Abstract
Context:
Gonadotrophin releasing hormone (GnRH) stimulation test is pivotal in the assessment of children with pubertal disorders. However, lack of availability and high cost often result in the test falling into disfavor. We routinely use the GnRH analogue stimulation test as an alternative at our center.
Aim:
To present the data on children with endocrine disorders who underwent GnRH agonist stimulation test in pediatric endocrine clinic of a tertiary care referral hospital.
Setting and Design:
Pediatric endocrine clinic of a tertiary care referral hospital. Retrospective analysis of case records.
Materials and Methods:
The details pertaining to clinical and radiological parameters and hormonal tests were retrieved from case records of 15 children who underwent GnRH agonist stimulation test from May 2010 to April 2011.
Results:
Indications for testing with GnRH analogue were evaluation of delayed puberty, diagnosis of precocious puberty, assessment of hormonal suppression in treatment of precocious puberty and micropenis in two, nine, three and one cases, respectively. The results of the test and clinical and radiological parameters were in concordance. The test was also crucial in diagnosing the onset of central precocious puberty in two children with congenital adrenal hyperplasia.
Conclusion:
GnRH agonist test is a convenient, safe test that can be performed on an out-patient basis and can help the clinicians in the correct diagnosis and appropriate treatment of various puberty-related disorders.
Keywords: Analogue, gonadotrophin releasing hormone, puberty
INTRODUCTION
Gonadotrophin releasing hormone (GnRH) stimulation test is an important tool in the evaluation of children with pubertal disorders.[1] It is commonly performed to establish the diagnosis of central precocious puberty, distinguish constitutional delay of growth and puberty (CDGP) from hypogonadotropic hypogonadism, assess adequacy of hormonal suppression in central precocious puberty (CPP) and evaluate hypogonadotrophic hypogonadism in infancy.[1–3] Stimulated gonadotrophin concentrations often score over basal values in their correlation with pubertal stage, pubertal progression and assessment of pituitary reserve. Luteinizing hormone-releasing hormone (LH-RH), first synthetized in 1971, has been available for clinical purposes since then.[1] Conventional GnRH stimulation test involves intravenous administration of LH-RH at doses of 100 μg/m2 or 2.5 μg/kg and determination of peak LH and follicle stimulating hormone (FSH) responses in five to eight blood samples in approximately 20–30 minutes, which makes it cumbersome and costly.[4–6] Hence, modifications of the test have been extensively investigated, such as lesser sampling after the injection,[7,8] baseline LH[9] and LH/FSH ratio[10] to determine the onset of puberty and the usage of GnRH analogues.[11,12]
There is profound difficulty in procuring LH-RH for routine clinical use in our country owing to logistic reasons. Further, comparison of GnRH stimulation using analogues versus conventional LH-RH has been evaluated earlier and found to have similar efficacy.[13] GnRH analogue testing also has the advantage of being performed on an outpatient basis with fewer sampling, when compared to the conventional GnRH stimulation test. Hence, at our center, we use the standard GnRH agonist test to evaluate children with pubertal disorders. Data on GnRH analogue tests from India are scarce. Hence, we present the utility of GnRH analogue test along with clinical and laboratory data on children who have been assessed at our pediatric endocrine unit with a brief review of relevant literature.
