also received tramadol (Poltram 50; Zaklady Farmaceutyczne POLPHARMA S.A., Starogard Gdanski, Poland), 4 mg/kg BW, q6h subcutaneously (SC) for 2 d and amoxicillin with clavulinic acid (Synulox injectable; Pfizer Polska Sp. z o.o., Warsaw, Poland), 12.5 mg/kg BW/d, SC for 7 d because of fever (39.6°C). Fever ceased after 24 h. A day after surgery, the dog started vomiting, had hypertension (systolic blood pressure 210 mmHg) and a potassium concentration below the reference interval (3.0 mmol/L). Maropitant citrate (Cerenia; Pfizer Polska Sp. z o.o.), 1 mg/kg BW/d was given SC for 3 d because of the vomiting. The administration of maropitant citrate allowed the use of spironolactone (2 mg/kg BW/d) and amlodipine (0.1 mg/kg BW/d) orally.
The potassium concentration normalized gradually over 4 d and the blood pressure returned to normal by the 6th day after surgery (4.7 mmol/L and 120 mmHg, respectively). On the 4th day after surgery the dog was able to stand, walk, and had a good appetite. Potassium supplementation was discontinued on the 5th day after surgery, and the values for potassium concentration, measured every 24 h for the next 5 d, were 4.8, 4.7, 4.8, 5.0, and 5.1 mmol/L. After 10 days (following the surgery) the doses of spironolactone and amlodipine were gradually reduced (by 0.5 mg/kg and 0.025 mg/kg, respectively every 7 d). Potassium concentration and blood pressure were measured every 7 days (a day after the dosage reduction). The measurements were within normal values and the dog was in good condition. No clinical signs of mineralocorticoid excess were evident.
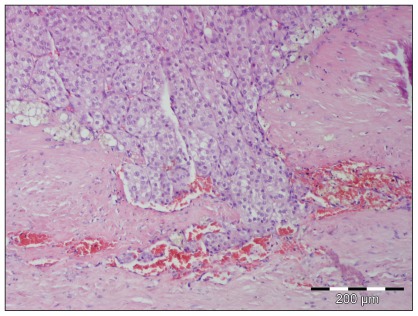
The histopathology identified an adrenal cortical carcinoma with inconclusive margin, capsular invasion, and tumor cell nests next to vascular spaces (Figure 1).
Figure 1.
Photomicrograph of a tumor from the left adrenal gland. Neoplastic cells are in the upper part of the figure, and normal tissue is at the bottom and right parts of the figure. Photomicrograph kindly provided by Dr. Martin Busch from Department of Histology, Vet Med Labor GmbH, Division of IDEXX Laboratories.
Five weeks after surgery, CBC, serum biochemical profile, electrolyte analysis, urinalysis, abdominal ultrasound and chest X-ray examination, and ACTH stimulation test for cortisol concentration were performed. The tests did not reveal any abnormalities except a mildly increased ALT activity (65 UI/L). There was no proteinuria or hypokalemia and metastases were not detected by radiography or ultrasonography. Urine specific gravity was normal (1.019). Systolic blood pressure was normal (130 mmHg). Plasma baseline and ACTH-stimulated cortisol concentration were both normal (113 nmol/L and 249 nmol/L). On physical examination the dog was in good condition and vital signs were normal. Four months later the same tests and the ACTH stimulation test for aldosterone, and abdominal ultrasound and chest X-ray examinations were performed. The tests again did not reveal any abnormalities, except for the ACTH stimulation test for aldosterone. Plasma baseline aldosterone was within reference range (19 pmol/L) and after ACTH stimulation amounted to only 26 pmol/L. No metastases were evident by radiography or ultrasonography. The dog was still in good condition and her weight was 29 kg.
Discussion
The dog had signs typical for mineralocorticoid excess, such as hypertension and hypokalemia refractory to potassium supplementation, but the ACTH stimulation test for aldosterone concentration did not indicate hyperaldosteronism. The ACTH stimulation test for cortisol concentration and the urine cortisol/ creatinine ratio allowed us to exclude glucocorticosteroid excess. The authors suspected deoxycorticosterone excess based on the results of histopathology, the ACTH stimulation test for aldosterone concentration, signs typical for mineralocorticoid excess, and good response to therapy with spironolactone and unilateral adrenalectomy. A similar case was described by Reine et al (4); however, in that case the authors were able to determine the concentration of deoxycorticosterone, which was almost 17 times higher than in a control group of 8 healthy dogs. Cases of deoxycorticosterone excess without an increase in plasma aldosterone concentration have also been observed in humans, who had hypertension and hypokalemia resulting from functioning adrenal tumors (5,6).
The results of the ACTH stimulation test for aldosterone concentration before and after adrenalectomy was similar to those reported by Reine et al (4). The authors of that case report also observed elevated deoxycorticosterone concentration even after adrenalectomy. Thus, it seems possible that in the present case, as in other reports, endocrinologically active metastases were present, especially in the liver and lungs (3). Therefore, the owner of the dog was advised to submit the dog to regular abdominal ultrasound and chest X-ray examinations, and electrolyte analysis.
The case reported here is one of a dog with adrenocortical carcinoma with typical signs of mineralocorticoid excess without elevated aldosterone concentration. Such cases are rare, and are caused by functioning adrenocortical tumors producing active precursors of aldosterone such as deoxycorticosterone. In this case the authors found a way of proceeding in veterinary practice in the absence of commercially available tests for deoxycorticosterone concentration or renin activity. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bailey DB, Page RL. Tumors of the Endocrine System. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 4th ed. St Louis, Missouri: Saunders Elsevier; 2007. pp. 583–609. [Google Scholar]
- 2.Galac S, Reusch CE, Kooistra HS, Rijnberk A. Adrenals. In: Rijnberk A, Kooistra HS, editors. Clinical Endocrinology of Dogs and Cats, An Illustrated Text. 2nd ed. Hannover, Germany: Schlütersche Verlagsgesellschaft; 2010. pp. 93–154. [Google Scholar]
- 3.Feldman EC, Nelson RW. Canine hyperadrenocorticism (Cushing’s syndrome) In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. 3rd ed. St. Louis, Missouri: Saunders; 2004. pp. 252–357. [Google Scholar]
- 4.Reine NJ, Hohenhaus AE, Peterson ME, Patnaik AK. Deoxycorticosterone-secreting adrenocortical carcinoma in a dog. J Vet Intern Med. 1999;13:386–390. doi: 10.1892/0891-6640(1999)013<0386:dsacia>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa SE, Saito T, Kaneko K, Okada K, Fukuda S, Kuzuya T. Hypermineralocorticism without elevation of plasma aldosterone: Deoxycorticosterone-producing adrenal adenoma and hyperplasia. Clin Endocrinol (Oxf) 1988;29:367–375. doi: 10.1111/j.1365-2265.1988.tb02885.x. [DOI] [PubMed] [Google Scholar]
- 6.Kondo K, Saruta T, Saito I, Yoshida R, Maruyama H, Matsuki S. Benign desoxycorticosterone-producing adrenal tumor. JAMA. 1976;236:1042–1044. [PubMed] [Google Scholar]