Abstract
Although thyroid scintigraphy and ultrasound continues to be the mainstay of the diagnostic imaging of the thyroid gland, there have been several recent advances that are of interest to both radiologists and endocrinologists. In this review article, the authors discuss recent progress in imaging of the thyroid by use of radionuclide imaging including single photon-emission computed tomography/positron emission tomography, ultrasonography (USG), USG elastography, computed tomography (CT), magnetic resonance imaging (MRI), and optical coherence tomography.
Keywords: Magnetic resonance imaging, optical coherence tomography, radionuclide imaging, thyroid imaging, recent advances, ultrasonography, ultrasound elastography, computed tomography
INTRODUCTION
The thyroid gland plays a critical role in regulating metabolic functions including heart rate and cardiac output, lipid metabolism, heat regulation, and skeletal growth. Recent advances in thyroid imaging have considerably improved the diagnosis, treatment, follow-up, and prognosis of high prevalence thyroid diseases such as thyroid nodule, goiter, thyroiditis, and thyroid cancer that affect the normal thyroid function. The relative roles of various imaging modalities in the evaluation of various thyroid diseases are discussed here.
TECHNIQUES OF THYROID IMAGING
Radionuclide imaging
Radionuclide imaging (RNI) has been a part of the thyroid evaluation for many years. Now, it plays a central role in the evaluation of thyroid disease as it provides excellent functional information about the thyroid gland. The most frequently used isotopes for thyroid scintigraphy are 99mTechnetium pertechnetate, 131Iodine, 18fluoro-deoxy-glucose, and gallium-67. Radionuclide scanning using 99mTechnetium pertechnetate and 131Iodine is used in the evaluation of focal thyroid nodule as hot, warm, or cold on the basis of relative uptake of radioactive isotope by the nodule [Figures 1–4]. 131Iodine, in addition, is also used in the treatment of patients with thyroid cancer to evaluate for residual/recurrent disease, to assess distant metastasis, and in the follow-up of patients after thyroidectomy. Gallium-67 is particularly useful in assessing thyroid lymphoma.[1,2]
Figure 1.
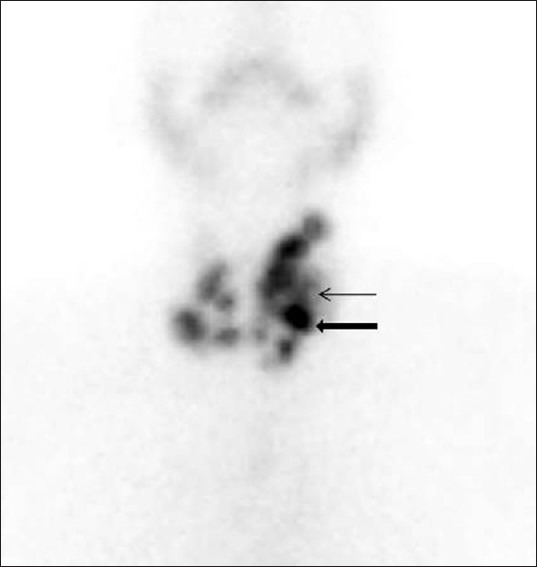
Multinodular goiter. 99mTechnetium pertechnetate (Tc-99m) scintigraphy image of thyroid gland, of a 48-year-old female patient with multinodular goiter, shows multiple hot (thick arrow) and cold nodules (thin arrow) involving both the lobes of the gland (left>right).
Figure 4.
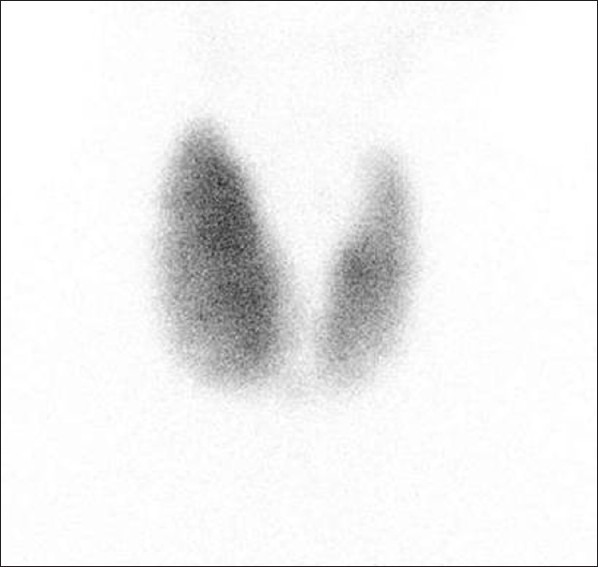
Graves’ disease. Tc-99m scintigraphy image of thyroid gland, of a 32-year-old male patient who presented with neck mass and proptosis, demonstrates enlarged thyroid gland with diffuse increased uptake of the tracer
Figure 2.
