Abstract
Context:
Prevalence of adrenal insufficiency (AI) is not uncommon in HIV infected population. However, AI is rarely diagnosed in clinical practice because many patients have non-specific symptoms and signs. Critical illness in such patients further complicates the evaluation of adrenal function. A 1μgm ACTH test can be used for diagnosis, since it results in more physiological levels of ACTH. A serum cortisol of <18 μg/dL, 30 or 60-minutes after ACTH test has been accepted as indicative of AI, but many experts advocate the normal cortisol response should exceed 25 μg/dL, in critically ill patients.
Aim:
To determine the prevalence of AI in critically ill AIDS patients, by using 1 μg ACTH test and also, to compare the diagnostic criteria for adrenal insufficiency between cortisol response of <18 μg/dL and <25 μg/dL.
Settings and Design:
This prospective study was done in the Department of Medicine.
Materials and Methods:
After taking blood for basal plasma cortisol from AIDS affected fifty adult men and women aged over 18 yrs, 1 μg ACTH was given intravenously, and blood samples were again collected at 30 and 60 minutes for plasma cortisol estimation.
Statistical analysis:
It was done by Mann-Whitney test.
Results:
Prevalence of AI was 74% (37 patients) and 92% (46 patients), when the peak stimulated cortisol level of <18 μg/dL and <25 μg/dL, respectively, was used.
Conclusion:
AI is more prevalent in critically ill AIDS patients. Hence, this test can be performed for early intervention and better management.
Keywords: 1-μgm adrenocorticotropic hormone test, adrenal Insufficiency, Human Immunodeficiency Virus
INTRODUCTION
Patients with the Acquired Immunodeficiency Syndrome (AIDS), caused by Human Immunodeficiency Virus Type-1 (HIV-1) infection, develop profound immunosuppression, particularly of their innate and T-helper 1-directed cellular immunity.[1] They may also develop dysfunction of many organ systems.[2] And, among the endocrine dysfunction, in patients with HIV, the most common is adrenocortical dysfunction.[3–6] The involvement can occur at any level of the hypothalamo-pituitary-adrenal (HPA) axis, but the adrenal gland is the level, most commonly involved.[7] The possible etiologies for adrenocortical dysfunction include HIV, opportunistic infections, neoplasms, medications, autoimmune diseases, and peripheral resistance to glucocorticoids.[3,8,9] Also, AIDS has been associated with changes related to circulating cytokines known to influence the function of the HPA axis,[10] hence the adrenocortical dysfunction. Glucocorticoids, the end products of the HPA axis have strong anti-inflammatory effects, are considered for reversing HIV-1 mediated depletion of circulating CD4+ lymphocytes and slowing progression to AIDS.[11]
Autopsy studies have documented adrenal gland damage in as many as two-thirds of patients with AIDS.[3,12,13] However, Adrenal Insufficiency (AI) is rarely diagnosed in clinical practice because many patients have non-specific symptoms and signs such as malaise, weight loss, and hypotension, which cannot be distinguished from those caused by chronic HIV infection, opportunistic infections, or neoplasms.[7] Also, AI is infrequent, since more than a 90% destruction of gland is needed for it to become clinically symptomatic.[14] Thus, diagnosis of AI requires high index of suspicion and confirmation by an appropriate laboratory evaluation.
In addition critical illness in such patients, further complicate the evaluation of adrenal function.[15–17] The traditional tests like insulin-induced hypoglycemia and the metyrapone tests, have controversial role, and are rarely used in clinical practice because they are inconvenient and dangerous especially in critically ill setting.[3]
A simple, and the most commonly used method for the diagnosis of AI is 250μgm Corticotropin Stimulation Test.[18] But, it being supra physiological high stimulus, results in circulating adrenocorticotropic hormone (ACTH) level of 100-fold greater than a maximal stress level.[7] Thus, this test may over stimulate the adrenal reserve, and mask the diagnosis of AI.[7] Maximum adrenal stimulation can be achieved with smaller doses of ACTH and these may reveal more subtle disturbances in the HPA axis. So 1-μgm ACTH test can replace 250 μgm ACTH for routine evaluation of HPA axis,[19] since it results in more physiological levels of ACTH.[7] Traditionally, a serum cortisol of <18 μg/dL, 30 or 60-minutes after ACTH test, has been accepted as indicative of adrenal insufficiency. However, most patients can achieve the cortisol level between 25-30 μg/dL during critical illness. Many experts advocate the normal cortisol response should exceed 25 μg/dL, in critically ill patients.[7]
In this study, we assessed the relevance of AI in critically ill AIDS patients in our region, by using 1-μg ACTH test (Synacthen test), and also compared the diagnostic criteria for adrenal insufficiency between cortisol response of <18 μg/dL and <25 μg/dL.
