Abstract
Osteoporosis is a condition of impaired bone strength that results in an increased risk of fracture. The current and most popular pharmacological options for the treatment of osteoporosis include antiresorptive therapy, in particular, oral bisphosphonates (alendronate, risedronate, ibandronate). Anabolic agents like teriparatide have widened our therapeutic options. They act by directly stimulating bone formation and improving bone mass quantity and quality. Two forms of recombinant human parathyroid hormone (PTH) are available : full-length PTH (PTH 1–84; approved in the EU only) and the 1–34 N-terminal active fragment of PTH (teriparatide, US FDA approved). This review aims to discuss the benefits of teriparatide beyond the currently licensed indications like fracture healing, dental stability, osteonecrosis of jaw, hypoparathyroidism, and hypocalcemia.
Keywords: Forsteo, osteoporosis, parathormone, teriparatide
INTRODUCTION
Osteoporosis is a condition of impaired bone strength that results in an increased risk of fracture, most importantly of the spine and hip, and its incidence increases with increasing age. Unfortunately, many individuals with osteoporosis are not diagnosed with the disease until a fracture occurs. Osteoporosis is usually diagnosed by assessing bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA). It is defined by the World Health Organization (WHO) criteria as a BMD T-score of 2.5 standard deviations (SD) or more below the average for young, healthy, premenopausal women [equating to a T-score of less than or equal to -2.5 (a normal T-score is greater than -1)].[1–3]
Current pharmacological options for the treatment of osteoporosis include antiresorptive agents (e.g. bisphosphonates, calcitonin, and raloxifene), antiresorptive agent with anabolic activity (strontium ranelate; not approved for use in the US), and osteoanabolic agents (teriparatide). Bisphosphonates are by large the most popularly prescribed anti-osteoporotic agents. They can increase the BMD by 4–8% by inhibiting bone resorption. This is far from sufficient, as patients with osteoporosis have lost more than 25% of their skeletal mass below the young adult mean. It, therefore, only makes sense that an osteoanabolic be used, as it provides the maximum gains with regard to BMD (7–14%).[4–9]
Anabolic agents like teriparatide have widened our therapeutic options. They act by directly stimulating bone formation and improving bone mass and quality, thereby reducing fracture rates significantly. It is the only anabolic agent currently approved for the treatment of post-menopausal osteoporosis, idiopathic or hypogonadal osteoporosis in men, and glucocorticoid-induced osteoporosis.[4–9] Its indications are growing and teriparatide is being tried virtually in any indication that requires bone growth, be it fracture healing or dental stability.
TERIPARATIDE
Human parathyroid hormone (PTH) is an 84-amino acid peptide produced by the parathyroid glands and is involved in maintenance of calcium and phosphate homeostasis. It is secreted in response to low serum calcium levels and acts directly to increase the release of calcium from the bone, increase renal tubular calcium reabsorption, and enhances intestinal calcium absorption by formation of 1,25-dihydroxyvitamin D.
Two forms of recombinant human PTH are available: Full-length PTH (PTH 1–84; Preotact 1, approved in the EU only) and the 1–34 N-terminal active fragment of PTH (teriparatide, Forteo™, Forsteo™).[2,9,10] Most clinical trials have used the 1–34 N-terminal recombinant fraction of PTH (teriparatide), from initial concerns of stimulating PTH-mediated bone resorption with the full-length 1–84 PTH molecule. A few independent studies using the full-length 1–84 PTH fraction, however, have shown similar benefits on BMD, comparable to the 1–34 PTH fraction.[11]
Teriparatide is a recombinant formulation of endogenous PTH, containing a 34 amino acid sequence [Figure 1], which is identical to the N-terminal portion of this hormone. The pharmacologic activity of teriparatide is similar to the physiologic activity of PTH. The full-length PTH 1-84 peptide contains both the N-terminal and C-terminal regions of the hormone. The N-terminal region binds to PTH receptor 1 which is believed to confer all biological actions of PTH. The C-terminal region binds to another receptor (CPTHR), which responds exclusively to the C-terminal of PTH.[12] It has been shown that the C-terminal fragments may have discrete biological properties acting on the osteocyte to enhance its apoptosis,[13] accounting for the rationale for creating the N-terminal 1–34 PTH fraction.
