Abstract
Aim:
The aim was to determine serum vitamin D levels in breast cancer patients and to assess its risk association with grade and stage of the tumor.
Materials and Methods:
Ninety breast cancer patients and equal number of age-matched healthy females were recruited into the study by consecutive sampling over a period of 6 months for this case control study. Serum 25(OH)2D levels and CT bone mineral density was done.
Results:
The mean age was 46±1.5 years. Age, marital status, menopausal, residential area, parda observing status, and body mass index were similar in distribution among cases and controls. The mean serum vitamin D level in the breast cancer patients was 9.3 ng/ml and in the control group was 14.9 ng/ml (P value <0.001). Vitamin D deficiency was seen in 95.6% (86) breast cancer patients and in 77% (69) of the control group (P value <0.001). Among the breast cancer patients the tumor characteristics (histology, grade, stage, and receptor status) did not show any significant associations with serum levels of vitamin D. Premenopausal breast cancer females had a mean serum vitamin D level of 10.5 ng/ml and postmenopausal females had a mean value of 13.5 ng/ml (P value 0.015). Low BMD did not correlate significantly with vitamin D deficiency (P value 0.787).
Conclusion:
Invariably almost all patients with breast cancer were vitamin D deficient. Tumor characteristics did not show any significant associations with serum levels of vitamin D. Bone mineral density did not correlate significantly with vitamin D deficiency.
Keywords: 25(OH)2D; (1,25 (OH)2D); bone mineral density; breast cancer; menopausal state; vitamin D deficiency
INTRODUCTION
In the cancer research field, vitamin D has emerged as the most prolific topic in the last decade with work connecting it with risk reduction in various epithelial cancers. Aside from calcium homeostasis, vitamin D exerts a wide range of immunogenic and antiproliferative activities in the body.[1] Of particular interest to the oncologists is the reduced incidence of breast, colon, and prostate cancers with higher sun exposure, higher intake, or higher serum levels of vitamin D.[2,3] Vitamin D exerts its antiproliferative effect by binding to vitamin D receptor (VDR) found in various tissues and cells of the body. Several human genes contain vitamin D response elements (specific DNA sequences) that encode for proteins important in regulation of cell proliferation, differentiation, apoptosis, and angiogenesis.[4,5] When the serum vitamin D levels are suboptimal these activities are impaired and as a result enhanced cellular growth, neoangiogensis, and cancer development takes place. The breast cells have VDRs in their nuclei and it is postulated that polymorphism of genes for these VDRs results in increased risk for breast cancer.[6,7]
Vitamin D from both diet and sun exposure is metabolized in the liver to 25-hydroxy vitamin D (25(OH)2D) and then further hydroxylated by 1 alpha hydroxylase enzyme in kidneys and other tissues like breast cells to 1,25- dihydroxy vitamin D (1,25 (OH)2D), the most biologically active form and the natural ligand for VDR.[4,6] Serum concentration of 25(OH)2D are more sensitive to exogenous sources (dietary and supplemental intake) and endogenous production (through synthesis in the skin) of vitamin D and have a long half-life of 3 weeks and is the predominant form of vitamin D in plasma and the major storage form; hence the circulating 25(OH)2D is the best indicator of vitamin D status of the body.[1,2]
Serum 25(OH)2D concentrations as well as treatment with vitamin D supplementation are significant independent predictors of breast cancer risk. Women with serum levels of 25(OH)2D more than 50 ng/ml had a 50% lower risk of breast cancer compared to those with serum values less than 30 ng/ml in various studies from the developing world.[5,8] It has been documented that consumption of oral calcium and serum levels of vitamin D are associated with reduced risk of breast cancer in premenopausal women but the results were not statistically significantly in postmenopausal women.[9] There are data showing that locally advanced breast cancer patients have more severe vitamin D deficiency than those with early stage disease.[10]
Low serum levels of vitamin D are common at breast cancer diagnosis and are associated with a poorer prognosis in terms of overall survival and distant disease free survival particularly in postmenopausal females.[9] In another group of breast cancer patients it was found that 94% women with serum levels of vitamin D less than 20 ng/ml were likely to develop metastases and 73% were likely to die of advance disease.[11]
Vitamin D deficiency is associated with secondary hyperparathyroidism which results in increased bone resorption, release of calcium from bones, and may precipitate or exacerbate osteoprosis with consequent ill effects on bone mineral density (BMD). There has been significant documentation of osteopenia and osteoprosis in breast cancer patients primarily due to early menopause and vitamin D deficiency and later amplified by chemotherapy and endocrine therapy particularly the aromatase inhibitors.[12] Thus breast cancer patients must undergo a baseline metabolic bone evaluation with serum vitamin D levels and bone mineral densitometry.[12,13]
PROJECT AIM
The aim of the study was to determine serum levels of 25-(OH)2D in Pakistani breast cancer patients at the time of presentation, to assess its risk association with grade and stage of the tumor and to evaluate the bone density in breast cancer patients.