MATERIALS AND METHODS
Fifty HIV seropositive adult men and women over 18 yrs of age, who fulfilled the AIDS defining CDC 1993 criteria,[20] those not on drugs known to alter HPA axis function, those not on corticosteroids in past three months, those with no history of HPA insufficiency/autoimmune disease and critically ill patients [in whom the Acute Physiology, Age, and Chronic Health Evaluation II (APACHE II) score was <20][21] were selected for the study, with their consent. Ethical clearance was obtained from the institute.
Adrenocorticotropic hormone preparation
A dilute solution of ACTH (Synacthen) was prepared, by adding 25 μg of Synacthen from a multi-dose vial (250 μg/ml) to 25 ml of 0.9% saline in a glass bottle. The resulting solution of 1 μg ACTH/ml was used immediately after reconstitution. The unused Synacthen in the multi-dose vial was stored at 4°C.
Study protocol
All the patients of the study were subjected to detailed clinical history, physical examination and laboratory findings. The intravenous catheter was inserted between 7 and 8 am. After taking blood for basal plasma cortisol at 8 am, 1 μg ACTH was given intravenously (I.V). From the indwelling I.V. catheter, the sample was again collected at 30 and 60 minutes, for plasma cortisol estimation. The samples were centrifuged, in a refrigerated centrifuge, at 4°C, for 10 minutes, and frozen at -70°C (±10° C), were sent to Acunova laboratory, after packing it in dry ice, wherein cortisol levels were determined by solid-phase radio-immunoassay.
The value of plasma cortisol of <18 μg/dl and <25 μg/dl, 30 or 60 minutes after the 1 μg ACTH test, was considered as indicative of AI.
Statistical analysis
For comparison of clinical parameters and laboratory data, the Fisher Exact and Mann Whitney test were used respectively. ‘P’ value of <0.05 was taken as statistically significant.
RESULTS
This study evaluated the clinical and biochemical profile of critically ill AIDS patients for Adrenal insufficiency. The fifty patients’ (38 males and 12 females) anthropometric characteristics are shown in Table 1. The mean CD4 count was 144 (Standard deviation, 49.78; range, 200-220) and the mean APACHE II score was 16.4 (Standard deviation, 3.6; range, 8-19). There was no statistically significant difference in age, gender and CD4 count for the diagnostic criteria of post-Cosyntropin serum cortisol levels of <18 μg/dL and <25 μg/dL, in AIDS patients without AI and with AI [Table 2].
Table 1.
Baseline characteristics of fifty critically ill Acquired Immunodeficiency Syndrome patients
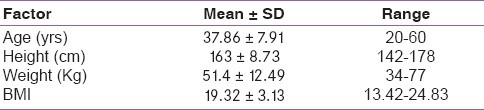
Table 2.
Statistical comparison of various parameters in critically ill patients (n = 50)
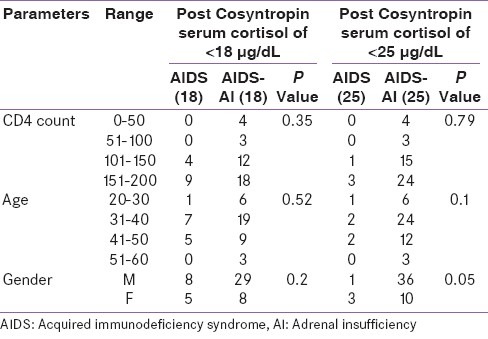
In the present study, patients predominantly had weakness, nausea, vomiting, fever, and weight loss, fatigue, anorexia, muscle pain and joint pain [Table 3]. It was also seen that the most common opportunistic infection was Pneumocystis Carinii Pneumonia (PCP), followed by tuberculosis, oral candidiasis, Cytomegalovirus (CMV) infection and cryptosporidiosis [Table 4].