Figure 1.
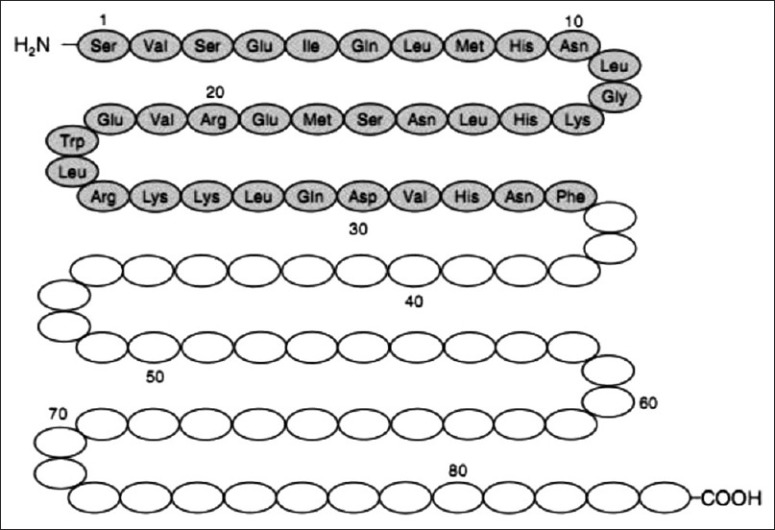
Human parathyroid hormone consists of 84 amino acids. Teriparatide represents the N-terminal portion of the first 34 amino acids. This fragment is believed to confer all the biological actions of the full-length peptide[14]
A continuous endogenous production of PTH (as in hyperparathyroidism) has a deleterious effect on the skeleton, but intermittent exposure to PTH (as in teriparatide use) leads to an increase in the number and activity of osteoblasts, leading to improved bone mass and skeletal architecture.[15]
After subcutaneous injection, PTH analogues are absorbed rapidly. The bioavailability of PTH 1–34 and PTH 1–84 is 95 and 55%, respectively.[16] The half-life after subcutaneous injection for PTH 1–34 is approximately 1 hour and that for PTH 1–84 is approximately 2.5 hours. The duration of action of PTH 1–84 is longer.[17]
ROLE AS AN OSTEOANABOLIC
Teriparatide leads to a rapid increase in bone formation markers, followed sometime thereafter by increases in bone resorption markers.[6] PTH is likely to first stimulate bone formation, and only later promotes the processes associated with bone remodeling in which bone resorption predominates, and hence the concept of the “anabolic window” [Figure 2] which is a period of time when the actions of PTH are maximally anabolic.[18]
Figure 2.