MATERIALS AND METHODS
The study was approved by the Scientific Research Committee and Institutional Review Board at Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore. All newly diagnosed breast cancer patients who presented to the Medical Oncology department were recruited into the study as “cases” after informed consent over a period of 6 months from November 2010 till May 2011. Age-matched healthy females who accompanied the nonbreast cancer patients to hospital were recruited as the “control group.” The socio-demographics were documented by direct questioning on to the proforma for the whole study population. Postmenopausal status of females was defined as last menstrual bleeding at least 12 months before the date of interview or a history of bilateral oophorectomy. The histopathological diagnosis of breast cancer, grade, stage of the tumor, and hormone receptor status (estrogen receptor - ER, progesterone receptor – PR, and Her2neu) was recorded from the pathology reports of breast cancer patients. Serum 25-(OH)2D levels were studied by the ELISA technique on the blood samples drawn of the study population at their initial presentation and the values were documented in ng/ml. Vitamin D deficiency was considered at serum level less than 20 ng/ml, suboptimal vitamin D levels were considered between 20 and 39 ng/ml and optimal levels were more than 40 ng/ml. Bone mineral density was calculated according to the WHO criteria from CT bone density scan of the breast cancer patients.
Statistical analysis
Data analysis was done by using SPSS version 19. The demographic variables and the descriptive measures in cases and controls were presented in frequency and percentages. Relation of vitamin D deficiency with grades, stages, histology, and receptor status of tumor was determined by using the chi-square test. Comparison of vitamin D levels among various histopathological parameters of tumor was done by using one-way Anova. Comparison of vitamin D levels between pre- and postmenopausal status was done by using a t-test. The association of vitamin D status with BMD in cases and the serum vitamin D levels among the cases and controls was calculated by using the chi-square. A P value of ≤ 0.05 was considered statistically significant.
RESULTS
The mean age of cases was 47.5th± 9.8 years and for the control group was 46.2th + 2.6 years. There were 46.7% premenopausal females and 53.3 % postmenopausal females among the breast cancer population. Age, marital status, menopausal, residential area, and parda observing status had almost similar distribution among cases and controls. A total of 70% of the cases were multiparous and the 50% of the healthy controls had more than three children. Regarding the occupational history, 92% of the cases were house wives while 33% of the controls were office workers. Seventy percent of the study population in both groups had BMI ≥25. Breast cancer females with BMI ≥30 were 28 versus 18 in the control group.
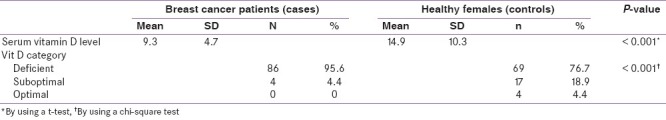
The mean serum vitamin D level in the breast cancer patient was 9.3 ng/ml and in the control group was 14.9 ng/ml and the P value calculated was <0.001. Vitamin D deficiency was seen in 95.6% (86) breast cancer patients while 77% (69) of the control group were deficient, the P value was <0.001. Suboptimal levels of vitamin D were seen in 4.4% (4) of the cases and 18.9% (17) control group, P value <0.001. None of the breast cancer patients had an optimal vitamin D level, while four patients in the control group had normal serum levels [Table 1].
Table 1.
Serum vitamin D level in cases and controls
Among the breast cancer patients the tumor characteristics (histology, grade, stage, and receptor status) did not show any significant associations with serum levels of vitamin D.