Table 3.
Symptomatology

Table 4.
Opportunistic infections

Synacthen test
The mean baseline, 30 and 60 minutes stimulated, serum cortisol levels of all fifty critically ill AIDS patients, are shown in Table 5.
Table 5.
Adrenocorticotropic hormone stimulation test (1 μgm) of 50 critically ill Acquired Immunodeficiency Syndrome patients

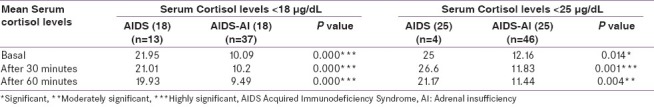
When patients with a peak stimulated cortisol level of <18 μg/dL were considered as having adrenal insufficiency, Synacthen test was positive in 37 (74%) patients, confirming the presence of AI and 13 (26%) were found to be negative, i.e., no AI. The patients with positive results were designated as AIDS-AI (18) and those with negative results as AIDS (18). The comparison of the mean basal, 30 and 60-minute cortisol levels, between the AIDS (18) and AIDS-AI (18) are shown in Figure 1, and the statistical comparison in Table 6.
Figure 1.

Cortisol levels between AIDS (18) and AIDS-AI (18) groups
Table 6.
Comparison of mean basal and post-cosyntropin cortisol levels
When patients with a peak stimulated cortisol level of <25 μg/dL, were considered as having AI, Synacthen test was positive in 46 (92%) patients, confirming the presence of AI and 4 (8%) were found to be negative, i.e., no AI. The patients with positive results were designated as AIDS-AI (25) and those with negative results as AIDS (25). The comparison of the mean basal, 30 and 60 minute cortisol levels, between the AIDS (25) and AIDS-AI (25) are shown in Figure 2, and the statistical comparison in Table 6.
Figure 2.
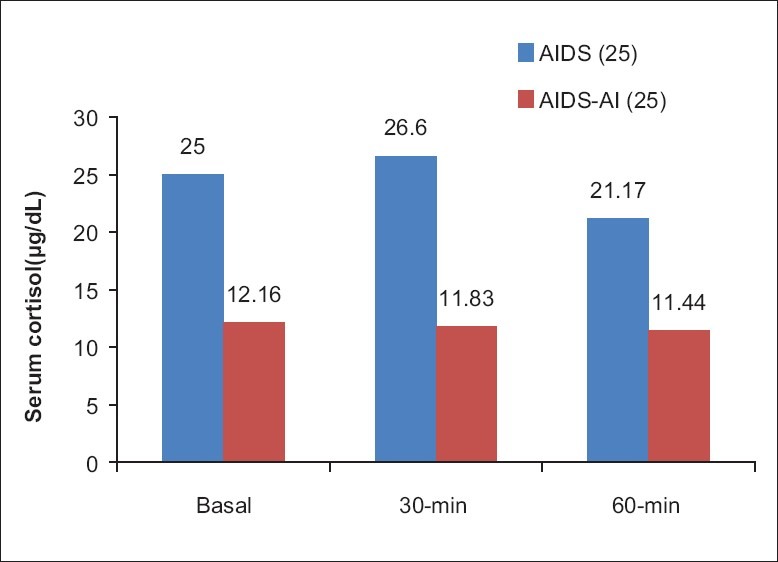
Cortisol levels between AIDS (25) and AIDS-AI (25) groups
DISCUSSION
The present prospective study, examined adrenal response to 1 μg ACTH test in 50 critically ill AIDS patients.