MATERIALS AND METHODS
Children presenting at the pediatric endocrine clinic of a tertiary care teaching hospital over 1 year, from May 2010 to April 2011, with pubertal disorders and evaluated using GnRH agonist Leupride stimulation test were included in the study. A retrospective analysis of their case records was performed and data pertaining to hormonal tests and the clinical parameters are presented. The GnRH agonist testing at our center is performed using a standard protocol[14] that involves taking venous blood samples at 0 hour (FSH and LH), administration of Inj. Leupride in a dose of 10 μg/kg subcutaneously, and collection of blood samples repeated at 4 hours (LH and FSH) and 24 hours [LH, FSH and testosterone (boys) or estradiol (girls)]. The onset of puberty is diagnosed if serum testosterone level is >25 ng/dl in boys or estrogen level is >10 pg/ml in girls.[5]
GnRH analogue, available as Inj. Lupride (Sun pharmaceuticals, Mumbai India) in 1 mg/0.5 ml, was administered in a dose of 10 μg/kg by subcutaneous route. Serum LH, FSH, estradiol and testosterone were measured by the nonisotopic, automated chemiluminescence immunoassay system, Architect (Abbott Laboratories, Chicago, IL, USA). The detection limit was 0.05 mIU/ml for FSH and 0.07 mIU/ml for LH. The minimum detectable levels of estradiol and testosterone were 10 and 0.08 ng/ml, respectively. The intra- and inter-assay coefficients of variation were <10% for all biochemical parameters.
Ethical clearance was obtained from the institutional ethics committee to review the case records of children who underwent GnRH analogue testing.
RESULTS
During the study period, 15 children presenting with pubertal problems at our endocrine clinic underwent GnRH agonist stimulation testing. Out of the 15 children, 6 were boys. The indications for GnRH stimulation testing were evaluation of delayed puberty, diagnosis of precocity, assessment of efficacy of suppressive therapy in precocious puberty and suspected hypogonadotrophic hypogonadism with micropenis in two, nine, three and one children, respectively. The relevant clinical, laboratory and radiological features are depicted in Table 1 and GnRH analogue testing results with final interpretation are illustrated in Table 2.
Table 1.
The clinical features and investigations of the study population
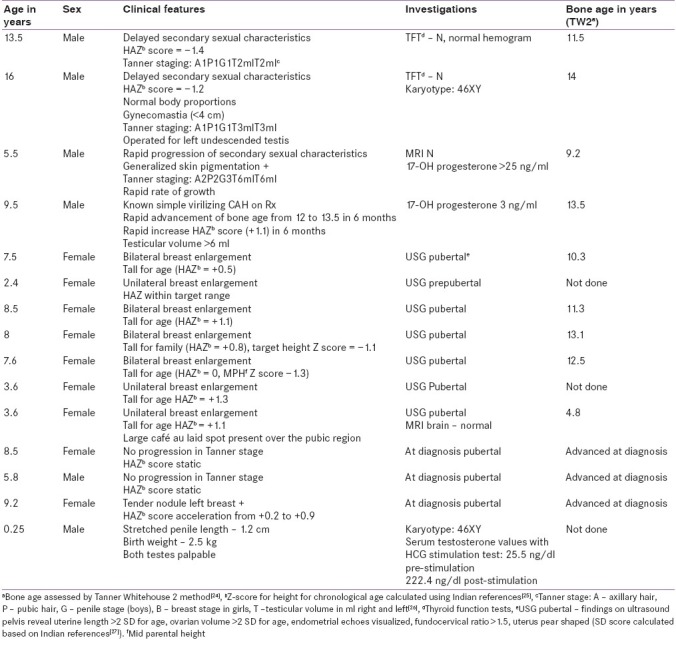
Table 2.
Clinical indications, GnRH analogue stimulation test results and their final interpretation
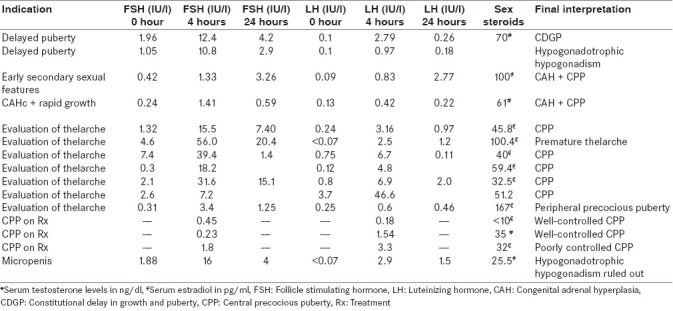
Two boys underwent testing to establish the etiology of delayed puberty. Case 1 had low baseline LH and FSH. The incremental response of LH to GnRH analogue was optimal in case 1 and suboptimal in case 2. Hence, we arrived at a diagnosis of constitutional delay in growth and puberty in case 1 and hypogonadotrophic hypogonadism in case 2.