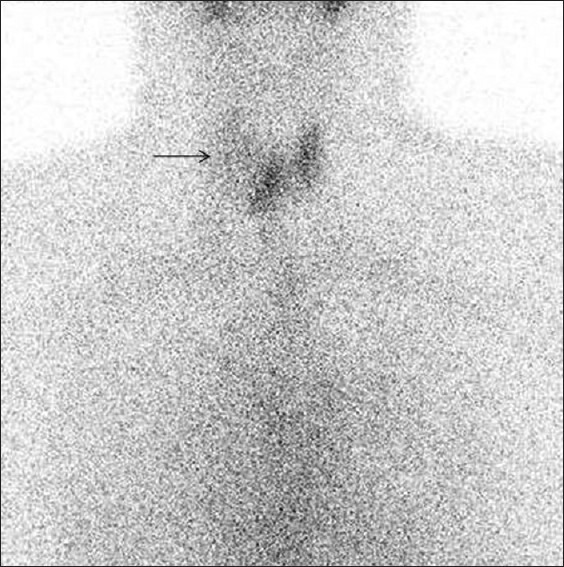
Folicular carcinoma. Tc-99m scintigraphy image of thyroid gland, of a 55-year-old female patient with a palpable right sided neck mass, reveals a large non-functioning cold nodule in right upper pole (arrow). The diagnosis of follicular carcinoma was made on histopathology after resection of the nodule
Figure 3.
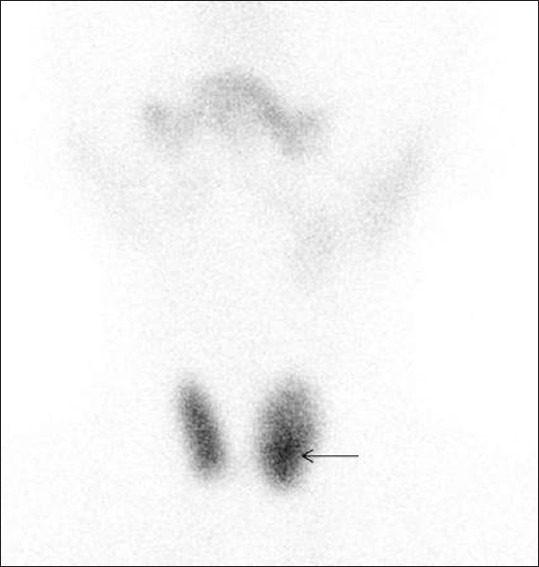
Follicular adenoma. Tc-99m scintigraphy image of thyroid gland, of a 47-year-old female patient with a palpable left-sided neck mass, demonstrates an ill-defined area of increased activity in left lower pole consistent with hot nodule (arrow). The diagnosis of follicular adenoma was made on histopathology after resection of the nodule
Positron emission tomography
Two new radionuclide techniques, namely positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are of potential interest in imaging of the thyroid.
F18-fluorodeoxyglucose-PET (18F-FDG-PET) is a well established imaging modality in oncology. Its greatest utility is in the evaluation of thyroid cancers with dedifferentiated tumors which are iodine scintigraphy negative but FDG-PET positive (in contrast to indolent slow growing thyroid tumors which are iodine scintigraphy positive but FDG-PET negative).[3,4] 18F-FDG-PET has been found to be the most accurate method for detecting recurrent or metastatic medullary thyroid carcinoma (MTC) in patients with an elevated calcitonin level (tumor marker for MTC) postoperatively, when other radionuclide and cross-sectional imaging techniques fail to localize the tumor or metastatic disease. It is also superior to other imaging modalities in localizing cervical and mediastinal lymph node involvement.[5–7]
The combination of PET with CT or MRI (PET/CT or PET/MRI) which allows fusion of functional and anatomic information has a very promising role in the evaluation of thyroid cancer. The sensitivity and specificity of FDG PET-CT to detect the suspected occult lesions and residual/recurrent well-differentiated thyroid cancer (DTC) is very high.