Several approaches to evaluate adrenal function in critically ill patients have been adopted by various investigators. It is seen that basal serum cortisol levels are frequently elevated in patients with HIV, and therefore, are not reliable to exclude adrenal insufficiency.[22] Although the insulin-induced hypoglycaemia is considered the gold standard in assessment of adrenal function, it is impractical and unsafe in the setting of critical illness.[23] Similarly, metyropone is not only hard to obtain, but the test is also very unsafe, and the data are difficult to interpret in the setting of critical illness.[23] Testing adrenal function in the setting of critical illness currently relies on measurements of co-syntropin-stimulated serum total cortisol levels. Very few have used low-dose (1 μg), although most have used the standard dose (250 μg) cosyntropin in defining normal adrenal function.[23] But, this being a supra-physiological dose, can induce the synthesis or mobilisation of cortisol from other pools producing a falsely normal test. Recently, it has been shown that a maximal cortisol response can be elicited with lower dose of ACTH, enhance the sensitivity of the procedure, and even reveal more subtle disturbances in the HPA axis.[19] Hence, 1μg of synthetic ACTH has been suggested as a replacement for the standard 250 μg test.[23]
When, the peak stimulated cortisol level of <18 μg/dL and <25 μg/dL, was used as the diagnostic criteria, prevalence of AI was 74% and 92%, respectively. Prevalence of adrenal insufficiency is not uncommon in HIV-infected population. A study has reported prevalence of adrenal insufficiency to be 21.2% in patients with HIV infection, with frequency of 26.4% and 9.4% in patients with AIDS and with asymptomatic HIV infection, respectively.[24] Another study, on 63 study population reported 19% prevalence of adrenal insufficiency.[25] Yet another study has reported the prevalence of abnormal adrenal response in patients with HIV, varying from 7% - 75%, depending on the criteria used.[26] One more study also has reported varied prevalence from 6% -45%, depending on the dose of ACTH used.[27] In a study, 26.7% of 60 HIV patients had abnormal adrenal function.[28] A retrospective study on 74 AIDS patients reported 22% prevalence of adrenal insufficiency.[29]
In the present study, prevalence of AI in critically ill AIDS patients was 74% and 92%, when the peak stimulated cortisol level of <18 μg/dL and <25 μg/dL was used as the diagnostic criteria, respectively. These results are consistent, though higher prevalence, with the previous study, wherein prevalence of AI in critically ill AIDS patients was 8.3% and 16.7%, when the peak stimulated cortisol level of <18 μg/dL and <25 μg/dL was used as the diagnostic criteria, respectively.[7] It is seen that, the prevalence of adrenal insufficiency in patients with critically ill AIDS patients is relatively high, compared to that of patients without HIV infection, and also to that of non-critically ill AIDS patients.[7] Another study, also has reported 19% prevalence of adrenal insufficiency in critically ill patients with AIDS based on low-dose ACTH test.[25]
Recent development and clinical use of combination therapy of three different types of anti-viral drugs (nucleoside, non-nucleoside analogues, and non-peptidic viral protease inhibitors), i.e., Highly Active Anti-Retroviral Therapy (HAART), have dramatically improved the clinical course of AIDS patients and prolonged their lives.[30–34] However, the prolongation of the life expectancy and/or the long-term use of the above antiviral agents have generated novel morbidities and complications, which influence the patients’ quality of life and add new risk factors for premature death. One among them is a typical biochemical picture of Addison's disease.[35] Also, the plasma from patients of critical illness contain some mediators that impair synthesis of corticosteroids,[26] a hypoadrenal state without obvious structural defects in HPA axis, i.e., “functional or relative adrenal insufficiency”- high cortisol levels but insufficient to control inflammatory responses.[7] These factors can be the explanation for such high prevalence of adrenal insufficiency in the present study.