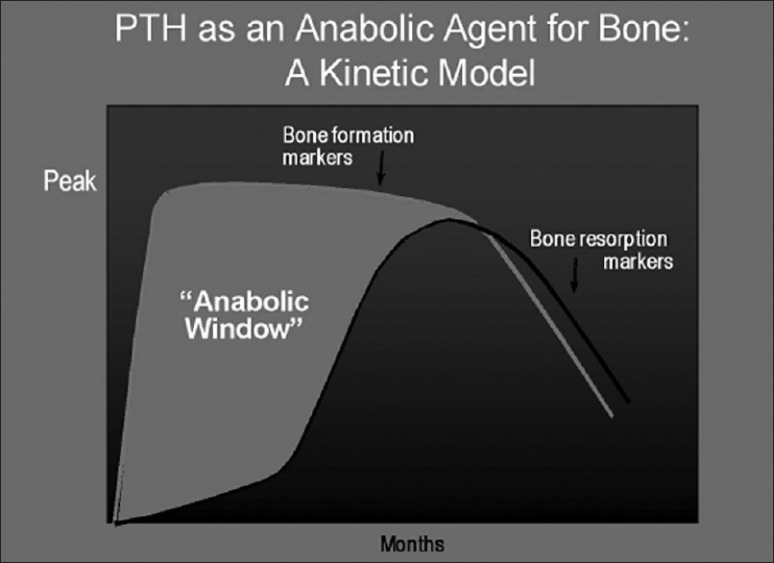
The anabolic window. Based upon the difference in kinetics of changes between bone formation and bone resorption markers, an “anabolic window” is formed during which the actions of parathyroid hormone are believed to be maximally anabolic
The beneficial effects of teriparatide on bone density, microarchitecture, and geometry are seen in both cancellous and cortical bones.[19] In cortical bones, it increases porosity in the inner one-third of bone, where the mechanical effect is minimal, and also improves bone microarchitecture and geometry at these sites. PTH stimulates periosteal apposition, which leads to an increase in cortical area, cortical thickness, and causes an overall increase in cross-sectional area.[20]
Teriparatide is licensed for use at a dose of 20 mcg/ day for approximately 18 months and no longer than 24 months. Absolute contraindications include primary and tertiary hyperparathyroidism, elevated alkaline phosphatase of uncertain cause, Paget's disease, open epiphysis in children, osteosarcoma, pregnancy, lactation, end-organ failure, metastatic skeletal malignancy and prior skeletal irradiation.[21]
When used alone, teriparatide results in an increase in BMD at the level of spine and hip but not at the radius. In the fracture prevention trial, BMD was shown to be increased by 9% more in recipients of teriparatide.[22] Teiparatide 20 mcg/day was compared to alendronate 10 mg/day over 30 months in a small study which showed that BMD increased by 25.8% at the lateral spine, 9.7% at the femoral neck, and 6.4% at the total hip in the teriparatide group, which were significantly greater than the values obtained in the alendronate group (11.1, 3.2, and 4.8%, respectively).[23] In another study that compared the efficacy of zoledronic acid with teriparatide in postmenopausal women, it was seen that the efficacy of teriparatide was greater than that of zoledronic acid at the level of the spine only.[24] Kung et al. showed that vertebral BMD values significantly increased from baseline to a greater extent with teriparatide 20 mg/ mL than with calcitonin.[25]
USES BEYOND CURRENTLY LICENSED INDICATIONS
It is well established that PTH, acting via the type 1 PTH receptor, induces matrix synthesis and suppresses maturation of chondrocytes. A study was done to test whether teriparatide would inhibit aberrant chondrocyte maturation and associated articular cartilage degeneration. Teriparatide was administered to mice after meniscal/ligamentous injury and knee joints were harvested at 4, 8, or 12 weeks after injury to examine the effects of teriparatide on cartilage degeneration and articular chondrocyte maturation. It was shown that teriparatide increased bone volume within joints and inhibited articular cartilage degeneration compared to controls.[26]
FRACTURE HEALING
Fracture involves healing via the following five stages of which 1–4 stages occur simultaneously: An immediate inflammatory response to injury; localized intramembranous bone formation induced at the periosteal surface adjacent to the fracture site; stabilization of the fracture site and re-establishment of mechanical stability and utility via endochondral bone formation in which the initial chondrogenic phase develops a mineralized callus providing initial stabilization of the fracture; osteoblast-mediated new bone formation that leads to the re-establishment of load-bearing function; and lastly bone remodeling leading to restoration of the original structural and geometric configurations of the bone.[27]
Preclinical studies on rat models have looked at the potential of PTH on fracture healing and spine fusion, and it has been shown that supra-physiological doses of PTH demonstrate increased fracture site strength and callus quantity,[28] with greater mineralization at the fracture site. In a study done on mice with closed femoral fractures, saline or PTH was administered for 14 days after the fracture with, PTH treatment induced a larger callus cross-sectional area, length, and total volume compared to the controls. Molecular analysis of the expression of extracellular matrix genes associated with chondrogenesis and osteogenesis showed that PTH treated fractures displayed a threefold greater increase in chondrogenesis relative to osteogenesis over the course of repair. This indicates that teriparatide treatment may influence both osteogenic and chondrogenic cell line development and activity.[29]