Currently, whole-body FDG PET-CT is recommended for assessing the metastases of DTC in patients with radioiodine negative scans and elevated serum thyroglobulin (Tg) levels. This is particularly useful in guiding treatment decisions.[8,9] FDG PET is also useful in differentiating incidentaloma (show focal uptake of FDG by the gland) from thyroiditis and/or hypothyroidism (show diffuse uptake of FDG by the gland); and malignant thyroid nodule (show high FDG avidity, approaching 100%) from benign nodule (show low FDG avidity, ~30%).[3,10,11]
Single photon emission computed tomography
SPECT is currently used with increased frequency due to its ability to provide the three-dimensional information which improves the overall sensitivity for detection and localization of a thyroid lesion. 131I SPECT-CT has been found to be more accurate than FDG PET-CT in localizing the regional and distant metastasis and in detecting residual/recurrent disease in the case of well-differentiated thyroid cancer. The most important advantage of fusion FDG PET-CT and 131I SPECT-CT is detection of metastasis in normal sized lymph nodes.[9]
The major drawback of FDG-PET is its poor specificity to differentiate between infective or inflammatory processes and the neoplasm, as well as its poor sensitivity to detect micrometastases and tumor sites in well-differentiated thyroid cancers that concentrate iodine. The major drawback with 131I SPECT is that various physiological variants may mimic disease.[9]
Ultrasonography
Ultrasonography is generally the first choice and the most sensitive imaging modality for diagnosing intrathyroid lesions. Because of its superficial location, the thyroid gland is ideally suited for high-frequency sonography (using 7-13 MHz transducer) which facilitates the detection of clinically non-palpable nodules of 2-3 mm size and allows a more accurate morphological characterization of the lesion. It is also used to determine the size and number of thyroid nodules, to assess the volume of thyroid tissue in cases of thyromegaly, and to differentiate thyroid masses from adjacent non-thyroid masses.
Addition of color and spectral Doppler imaging that determines the vascular pattern of thyroid diseases has been found to be very useful tool in screening the thyroid nodule for malignancy. A nodule with exclusively central vascular pattern is characterized as malignant, while a nodule with a predominantly perinodular pattern is generally benign. The gray scale ultrasonographic pattern associated with thyroid carcinoma includes a solid, hypoechoic mass that is taller than wide, has an irregular margin and microcalcifications but absent halo sign. The improved gray scale and Doppler sonography has increased the accuracy and specificity of ultrasound for thyroid diseases [Figures 5–8]. This combination is also helpful in evaluation of cervical lymphadenopathy. Metastatic lymph nodes appear hypoechoic and show lack of central hilar echogenicity and vascularity.[3,12–15]
Figure 5.
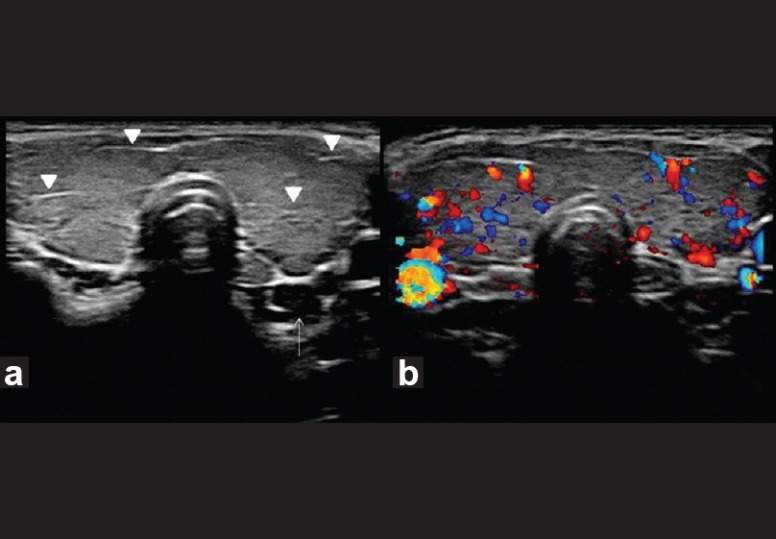
Hashimoto's thyroiditis. Transverse gray-scale ultrasound (a) and color Doppler (b) neck, of a 35-year-old female patient, who presented with features of hypothyroidism and had antithyroid antibodies positive for the disease, demonstrates diffuse enlargement of thyroid gland with linear echogenic fibrous bands (arrowheads) but normal vascularity. Note a small hypoechoic lymph node (arrow) in posterior aspect of inferior pole of left lobe of the thyroid gland
Figure 8.