Case 3–11 underwent testing for early onset of secondary sexual characteristics. Subjects 5, 7, 8, 9 and 10 were diagnosed as CPP as they had advanced skeletal age, height age, pubertal status of reproductive organs on ultrasound and pubertal response in GnRH stimulation test, and were started on GnRH analogue therapy as their predicted adult height was <–2 SD as per the skeletal age. Subject 6 had a predominant FSH response on stimulation, no undue advancement of height age and pre-pubertal state of reproductive organs on ultrasonography. Hence, a diagnosis of premature thelarche was made. Subject 11 was diagnosed as peripheral precocious puberty as the estradiol levels were pubertal, but gonadotrophins were not showing a pubertal increment on GnRH stimulation. A diagnosis of Mccune–Albright syndrome was made owing to the presence of café au laid spots. Subject 3 had suppressed gonadotrophins and generalized skin pigmentation, hence isosexual precocity arising due to missed congenital adrenal hyperplasia (CAH) was considered and confirmed by investigations and hydrocortosine replacement was started. However, in view of the increased testicular volume, a GnRH test was performed to confirm the associated CPP, which revealed a threefold increase in the stimulated gonadotrophin concentrations over the baseline.[5] Hence, additional therapy with suppressive GnRH analogues was initiated. Subject 4 was a known case of CAH on treatment, presenting with rapid advancement of height age and skeletal age and testicular volumes in spite of adequate glucocorticoid therapy. Hence, GnRH stimulation test was performed which showed the patient had entered true central precocity, and GnRH suppression therapy was advised to augment final height.
Case 12, 13 and 14 were children with CPP on GnRH suppressive therapy with depot preparations of Inj. Leupride. Central suppression of gonadotrophins was assessed in these children by measuring LH, FSH and sex steroids, 4 hours post-analogue therapy. Adequate suppression of hormones in case 12 and 13 was seen, hence the same regimen was continued. However, in child 14, the LH and estradiol were unsuppressed [Table 2], which was correlated with the clinical picture. Child 14 had developed tenderness in the breast and progression of secondary sexual characteristics with acceleration of height. Therefore, the dose was increased from 11.25 to 22.5 mg. The advancement of skeletal age, height and secondary sexual characteristics was well controlled with this dosage when she came for follow-up after 3 months.
Case 15 was a child with micropenis (penile length < –2.5 SD)[15] and had no ambiguity of genitalia. Basal gonadotrophins revealed low LH levels, and hence GnRH stimulation was performed which demonstrated an incremental response of LH [Table 2].
DISCUSSION
We have presented the results of GnRH analogue testing in 14 children with puberty related disorders. GnRH analogue test is based on the principle that the 4-hour sample would indicate the gonadotrophin reserve and the 24-hour sample would gauge the gonadal response to endogenous gonadotrophins.[15] Cost-effectiveness of GnRH analogue test (approximately INR 2500) is thrice that of the conventional GnRH test (INR 7500). GnRH analogue test also scores over conventional GnRH testing in terms of availability and convenience as the former can be performed on an out-patient basis with fewer sampling (three in former versus six to eight in the latter). Also, in patients who are on treatment with GnRH analogues, LH and FSH levels at 4 hours after the therapeutic injection can be used to assess the adequacy of suppression.