The reliance of total serum cortisol concentrations in critically ill patients with a high likelihood for being hypoproteinemic introduces a significant limitations of these data.[36] It has been showed that 40% of hypoproteinemic critically ill subjects would have a co-syntropin-stimulated serum cortisol of less than 18.5 μg/dL. In contrast, all of the critically ill with near-normal serum proteins had a peak cosyntropin-stimulated serum cortisol levels more than 20 μg/dL.[36] Importantly, the serum free cortisol levels in the two groups were similar. Hence, measurement of serum free cortisol represents the most ideal approach.[37] Current assays for determining serum free cortisol concentrations are difficult, time consuming, results are not immediately available to clinicians, and are labour intensive. Until rapid assays for measurements of serum cortisol are available for routine clinical care, alternate approaches could be explored like measurements of salivary cortisol concentrations and determination of other ACTH-dependent adrenal steroids such as DHEA and DHEA-S. More studies are needed on the value of DHEA and DHEA-S in the critically ill before they become part of the standard assessment of adrenal function in this setting.[23]
A recent study investigated the reproducibility of two cosyntropin tests (1d apart) in critically ill patients and found discordance in results in patients with septic shock.[38] It is not known whether fluid administration to these patients contributed to the discordance in results.[23] It is commonly believed that glucocorticoid secretion during critical illness is generally proportionate to the degree of stress.[10] Studies have demonstrated decreased adrenal glucocorticoid secretion with advancing chronicity of critical illness were based on measurements of serum total cortisol levels. But, these measurements have serious limitations, especially during chronic illnesses in which malnutrition and hypoproteinemia are more common and serum free cortisol levels show continuous elevation throughout the critical illness.[36] This raises the question of the presence of other factors that are stimulating and regulating glucocorticoid secretion, like endothelin, ANF, vasopressin, and other cytokines.[39] Also, published studies involving evaluation of adrenal function in critically ill subjects have consistently demonstrated that there was a wide range in measured cosyntropin-stimulated serum cortisol levels.[23] It is not known whether variation in ACTH and CRH receptor activities contributes to the observed individual differences among patients. Similarly, another area that has not been investigated is the variability of the 11β-hydroxysteriod dehydrogenase enzyme.[23]
Adrenal insufficiency reported in AIDS patients is mainly induced by specific causes that target the adrenal glands.[35] The possible etiologies include HIV, opportunistic infections, neoplasms, medications, autoimmune diseases, and peripheral resistance to glucocorticoids.[3,8,9] Also, AIDS has been associated with changes related to circulating cytokines known to influence the function of the HPA axis.[10] Many medications alter the baseline and/or the cosyntropin-stimulated cortisol levels. Such medications often influence binding proteins, directly interfere with glucocorticoid synthesis, have direct inhibitory effects on CRH/ACTH secretion, direct anti-glucocorticoid effects suppressing HPA axis just like glucocorticoids. In the present study, patients on medications known to alter HPA axis and with history of autoimmune disease were excluded. In present study, we could not screen the patients for glucocorticoid resistance and cytokine levels. But, patients in this study had HIV-associated infections [Table 3], a possible explanation for adrenal dysfunction. Previous study has reported that most patients had chronic opportunistic infections like mycobacteriosis, cryptococcosis, and CMV diseases to be associated with adrenal insufficiency.[7,3,26] It is seen that, CMV may involve the adrenal glands in 33% to 88% of patients with AIDS.[40,41] But still, the role of CMV in adrenal insufficiency is controversial, since most patients with CMV disease had symptomatic HIV infection, and thus may have other associated opportunistic infections involving the adrenal glands. In addition, there are no previous studies that have investigated thoroughly for CMV/other opportunistic diseases in adrenal insufficient AIDS patients. Hence, well-designed studies may be useful in clarifying the association between CMV/other opportunistic infections, glucocorticoid resistance, circulating cytokines, and adrenal insufficiency in patients with AIDS.