Many case reports of fracture healing with teriparatide use have been published. A summary of fracture healing by teriparatide is as follows:
Atypical subtrochanteric femoral fractures associated with use of alendronate wherein the authors demonstrated much improved healing and relief from use of narcotic analgesics within 1 year of continuous use of teriparatide.[30]
Adult hypohposphatasia (HPP) is characterized by low serum alkaline phosphatase (S-ALP), defective bone and tooth mineralization, and poorly healing fractures due to ALPL gene mutations. The authors demonstrate the effective use of 1–84 full-length PTH for adult HPP patients with longstanding, non-healing fractures.[31]
Distal radius fractures: The authors showed that teriparatide possibly appeared to improve early callus formation in distal radial fractures.[32]
Odontoid fractures: The authors reported patients with type III odontoid fractures with substantial, activity-limiting neck pain, which failed to unite despite external immobilization. Use of teriparatide resulted in a remarkable resolution of chronic neck pain and union of the fractures.[33]
Sternal fractures: Sternal fractures associated with non-union, although rare, are particularly onerous for the patient. The authors described the successful use of teriparatide in this situation.[34]
Atrophic femur shaft fracture with nonunion.[35]
Clinical studies have shown mixed results, and only one randomized, double-blind, placebo-controlled study of teriparatide in fracture healing has been published.[36] In this study, postmenopausal women with a dorsally angulated distal radial fracture in need of closed reduction but no surgery were randomized to 8 weeks of teriparatide 20 mcg, 40 mcg, or placebo. Teriparatide was shown to achieve the primary endpoint of accelerated healing with improved early callus formation compared to placebo.[32]
HYPOPARATHYROIDISM
A study of teriparatide on the effects of calcium showed that following teriparatide 20 mcg/day, serum calcium levels increased from approximately 2 hours post-dose, reaching peak levels at 4–6 hours post-dose [median increase 0.4 mg/dL (0.1 mmol/L)]. Serum calcium levels start to decline approximately 6 hours post-dose, reaching baseline levels at 16–24 hours after each dose. Median peak serum levels were 9.68 mg/dL (2.42 mmol/L) in postmenopausal women with osteoporosis and 9.44 mg/ dL (2.35 mmol/L) in men with primary or hypogonadal osteoporosis.[9,37] Several healthcare physicians have used this ability of teriparatide to treat resistant hypocalcemia.
In 1996, Winer et al. demonstrated that subcutaneous injections of PTH might be superior to calcitriol in the treatment of hypoparathyroidism.[38] A twice daily dose of PTH was shown to be effective in maintaining urine calcium excretion within the normal range, with less fluctuation in serum calcium.[39] To further exemplify its effects on serum calcium, teriparatide was used as a multi-pulse infusion effectively to restore serum calcium in a patient with severe vitamin D resistant hypoparathyroidism, post thyroid surgery.[40] Similar results were noticed in patients with severe refractory hypocalcemia, post renal transplantation, who had undergone parathyroidectomy for severe secondary hyperparathyroidism.[41]
A 3-year study was done where 27 patients with hypoparathyroidism were randomized to twice daily PTH treatment or caicitriol and calcium. Throughout the study period, serum calcium levels were similar in both groups within or just below the normal range. Mean urinary calcium excretion was within the normal range in PTH-treated patients, but remained above normal in the calcitriol group. Bone mineral content and BMD showed no significant between-group differences over the study period.[42] A study conducted in 12 children, aged 5–14 years, with chronic hypoparathyroidism was done to compare the effects of PTH versus calcitriol. Although treatment with PTH (1–34) may be superior to conventional therapy, it is contraindicated in children, as long-term effects on the skeleton are unknown. There was no significant difference in serum and urine calcium levels, creatinine clearance, and mean BMD Z-scores (at the anterior–posterior lumbar spine, femoral neck, distal radius, and whole body) between both groups throughout the study. Additionally, PTH 1–34 treatment allowed normal skeletal development because there were no differences in bone mineral accrual, linear growth, or weight gain between the two treatment arms over the 3-year study period,[43] suggesting that it may be used safely in children.