The rationale of using GnRH testing to distinguish CDGP from hypogonadotrophic hypogonadism would be that priming of the former in minipuberty during infancy with sex steroids is absent in the latter. The stimulated values of LH to discriminate the two conditions are variable: 2.8 IU/l,[16] 5.8 IU/l,[17] 4.2 IU/l.[6] The response of the gonadotrophins to GnRH in case 1 (4 hour LH 2.79 IU/l) was close to a pubertal response, and hence labeled as CDGP.[17] However, a suboptimal increment of LH in case 2 (0.97 IU/l) is in line with the clinical features of hypogonadotrophic hypogonadism in this child, like gynecomastia, undescended testis and normal karyotype. Based on the results, we counseled case 2 for lifelong hormone replacement therapy and case 1 on the benign nature of his condition and need for follow-up at our clinic.
GnRH testing in precocious puberty has to be interpreted on the background of the clinical picture pertaining to skeletal age advancement, height advancement and rate of progression of secondary sexual characteristics. Although numerous interpretations of stimulated values of gonadotrophins have been reported in literature, at our center we follow a stimulated LH cut-off of >3.3 IU/l[18] or a threefold rise in the stimulated gonadotrophin level[5] to diagnose CPP. Usage of this test helped us rightly distinguish CPP in cases 5, 7, 8, 9, 10 (who had advanced skeletal maturation >2 SD over chronological age, advanced height SD scores, mature pelvic reproductive organs), premature thelarche in case 6 (who had less advancement in height age and had pre-pubertal pelvic organs) and peripheral precocious puberty in case 11. We diagnosed the onset of central precocity in two children with CAH (case 3, 4) as the testosterone levels were >25 ng/dl, despite 17-hydroxyprogesterone being normal (which indicates that the CAH is effectively controlled by hydrocortisone suppression therapy).[19] Subsequently, clinical control in growth velocity and secondary sexual characteristics has been noted.
The pharmacokinetic properties of the depot preparation show that it contains two parts – one in the microspheres causing long-term action and some free particles causing rapid onset of action. The efficacy of suppression can be assessed by gonadotrophin concentrations shortly after a depot injection. There is no consensus on the timing of the hormonal estimation and the cut-off value to quantify adequate suppression. Recommendations on timing of sample after injection include 40 minutes[20] and 2 hours[21] from western studies and 3 hours from Indian studies.[22] The recommended cut-off for LH varies from 2[20] to 6.6 IU/l.[21] At our center, we draw blood sample of children 4 hours after the injection of depot. The interpretations of the hormonal tests [Table 2] are in conjunction with the clinical picture [Table 1]. Hence, the same dosage was continued for case 11 and 12, whereas it was increased for case 13.
Case 15 with micropenis had low gonadotrophin levels. Hence, the possibility of hypogonadotrophic hypogonadism was considered in the differential diagnoses. However, stimulated LH levels were normal. Hence, hypogonadotrophic hypogonadism was ruled out in this infant using the GnRH analogue testing. Results of the human chorionic gonadotrophin (HCG) stimulation test were suggestive of androgen receptor insensitivity. High basal testosterone concentration is not a universal finding in Androgen Insensitivity Syndrome,[3] the surge in LH with GnRH is also described in healthy infants,[23] and growth hormone deficiency can present with normal growth in the first year of life, and micropenis – these entities were considered for the child. The child was treated with three monthly doses of testosterone 25 mg intramuscularly.[15]
Our study is not without limitations. An ideal methodology would have been to subject all the children to an intravenous GnRH stimulation test after an analogue test and look for similar results. However, retrospective nature of the study and nonavailability of LH-RH prevented us from its usage. However, the concordance between the clinical parameters, progress and GnRH analogue stimulation test results is an indicator of applicability of this test in routine practice. Also, a cut-off of stimulated LH that distinguishes CPP and Gonadotrophin Independent Precocious puberty (GIPP) for our children could not be arrived at owing to the small sample size.