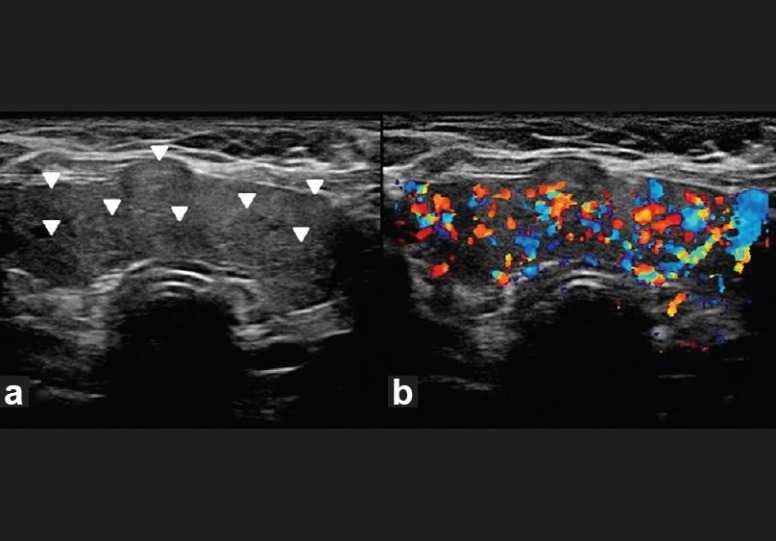
Multinodular goiter. Transverse gray-scale ultrasound (a) and color Doppler (b) neck, of a 50-year-old female patient, shows enlargement of both thyroid lobes and isthmus by multiple iso-hyperechoic solid nodules (arrowheads). There is marked increase in vascularity within the gland
Figure 6.
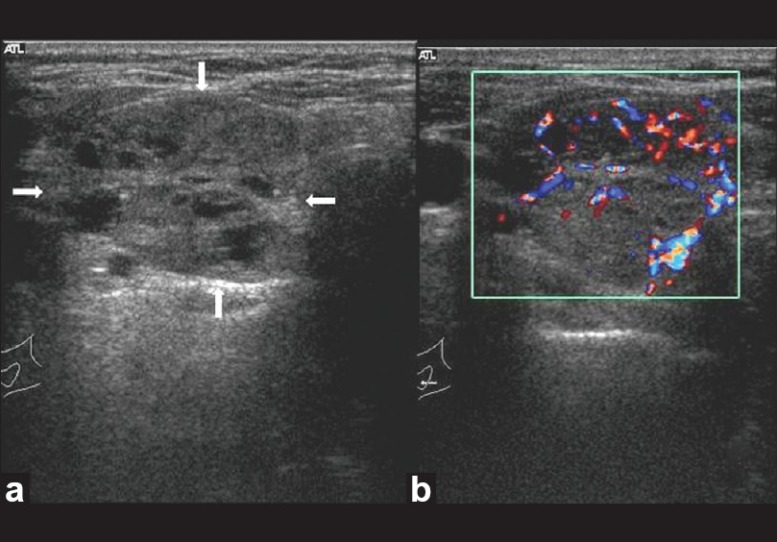
Follicular lesion thyroid. Transverse gray-scale ultrasound (a) and color Doppler (b) neck, of a 40-year-old female patient, shows a large well circumscribed iso-hypoechoic solid thyroid nodule with multiple internal cystic spaces (arrow) and both central and peripheral vascularity
Figure 7.