HYPERPARATHYROIDISM
Teriparatide can be used effectively in patients with cured primary hyperparathyroidism (those who had undergone parathyroidectomy) but still at residual risk of fracture particularly at the spine. An increase of up to 7.1% in BMD can be seen with use of 18 months of teriparatide in these patients. Teriparatide should therefore be considered as a viable alternative for the treatment of these patients as it may help in the prevention of fractures and their complications.[44]
DENTAL INDICATIONS INCLUDING OSTEONECROSIS OF JAW
Teriparatide has been shown to be useful in periodontal disease. In a trial where 40 adults with chronic periodontitis were subjected to periodontal surgery and teriparatide, it resulted in greater radiographic resolution of the periodontal bone defects compared to placebo with accelerated osseous wound healing in the oral cavity.[45]
Preclinical studies have suggested greater stability of orthodontic prosthesis/implants with use of teriparatide. Since teriparatide causes augmentation of bone mass, it only makes sense that cosmetic dentists may consider this therapy more often in the future in order to improve their success rates for dental procedures.[46,47]
Osteonecrosis of jaw (ONJ) involves necrotic, exposed bone in the jaw, pain, swelling, and various dysesthesias associated with long-term bisphosphonate use. The precise mechanism underlying the development of ONJ with bisphosphonate use is not clear. Risk factors include old age, cancer (multiple myeloma), poor oral hygiene, recent dental trauma, and steroid use. ONJ prevalence appears to be associated with the duration of bisphosphonate use in most patients.[48–50] Bisphosphonates are able to localize to bone where they inhibit osteoclast function,[51] and due to their very long half-life, continue to persist in the bone.[52] The standard of care for ONJ includes symptomatic control, treatment of dental infections, and conservative surgical intervention. Teriparatide has been reported to be an adjunctive treatment modality for ONJ.[53] As teriparatide promotes osteoblast differentiation and activity, it would seem to be beneficial by exerting its physiologic effect to promote bone formation and anabolic activity against a background of the suppressed turnover common to long-term bisphosphonate use.[54]
ADRENAL EFFECTS
Teriparatide has shown to have a secretagogue effect on the adrenal glands, causing a significant elevation of plasma and urinary cortisol, which seems to persist throughout the duration of teriparatide use (1 year). Although the importance if this is unknown, an optimist would believe that it could have therapeutic implications in the future.[55]
SAFETY CONCERNS
Teriparatide has both short- and long-term adverse effects. Hypercalcemia and hypercalciuria are the two most common side effects, which have been used to the advantage of the healthcare physician, as cited above.[56] Although hypercalciuria can be detected with teriparatide use, the urinary calcium/creatinine ratio rarely exceeds 0.4 and there are no reported cases of nephrocalcinosis associated with teriparatide therapy.[57] Teriparatide may have to be used with caution when co-administered with drugs such as digoxin, high-dose hydrochlorothiazide (>25 mg/day), or intravenous furosemide, as it may cause a transient mild hypercalcemia (2%) and hypercalciuria (37%). A mild hypercalcemia can risk digitalis toxicity in those receiving digitalis therapy. The serum uric acid concentrations can increase by 2.8%; however, it does not seem to be clinically relevant.[21]
In practice, very few long-term side effects have been reported with PTH. However, the development of osteosarcoma is a cause for concern. In 2008, Harper et al. reported the first case of osteosarcoma in a patient treated with teriparatide with,[57] among more than 250,000 patients in the United States and more than 300,000 patients worldwide. Based on SEER registry data, the background incidence of osteosarcoma in the general population is 1 in 250,000 per year. Thus, the case would not appear to change the risk–benefit profile for teriparatide.[58] Prior to this case, the only reports of osteosarcoma associated with teriparatide use has been in preclinical studies.[59]