To conclude, GnRH agonist stimulation test is a useful tool in the diagnosis children with pubertal disorders. The response of our patients to GnRH agonist test was in concordance with the clinical diagnosis and response to therapy. The test can be carried out on an out-patient basis, requires no intravenous medications and requires fewer samples. Hence, GnRH agonist test is more cost-effective, safe, easily available, and yet, as efficacious as the conventional GnRH testing in helping the clinician arrive at the correct diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Job JC, Chaussain JL, Garnier PE. The use of luteinizing hormone-releasing hormone in pediatric patients. Horm Res. 1977;8:171–87. doi: 10.1159/000178795. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SF, Cheng A, Hughes IA. Assessment of the gonadotrophin–gonadal axis in androgen insensitivity syndrome. Arch Dis Child. 1999;80:324–9. doi: 10.1136/adc.80.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kletter GB, Rolfes-Curl A, Goodpasture JC, Solish SB, Scott L, Henzl MR, et al. Gonadotropin-releasing hormone agonist analog (nafarelin): A useful diagnostic agent for the distinction of constitutional growth delay from hypogonadotropic hypogonadism. J Pediatr Endocrinol Metab. 1996;9:9–19. doi: 10.1515/jpem.1996.9.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Garibaldi L. Disorders of pubertal development. In: Behrman RE, Kleigman RM, Jenson HB, editors. Nelson Text Book of Pediatrics. 18th ed. Philadelphia: WB Saunders; 2008. pp. 2309–11. [Google Scholar]
- 5.Bajpai A. Precocious puberty. In: Desai MP, Bhatia V, Menon PS, editors. Pediatric Endocrine Disorders. 1st ed. Hyderabad: Orient Longman; 2001. pp. 217–41. [Google Scholar]
- 6.Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in a female. In: Sperling MA, editor. Pediatric Endocrinology. 3rd ed. Philadelphia: WB Saunders; 2008. pp. 573–90. [Google Scholar]
- 7.Cavallo A, Richards GE, Busey S, Michaels SE. A simplified gonadotrophin-releasing hormone test for precocious puberty. Clin Endocrinol (Oxf) 1995;42:641–6. doi: 10.1111/j.1365-2265.1995.tb02692.x. [DOI] [PubMed] [Google Scholar]
- 8.Eckert KL, Wilson DM, Bachrach LK, Anhalt H, Habiby RL, Olney RC, et al. A single-sample, subcutaneous gonadotropin-releasing hormone test for central precocious puberty. Pediatrics. 1996;97:517–9. [PubMed] [Google Scholar]
- 9.Houk CP, Kunselman AR, Lee PA. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics. 2009;123:e1059–63. doi: 10.1542/peds.2008-1180. [DOI] [PubMed] [Google Scholar]
- 10.Supornsilchai V, Hiranrat P, Wacharasindhu S, Srivuthana S, Aroonparkmongkol S. Basal luteinizing hormone/follicle stimulating hormone ratio in diagnosis of central precocious puberty. J Med Assoc Thai. 2003;86(Suppl 2):S145–51. [PubMed] [Google Scholar]
- 11.Poomthavorn P, Khlairit P, Mahachoklertwattana P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm Res. 2009;72:114–9. doi: 10.1159/000232164. [DOI] [PubMed] [Google Scholar]
- 12.Sathasivam A, Garibaldi L, Shapiro S, Godbold J, Rapaport R. Leuprolide stimulation testing for the evaluation of early female sexual maturation. Clin Endocrinol (Oxf) 2010;73:375–81. doi: 10.1111/j.1365-2265.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 13.Ibáñez L, Potau N, Zampolli M, Virdis R, Gussinyé M, Carrascosa A, et al. Use of leuprolide acetate response patterns in the early diagnosis of pubertal disorders: Comparison with the gonadotropin-releasing hormone test. J Clin Endocrinol Metab. 1994;78:30–5. doi: 10.1210/jcem.78.1.7507123. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfield RL. Menstrual disorders and hyperandrogenism in adolescence. In: Radovick S, MacGillivray MH, editors. Pediatric Endocrinology: A practical clinical guide. Totowa, NJ: Humana Press; 2003. pp. 451–78. [Google Scholar]