CONCLUSION
Adrenal insufficiency in patients with AIDS is more prevalent than those without HIV infection[7] and even more in critically ill group. This becomes more relevant in present situations, wherein life span post-infection has increased due to HAART, but at the same time, bringing in new morbidity factors and early death, one of it being adrenal insufficiency. It is difficult to exclude adrenal insufficiency during critical illness, on clinical grounds alone, and laboratory evaluation is difficult to interpret because there are many controversies and a large number of confounding factors. Among all the tests available for evaluation, low-dose corticotropin stimulation test seems to be relevant, in such situations, yet limitations of these measurements should be considered, until serum free cortisol determination becomes widely available. Hence, this test can be performed, in suspected critically ill AIDS patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–35. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 2.Sellmeyer DE, Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996:518–32. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon SK, Bilezikian JP. HIV disease and endocrine system. N Engl J Med. 1992;327:1306–5. doi: 10.1056/NEJM199211053271906. [DOI] [PubMed] [Google Scholar]
- 4.Eledrisi MS, Verghese AC. Adrenal insufficiency in HIV infection: A review and recommendations. Am J Med Sci. 2001;321:137–44. doi: 10.1097/00000441-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow BJ, Steinsapir KD, Anders K, Layfield LJ. Adrenal pathology in the acquired immune deficiency syndrome. Am J Clin Pathol. 1985;84:594–7. doi: 10.1093/ajcp/84.5.594. [DOI] [PubMed] [Google Scholar]
- 6.Aron DC. Endocrine complications of acquired immunodeficiency syndrome. Arch Intern Med. 1989;149:330–3. [PubMed] [Google Scholar]
- 7.Prasanthai V, Sunthornyothin S, Phowthongkum P, Suankratay C. Prevalence of adrenal insufficiency in critically ill patients with AIDS. J Med Assoc Thai. 2007;90:1768–74. [PubMed] [Google Scholar]
- 8.Greene LW, Cole W, Greene JB, Levy B, Louie E, Raphael B, et al. Adrenal insufficiency as a complication of the adrenal insufficiency syndrome. Ann Intern Med. 1984;101:497–8. doi: 10.7326/0003-4819-101-4-497. [DOI] [PubMed] [Google Scholar]
- 9.Mayo J, Collazos J, Martinez E, Ibarra S. Adrenal function in human immunodeficiency virus-infected patient. Arch Intern Med. 2002;162:1095–8. doi: 10.1001/archinte.162.10.1095. [DOI] [PubMed] [Google Scholar]
- 10.Chousos GP. The hypothalamo-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 11.Andrieu JM, Lu W, Levy R. Sustained increase in CD$ cell counts in asymptomatic human immunodeficiency virus type 1-seropositive patients treated with prednisolone for 1 year. J Infect Dis. 1995;171:523–30. doi: 10.1093/infdis/171.3.523. [DOI] [PubMed] [Google Scholar]
- 12.Welch K, Finkbeiner W, Alpers CE, Blumenfeld W, Davis RL, Smuckler EA, et al. Autopsy findings in the acquired immune deficiency syndrome. JAMA. 1984;252:1152–9. [PubMed] [Google Scholar]
- 13.Niedt GW, Schinella RA. Acquired immunodeficiency syndrome: Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985;109:727–34. [PubMed] [Google Scholar]
- 14.Baraia-Etxaburu J, Astigarraga B, Zubero Z, Munoz J, Teira R, Santamaria JM. Int Conf AIDS. 1994. [accessed on 2011 Jul 23]. Available from: http://gateway.nlm.nih.gov/meetingAbstracts/ma?f=102210258.html .
- 15.Cooper MS, Stewart PM. Corticosterol insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–34. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 16.Drucker D, McLaughlin J. Adrenocortical dysfunction in acute medical illness. Crit Care Med. 1986;14:789–91. doi: 10.1097/00003246-198609000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Beishuizen A, Thijs LG. Relative adrenal failure in intensive care: An identifiable problem requiring treatment? Best Prac Res Clin Endocrinol Metab. 2001;15:513–31. doi: 10.1053/beem.2001.0167. [DOI] [PubMed] [Google Scholar]
- 18.Grinspoon SK, Biller BM. Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923–31. doi: 10.1210/jcem.79.4.7962298. [DOI] [PubMed] [Google Scholar]
- 19.Tordjman K, Jaffe A, Grazas N, Apter C, Stern N. The role of the low dose (1μg) adreno-corticotropin test in the evaluation of patients with Pituitary diseases. J Clin Endocrinol Metab. 1995;80:1301–5. doi: 10.1210/jcem.80.4.7714104. [DOI] [PubMed] [Google Scholar]