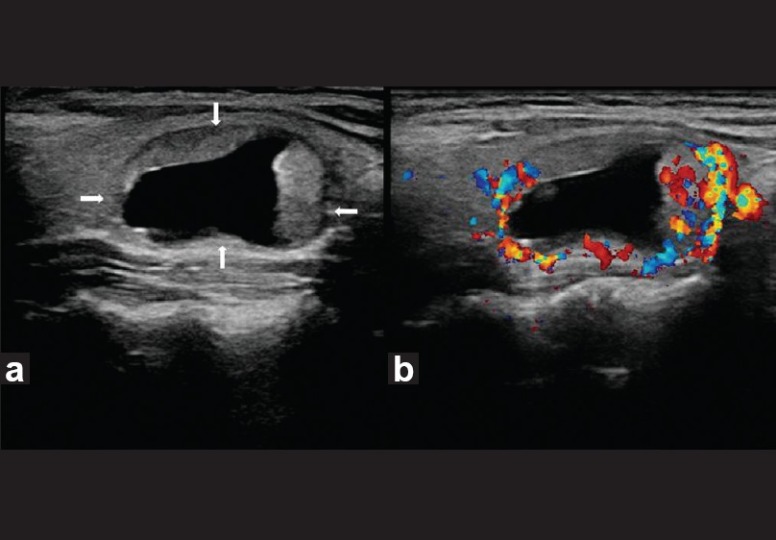
Benign thyroid nodule with large intratumoral cyst. Transverse gray-scale ultrasound (a) and color Doppler (b) neck, of a 55-year-old female patient, reveal a well-circumscribed right-sided thyroid nodule (arrow) with a large intratumoral cyst and solid peripheral component which shows increased vascularity. The lesion demonstrates a thin hypoechoic rim and posterior acoustic enhancement
Ultrasound elastography
Ultrasound (US) elastography measures the tissue elasticity and differentiates between benign and malignant nodule on the basis of consistency of the lesion. A benign nodule is softer and hence deforms more easily, whereas the malignant nodule is harder and hence deforms less when compressed by ultrasound probe. Cystic nodules and nodules having calcified shell are excluded from the US elastographic evaluation. US elastography has a high predictive value in characterizing an indeterminate nodule as malignant with its effectiveness almost comparable to fine-needle aspiration cytology (FNAC).[3,15–17]
Other improvements in ultrasonography
Use of specific contrast (e.g. SonoVue) and pulse inversion harmonic imaging has further improved the efficacy of ultrasound to characterize a thyroid nodule.[18] Ultrasonography-guided fine needle aspiration biopsy (FNAB) is particularly helpful in the case of nonpalpable or small nodules or the nodules that are difficult to access and is preferable to palpation-guided FNAB by many pathologists for appropriate nodule selection.[19]
Main limitation with use of USG is a significant overlap between benign and malignant thyroid nodules; hence, FNAB is necessary in many cases with equivocal findings.[19] US elastography also has limitation in assessing lesions that are not surrounded by adequate normal tissue.[15]
Computed tomography and magnetic resonance imaging
Computed tomography (CT) and magnetic resonance imaging (MRI) have an adjuvant role in the evaluation of thyroid disease. CT and MRI are less sensitive than USG in detecting and characterizing intrathyroid lesions as benign and malignant. They are particularly used for staging thyroid cancer as they are useful in evaluating regional lymphadenopathy, assessing loco-regional extension of the tumor (particularly involving trachea and esophagus), spread of disease into the mediastinum or retrotracheal region and detecting pulmonary and hepatic metastases. CT and MR imaging are also recommended to evaluate the occult metastases (mediastinal or retrophayngeal) in post-thyroidectomy follow-up cases with elevated serum Tg level and negative sonographic finding.[1,20,21]
CT, in particular, is most sensitive in detecting intra-glandular calcification [Figure 9]. Fusion PET-CT and PET-MRI play a promising role in the evaluation of thyroid cancer.[9] Diffusion-weighted imaging (DWI) may be helpful in differentiating benign and malignant nodules. Benign nodules have higher apparent diffusion coefficient (ADC) values than malignant ones.[22] MR spectroscopy using long echo-time (TE) has been proved to be a sensitive method in differentiating thyroid carcinoma from benign follicular lesion. Choline peak is identified in almost all carcinomas, with raised choline/creatine ratio ranging from 1.6 in well differentiated carcinoma to 9.4 in anaplastic carcinoma. The normal thyroid tissue and benign follicular lesions generally demonstrate no choline peak.[23]
Figure 9.