- 15.Menon PS, Khatwa UA. The child with micropenis. Indian J Pediatr. 2000;67:455–60. doi: 10.1007/BF02859468. [DOI] [PubMed] [Google Scholar]
- 16.Grinspon RP, Ropelato MG, Gottlieb S, Keselman A, Martínez A, Ballerini MG, et al. Basal follicle-stimulating hormone and peak gonadotropin levels after gonadotropin-releasing hormone infusion show high diagnostic accuracy in boys with suspicion of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2010;95:2811–8. doi: 10.1210/jc.2009-2732. [DOI] [PubMed] [Google Scholar]
- 17.Segal TY, Mehta A, Anazodo A, Hindmarsh PC, Dattani MT. Role of gonadotropin-releasing hormone and human chorionic gonadotropin stimulation tests in differentiating patients with hypogonadotropic hypogonadism from those with constitutional delay of growth and puberty. J Clin Endocrinol Metab. 2009;94:780–5. doi: 10.1210/jc.2008-0302. [DOI] [PubMed] [Google Scholar]
- 18.Carel JC. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123:e752–62. doi: 10.1542/peds.2008-1783. [DOI] [PubMed] [Google Scholar]
- 19.Pescovitz OH, Comite F, Cassorla F, Dwyer AJ, Poth MA, Sperling MA, et al. True precocious puberty complicating congenital adrenal hyperplasia: Treatment with a luteinizing hormone-releasing hormone analog. J Clin Endocrinol Metab. 1984;58:857–61. doi: 10.1210/jcem-58-5-857. [DOI] [PubMed] [Google Scholar]
- 20.Lawson ML, Cohen N. A single sample subcutaneous luteinizing hormone (LH)-releasing hormone (LHRH) stimulation test for monitoring LH suppression in children with central precocious puberty receiving LHRH agonists. J Clin Endocrinol Metab. 1999;84:4536–40. doi: 10.1210/jcem.84.12.6181. [DOI] [PubMed] [Google Scholar]
- 21.Brito VN, Latronico AC, Arnhold IJ, Mendonca BB. A Single luteinizing hormone determination 2 hours after depot leuprolide is useful for therapy monitoring of gonadotropin-dependent precocious puberty in girls. J Clin Endocrinol Metab. 2004;89:4338–42. doi: 10.1210/jc.2003-031537. [DOI] [PubMed] [Google Scholar]
- 22.Acharya SV, Gopal RA, George J, Bandgar TR, Menon PS, Shah NS. Utility of single luteinizing hormone determination 3h after depot leuprolide in monitoring therapy of gonadotropin-dependent precocious puberty. Pituitary. 2009;12:335–8. doi: 10.1007/s11102-009-0184-0. [DOI] [PubMed] [Google Scholar]
- 23.Winter JS, Faiman C, Hobson WC, Prasad AV, Reyes FI. Pituitary–gonadal relations in infancy: Patterns of serum gonadotrophin concentrations from birth to four years of age in man and chimpanzee. J Clin Endocrinol Metab. 1975;40:545–51. doi: 10.1210/jcem-40-4-545. [DOI] [PubMed] [Google Scholar]
- 24.Tanner JM, Healy MJ, Goldstein H, Cameron N. Assessment of Skeletal Maturity and Prediction of Adult Height (TW3 method) 3rd ed. London: WB Saunders; 2001. [Google Scholar]
- 25.Khadilkar VV, Khadilkar AV, Kinare AS, Tapasvi HS, Deshpande SS, Maskati GB. Ovarian and uterine ultrasonography in healthy Indian girls between birth to 18 years. Indian Pediatr. 2006;43:625–30. [PubMed] [Google Scholar]
- 26.Tanner JM. 2nd ed. Oxford, England: Blackwell Scientific Publications; 1962. Growth and Adolescence. [Google Scholar]
- 27.Khadilkar VV, Khadilkar AV, Cole TJ, Sayyad MG. Cross sectional growth curves for height, weight and body mass index for affluent Indian children, 2007. Indian Pediatr. 2009;46:477–89. [PubMed] [Google Scholar]