- 20.Data from Centre of Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adults and adolescents. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 22.Eledrisi MS, Vergese AC. Adrenal insufficiency in HIV infection: A review and recommendations. Am J Med Sci. 2001;321:137–44. doi: 10.1097/00000441-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Arafah BM. Review: Hypothalamic pituitary adrenal function during critical illness: Limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–45. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Gonzalez JG, de la Garza-Hernandez NE, Garza-Moran RA, Rivera-Morales IM, Montes-Villarreal J, Valenzuela-Rendon J, et al. Prevalence of abnormal adrenocortical function in human immunodeficiency virus infection by low-dose cosyntropin test. Int J STD AIDS. 2001;12:804–10. doi: 10.1258/0956462011924434. [DOI] [PubMed] [Google Scholar]
- 25.Wolff FH, Nhuch C, Cadore LP, Glitz CL, Lhullier F, Furlanetto TW. Low-dose adrenocorticotropin test in patients with the acquired immunodeficiency syndrome. Braz J Infect Dis. 2001;5:53–9. doi: 10.1590/s1413-86702001000200002. [DOI] [PubMed] [Google Scholar]
- 26.Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30:1267–73. doi: 10.1097/00003246-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Smolyar D, Tirado-Bernardini R, Landman R, Lesser M, Young I, Poretsky L. Comparision of 1-micro g and 250-micro g corticotropin stimulation test for the evaluation off adrenal function in patients with acquired immunodeficiency syndrome. Metabolism. 2003;52:647–51. doi: 10.1053/meta.2003.50099. [DOI] [PubMed] [Google Scholar]
- 28.Hoshino Y, Yamashita N, Nakamura T, Iwamoto A. Prospective examination of adrenocortical function in advanced AIDS patients. Endocr J. 2002;49:641–7. doi: 10.1507/endocrj.49.641. [DOI] [PubMed] [Google Scholar]
- 29.Piedrola G, Casado JL, Lopez E, Moreno A, Perez-Elias MJ, Garcia-Robles R. Clinical features of adrenal insufficiency in patients with acquired immunodeficiency syndrome. Clin Endocrinol (Oxf) 1996;45:97–101. doi: 10.1111/j.1365-2265.1996.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 30.Morris AB, CU-Uvin S, Harwell JI, Garb J, Zorrilla C, Vajaranant M, et al. Multicenter review of protease inhibitors in 89 pregnancies. J Acquir Immune Defic Syndr. 2000;25:306–11. doi: 10.1097/00042560-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Tashima KT, Flanigan TP. Antiretroviral therapy in the year 2000. Infect Dis Clin North Am. 2000;14:827–49. doi: 10.1016/s0891-5520(05)70136-7. [DOI] [PubMed] [Google Scholar]
- 32.Vella S, Palmisano L. Antiretroviral therapy: State of the HAART. Antiviral Res. 2000;45:1–7. doi: 10.1016/s0166-3542(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 33.Harrington M, Carpenter CC. Hit HIV-1 hard, but only when necessary. Lancet. 2000;355:2147–52. doi: 10.1016/S0140-6736(00)02388-6. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann GR, Cooper DA. Antiretroviral therapy of HIV-1: Established treatment strategies and new therapeutic options. Curr Opin Microbiol. 2000;3:508–14. doi: 10.1016/s1369-5274(00)00131-4. [DOI] [PubMed] [Google Scholar]
- 35.Tomoshige K, George P, Chrousos AIDS/HPA Axis. [accessed on 2011 Jul 23]. Available from: http://www.endotext.org/adrenal/aadrenal19/adrenalframe19.html .
- 36.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–38. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 37.Loriaux L. Glucocorticoid therapy in the intensive care unit. N Engl J Med. 2004;350:1601–2. doi: 10.1056/NEJMp048052. [DOI] [PubMed] [Google Scholar]
- 38.Loisa P, Uusaro A, Ruokonen E. A single adrenocorticotropic hormone stimulation test does not reveal adrenal insufficiency in septic shock. Anesth Analog. 2005;101:1792–8. doi: 10.1213/01.ANE.0000184042.91452.48. [DOI] [PubMed] [Google Scholar]
- 39.Vermis I, Beishuizen A, Hampsink RM, Haanen C. Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: Possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab. 1995;80:1238–42. doi: 10.1210/jcem.80.4.7714094. [DOI] [PubMed] [Google Scholar]
- 40.Pulakhandam U, Dincsoy HP. Cytomegaloviral adrenalitis and adrenal insufficiency in AIDS. Am J Clin Pathol. 1990;93:651–6. doi: 10.1093/ajcp/93.5.651. [DOI] [PubMed] [Google Scholar]
- 41.Muhlhofer JE, Jung C, Gross M. Succesful treatment with ganciclovir of a HIV endstage patient with adrenal insufficiency. Eur J Med Res. 1997;2:469–72. [PubMed] [Google Scholar]