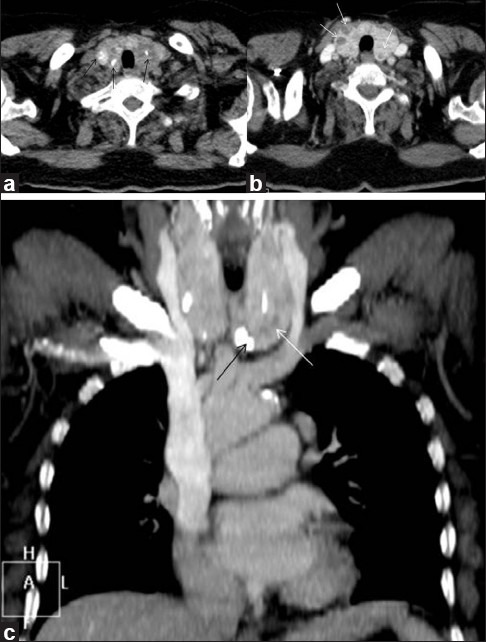
Multinodular goiter. Plain axial (a) and contrast-enhanced axial (b) and coronal (c) CT scan neck region, of a 52-year-old female patient, shows enlargement of bilateral thyroid lobes due to presence of multiple non-enhancing hypodense (thin white arrows) and calcified thyroid nodules (thin black arrows)
Optical coherence tomography and optical coherence microscopy
Optical coherence tomography (OCT) and optical coherence microscopy (OCM) are emerging imaging technologies based on inherent optical contrast. OCT provides high-resolution, real-time, cross-sectional imaging of tissues. OCM is an extension of OCT and provides high magnification resulting in cellular imaging. OCT/OCM system uses infrared light in fiber active device that allows visualization of microstructures of gland (1-15 μm cellular range) and provides high resolution images comparable with those obtained using histopathologic methods. OCT and OCM can clearly differentiate between benign and malignant thyroid tissue using intrinsic optical contrast.[24,25]
CONCLUSION
In general, the thyroid imaging has evolved from early radionuclide thyroid scanning to the development of the advanced technique of SPECT, PET and fusion imaging. The advancement in cross-sectional techniques such as USG, CT and MRI has further improved the evaluation of intrathyroid pathologies. OCT has supplemented the imaging for better selection of patients for operation.
ACKNOWLEDGEMENT
The authors would like to thank Dr. Deepa Prajapati for providing Tc-99m scintigraphy images. We also thank Dr. Deepti Jain for the unconditional support.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Hopkins CR, Reading CC. Thyroid and parathyroid imaging. Semin Ultrasound CT MR. 1995;16:279–95. doi: 10.1016/0887-2171(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 2.Gates JD, Benavides LC, Shriver CD, Peoples GE, Stojadinovic A. Preoperative thyroid ultrasound in all patients undergoing parathyroidectomy? J Surg Res. 2009;155:254–60. doi: 10.1016/j.jss.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Soto GD, Halperin I, Squarcia M, Lomena F, Domingo MP. Update in thyroid imaging: The expanding world of thyroid imaging and its translation to clinical practice. Hormones (Athens) 2010;9:287–98. doi: 10.14310/horm.2002.1279. [DOI] [PubMed] [Google Scholar]
- 4.Feine U, Lietzenmayer R, Hanke JP, Held J, Wöhrle H, Müller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996;37:1468–72. [PubMed] [Google Scholar]
- 5.Ersoy R, Karako A, Atasever T. Imaging techniques for metastatic thyroid medullary cancer. Turk J Endocrinol Metab. 2002;4:149–53. [Google Scholar]
- 6.Reading CC, Gorman CA. Thyroid imaging techniques. Clin Lab Med. 1993;13:711–24. [PubMed] [Google Scholar]
- 7.Heshmati HM, Gharib H, Van Heerden JA, Sizemore GW. Advances and controversies in the diagnosis and management of medullary thyroid carcinoma. Am J Med. 1997;103:60–9. doi: 10.1016/s0002-9343(97)00024-7. [DOI] [PubMed] [Google Scholar]
- 8.Zoller M, Kohlfuerst S, Igerc I, Kresnik E, Galloeitsch HJ, Gomez I, et al. Combined PET/CT in the follow-up of differentiated thyroid carcinoma: What is the impact of each modality? Eur J Nucl Med Mol Imaging. 2007;34:487–95. doi: 10.1007/s00259-006-0276-2. [DOI] [PubMed] [Google Scholar]