CONCLUSION
The introduction of anabolic agent has widened our treatment armamentarium in the management of osteoporosis. Its use is being attempted in any pathological condition requiring an increase in bone mass (ONJ, fracture healing, dental defects, etc). Being a fraction of full-length PTH, healthcare physicians have used it successfully in hypoaparathyroid patients or in patients with hypocalcemia. It is a molecule with tremendous potential, which seems to be unraveling itself slowly, much to the excitement of the healthcare provider.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.National Institute of Health. Osteoporosis prevention, diagnosis and therapy. NIH Consens Statement. 2000;17:1–45. [PubMed] [Google Scholar]
- 2.Blick SK, Dhillon S, Keam SJ. Teriparatide: A review of its use in osteoporosis. Drugs. 2008;68:2709–37. doi: 10.2165/0003495-200868180-00012. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 4.US FDA; Teriparatide (rDNA origin) injection 750 mcg/3 ml. [Last accessed on 2011 Nov 13]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021318s015lbl.pdf .
- 5.Bogado CE, Massari FE, Zanchetta JR. Parathyroid hormone (1–84) and teriparatide in the treatment of postmenopausal osteoporosis. Womens Health. 2006;2:447–57. doi: 10.2217/17455057.2.3.447. [DOI] [PubMed] [Google Scholar]
- 6.Girotra M, Rubin MR, Bilezikian JP. The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord. 2006;7:113–21. doi: 10.1007/s11154-006-9007-z. [DOI] [PubMed] [Google Scholar]
- 7.Rosen CJ, Bilezikian JP. Anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 2001;86:957–64. doi: 10.1210/jcem.86.3.7366. [DOI] [PubMed] [Google Scholar]
- 8.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis : A0 review of the evidence and suggested guidelines for its use. Endocrine Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 9.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 10.Moen MD, Scott LJ. Recombinant full-length parathyroid hormone (1-84) Drugs. 2006;66:2371–81. doi: 10.2165/00003495-200666180-00008. [DOI] [PubMed] [Google Scholar]
- 11.Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, et al. Efficacy and safety of human parathyroid hormone-(1-84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metabol. 2003;88:5212–20. doi: 10.1210/jc.2003-030768. [DOI] [PubMed] [Google Scholar]
- 12.Inomata N, Akiyama M, Kubota N, Jüppner H. Characterization of a novel parathyroid hormone (PTH) receptor with specificity for the carboxyl-terminal region of PTH-(1–84) Endocrinology. 1995;136:4732–40. doi: 10.1210/endo.136.11.7588200. [DOI] [PubMed] [Google Scholar]
- 13.Divieti P, Inomata N, Singh R, Juppner H, Bringhurst FR. Receptors for the carboxyl-terminal region of PTH (1–84) are highly expressed in osteocytic cells. Endocrinology. 2001;142:916–25. doi: 10.1210/endo.142.2.7955. [DOI] [PubMed] [Google Scholar]
- 14.Niall HD, Sauer RT, Jacobs JW, Keutmann HT, Segre GV, O’Riordan JL, et al. The amino acid sequence of the amino-terminal 37 residues of human parathyroid hormone. Proc Natl Acad Sci U S A. 1974;71:384–8. doi: 10.1073/pnas.71.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuprez A, Reginster JY. Bone-forming agents in the management of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22:869–83. doi: 10.1016/j.beem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Verhaar HJ, Lems WF. PTH analogues and osteoporotic fractures. Expert Opin Biol Ther. 2010;10:1387–94. doi: 10.1517/14712598.2010.506870. [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–70. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 18.Rubin M, Bilezikian J. The anabolic effects of parathyroid hormone therapy. Clin Geriat Med. 2002;19:415–32. doi: 10.1016/s0749-0690(02)00074-5. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–41. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 20.Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroidhormone(1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001;16:157–65. doi: 10.1359/jbmr.2001.16.1.157. [DOI] [PubMed] [Google Scholar]
- 21. [Last accessed on 2011 Nov 13]. Available from: http://pi.lilly.com/us/forteo-pi.pdf .
- 22.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–8. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–26. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 24.Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–11. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 25.Kung AW, Pasion EG, Sofiyan M, Lau EM, Tay BK, Lam KS, et al. A comparison of teriparatide and calcitonin therapy in postmenopausal Asian women with osteoporosis : A0 6-month study. Curr Med Res Opin. 2006;22:929–37. doi: 10.1185/030079906X104768. [DOI] [PubMed] [Google Scholar]
- 26.Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal develop- mental process : M0 olecular, spatial and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 28.O’Loughlin PF, Cunningham ME, Bukata SV, Tomin E, Poynton AR, Doty SB, et al. Parathyroid hormone (1-34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine (Phila Pa 1976) 2009;34:121–30. doi: 10.1097/BRS.0b013e318191e687. [DOI] [PubMed] [Google Scholar]
- 29.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–12. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 30.Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96:1627–32. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 31.Schalin-Jäntti C, Mornet E, Lamminen A, Välimäki MJ. Parathyroid hormone treatment improves pain and fracture healing in adult hypophosphatasia. J Clin Endocrinol Metab. 2010;95:5174–9. doi: 10.1210/jc.2010-1168. [DOI] [PubMed] [Google Scholar]
- 32.Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 2010;81:234–6. doi: 10.3109/17453671003761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubery PT, Bukata SV. Teriparatide may accelerate healing in delayed unions of type III odontoid fractures: A report of 3 cases. J Spinal Disord Tech. 2010;23:151–5. doi: 10.1097/BSD.0b013e31819a8b7a. [DOI] [PubMed] [Google Scholar]
- 34.Chintamaneni S, Finzel K, Gruber BL. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporosis Int. 2010;21:1059–63. doi: 10.1007/s00198-009-1061-4. [DOI] [PubMed] [Google Scholar]
- 35.Oteo-Alvaro A, Moreno E. Atrophic humeral shaft nonunion treated with teriparatide (rh PTH 1-34) : A0 case report. J Shoulder Elbow Surg. 2010;19:e22–8. doi: 10.1016/j.jse.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, et al. Teriparatide for acceleration of fracture repair in humans: A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–14. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 37.Eli L. Forteo, teriparatide (rDNA)injection 750 mgs/3 (online) [Last accessed on 2011 Nov 13]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm085729.htm .