- 9.Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM. Hybrid SPECT-CT and PET-CT imaging of differentiated thyroid carcinoma. Br J Radiol. 2009;82:860–76. doi: 10.1259/bjr/25645894. [DOI] [PubMed] [Google Scholar]
- 10.Van den Bruel A, Maes A, De Potter T, Mortelmans L, Drijkoningen M, Van DB, et al. Clinical relevance of thyroid fluorodeoxyglucose-whole body positron emission tomography incidentaloma. J Clin Endocrinol Metab. 2002;87:1517–20. doi: 10.1210/jcem.87.4.8371. [DOI] [PubMed] [Google Scholar]
- 11.De Geus-Oei LF, Pieters GF, Bonenkamp JJ, Mudde AH, Bleeker-Rovers CP, Corstens FH, et al. 18F-FDG PET reduces unnecessary hemithyroidectomies for thyroid nodules with inconclusive cytologic results. J Nucl Med. 2006;47:770–5. [PubMed] [Google Scholar]
- 12.Gooding GA. Sonography of the thyroid and parathyroid. Radiol Clin North Am. 1993;31:967–89. [PubMed] [Google Scholar]
- 13.Kerr L. High resolution thyroid ultrasound: The value of color doppler. Ultrasound Q. 1994;12:21–43. [Google Scholar]
- 14.Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US Features of thyroid malignancy: Pearls and pitfalls. Radiographics. 2007;27:847–65. doi: 10.1148/rg.273065038. [DOI] [PubMed] [Google Scholar]
- 15.Rago T, Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:913–28. doi: 10.1016/j.beem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: New developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92:2917–22. doi: 10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]
- 17.Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–11. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 18.Bartolotta TV, Midiri M, Quaia E, Bertolotto M, Galia M, Cademartiri F, et al. Benign focal liver lesions: Spectrum of findings on SonoVue enhanced pulse-inversion ultrasonography. Eur Radiol. 2005;15:1643–9. doi: 10.1007/s00330-005-2668-2. [DOI] [PubMed] [Google Scholar]
- 19.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 20.Imanishi Y, Ehara N, Mori J, Shimokawa M, Sakuyama K, Ishikawa T, et al. Measurement of thyroid iodine by CT. J Comput Assist Tomogr. 1991;15:287–90. doi: 10.1097/00004728-199103000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. [DOI] [PubMed] [Google Scholar]
- 22.Bozgeyik Z, Coskun S, Dagli AF, Ozkan Y, Sahpaz F, Ogur E. Diffusion-weighted MR imaging of thyroid nodules. Neuroradiology. 2009;51:193–8. doi: 10.1007/s00234-008-0494-3. [DOI] [PubMed] [Google Scholar]
- 23.King AD, Yeung DK, Ahuja AT, Tse GM, Chan AB, Lam SS, et al. In vivo 1H MR spectroscopy of thyroid carcinoma. Eur J Radiol. 2005;54:112–7. doi: 10.1016/j.ejrad.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhou C, Wang Y, Aguirre AD, Tsai TH, Cohen DW, Connolly JL. Ex vivo imaging of human thyroid pathology using integrated optical coherence tomography and optical coherence microscopy. J Biomed Opt. 2010;15:016001. doi: 10.1117/1.3306696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantanowitz L, Hsiung PL, Ko TH, Schneider K, Herz PR, Fujimoto JG, et al. High-resolution imaging of the thyroid gland using optical coherence tomography. Head Neck. 2004;26:425–34. doi: 10.1002/hed.10392. [DOI] [PubMed] [Google Scholar]