- 38.Winer KK, Yanovski JA, Cutler GB. Synthetic human parathyroid hormone 1-34 vs.calcitriol and calcium in the treatment of hypoparathyroidism : R0 esults of a randomized crossover trial. JAMA. 1996;276:631–6. [PubMed] [Google Scholar]
- 39.Winer KK, Yanovski JA, Sarani B, Cutler GB. A randomized, crossover trial of once daily vs.twice-daily human parathyroid hormone 1-34 in the treatment of hypoparathyroidism. J Clin Endocrinol Metab. 1998;83:3480–6. doi: 10.1210/jcem.83.10.5185. [DOI] [PubMed] [Google Scholar]
- 40.Puig-Domingo M, Díaz G, Nicolau J, Fernández C, Rueda S, Halperin I. Successful treatment of vitamin D unresponsive hypoparathyroidism with multipulse subcutaneous infusion of teriparatide. Eur J Endocrinol. 2008;159:653–7. doi: 10.1530/EJE-08-0269. [DOI] [PubMed] [Google Scholar]
- 41.Nogueira EL, Costa AC, Santana A, Guerra JO, Silva S, Mil-Homens C, et al. Teriparatide efficacy in the treatment of severe hypocalcemia after kidney transplantation in parathyroidectomized patients: A series of five case reports. Transplantation. 2011;92:316–20. doi: 10.1097/TP.0b013e3182247b98. [DOI] [PubMed] [Google Scholar]
- 42.Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, et al. Long-term treatment of hypoparathyroidism : A0 randomized controlled study comparing parathyroid hormone- (1-34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88:4214–20. doi: 10.1210/jc.2002-021736. [DOI] [PubMed] [Google Scholar]
- 43.Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, Cutler GB., Jr Long-term treatment of 12 children with chronic hypoparathyroidism : A0 randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium. J Clin Endocrinol Metab. 2010;95:2680–8. doi: 10.1210/jc.2009-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horowitz BS, Horowitz ME, Fonseca S, Ruiz M, Kaye WA. An 18 month open-label trial of teriparatide in patients with previous parathyroidectomy at continued risk for osteoporotic fractures: An exploratory study. Endocr Pract. 2011;17:377–83. doi: 10.4158/EP10247.OR. [DOI] [PubMed] [Google Scholar]
- 45.Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363:2396–405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar M, Hernandes L, Ramos AL, Micheletti KR, Albino CC, Nakamura Cuman RK. Effect of teriparatide on induced tooth displacement in ovariectomized rats: A histomorphometric analysis. Am J Orthod Dentofacial Orthop. 2011;139:e337–44. doi: 10.1016/j.ajodo.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal P, Zavras A. Parathyroid hormone and its effects on dental tissues. Oral Dis. 2012;18:48–54. doi: 10.1111/j.1601-0825.2011.01850.x. [DOI] [PubMed] [Google Scholar]
- 48.Shannon J, Shannon J, Modelevsky S, Grippo AA. Bisphosphonates and osteonecrosis of the Jaw. J Am Geriatr Soc. 2011;59:2350–5. doi: 10.1111/j.1532-5415.2011.03713.x. [DOI] [PubMed] [Google Scholar]
- 49.Garg A, Guez G. Study investigates novel approach to bone loss in the jaw. Dent Implantol Update. 2011;22:9–15. [PubMed] [Google Scholar]
- 50.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 51.Fleisch H. Bisphosphonates: Mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 52.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 53.Harper RP, Fung E. Resolution of bisphosphonate-associated osteonecrosis of the mandible : P0 ossible application for intermittent low-dose parathyroid hormone [rhPTH(1–34)] J Oral Maxillofac Surg. 2007;65:573–80. doi: 10.1016/j.joms.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 54.Narongroeknawin P, Danila MI, Humphreys LG, Jr, Barasch A, Curtis JR. Bisphosphonate-associated osteonecrosis of the jaw, with healing after teriparatide: A review of the literature and a case report. Spec Care Dentist. 2010;30:77–82. doi: 10.1111/j.1754-4505.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lasco A, Catalano A, Morabito N, Gaudio A, Basile G, Trifiletti A, et al. Adrenal effects of teriparatide in the treatment of severe postmenopausal osteoporosis. Osteoporosis Int. 2011;22:299–303. doi: 10.1007/s00198-010-1222-5. [DOI] [PubMed] [Google Scholar]
- 56.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: A review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 57.Harper KD, Krege JH, Marcus R, Mitlak BH. Osteosarcoma and teriparatide? J Bone Miner Res. 2007;22:334. doi: 10.1359/jbmr.061111. [DOI] [PubMed] [Google Scholar]
- 58.Surveillance Research Program. National Cancer Institute SEER_Stat Software. 2008 [Google Scholar]
- 59.Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections o recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–21. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]