On analysis of the individual grade of breast cancer with serum vitamin D levels, it was seen that grade III tumors had a mean vitamin D level of 8.6 ng/ml + SD 3.44, while similar low levels (mean 8.5 ng/ml + SD 3.54) were also seen in grade I tumors. Grade II breast cancer patients had a mean serum vitamin D level of 10.28 + SD 6.23. The P value calculated by Anova was 0.26.
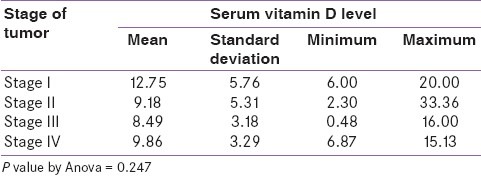
Serum vitamin D levels were found to be lower (mean 8.49 ng/ml and 9.86 ng/ml) in stage III and IV breast cancer respectively and 12.75 ng/ml in stage I disease but the P value was 0.247 [Table 2].
Table 2.
Association of serum vitamin D level with Stage of breast cancer
On comparing serum vitamin D levels with receptor status, patients with Her2neu over expression had a mean vitamin D level of 8.28 ng/ml + SD 2.3, patients with triple negative tumors had mean serum vitamin D levels 10.3 ng/ml + SD 4.65, triple positive and ER positive/Her2 negative had 9.04 ng/ml + SD 3.97 and 9.06 ng/ml + SD 5.5 respectively. The calculated P value was 0.681.
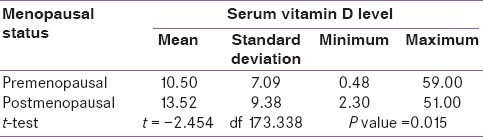
According to the menopausal state of breast cancer patients, premenopausal females had a mean serum vitamin D level of 10.5 ng/ml and postmenopausal females had a mean value of 13.5 ng/ml. The P value by t-test was 0.015 [Table 3].
Table 3.
Distribution of serum vitamin D level according to menopausal status of breast cancer patients
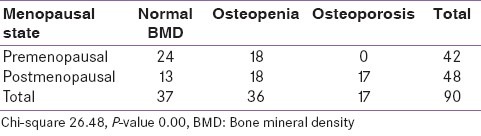
On estimation of bone mineral density (BMD) in vitamin D deficient breast cancer patients, 36 patients had normal bone density, 34 patients had Osteopenia, and 16 patients had Osteoporosis. Four patients had suboptimal vitamin D levels, out of which 2 had osteopenia and 1 had normal bone density and Osteoporosis each. The P value by chi-square test was 0.787.
Low BMD (Osteopenia and Osteoprosis) among the postmenopausal breast cancer patients was found in 35/45 (73%) females while only 18/42 (43%) premenopausal females had low BMD (osteopenia only) with a P value of <0.001 [Table 4].
Table 4.
Correlation of bone mineral density with menopausal state of breast cancer patients
DISCUSSION
Low levels of vitamin D are the norm rather than an exception in South East Asia. The prevalence of vitamin D deficiency in healthy asymptomatic people is reported in the range of 70-97% in Pakistan and this is more common in the urban population.[14] The incidence is twice that in western population. Postmenopausal women with osteoporosis are especially likely to exhibit the deficiency. Studies from United States report 50-74% vitamin D deficiency in newly diagnosed premenopausal breast cancer patients.[15] In our study we found that 95.6% breast cancer females and 77% healthy females were vitamin D deficient. Our results for the healthy control group lie within the range reported in the Pakistani literature. Serum levels of vitamin D in breast cancer patients were significantly lower than in non breast cancer Pakistani women. Hence, the association between breast cancer risk and serum levels of vitamin D parallels other studies from the developed world.
Our two groups of population were comparable in age, menopausal state, residential area (rural vs. urban) and parda observation characteristics. The study consisted of middle-aged females with equal distribution of pre- and postmenopausal females. Multiparty was high in the breast cancer women and we expect that the deficiency of vitamin D was compounded by the suboptimal nutrition in this group. Ninety-two percent of the breast cancer females were housewives looking after their children and homes and expected to have minimal sun exposure. In the control group 66% females were housewives while 34% were office going; thus assuming that the healthy subjects did get some solar exposure as they went out of the house, in this study we did not categorically ask about the direct amount of sun exposure. None of the females we interviewed was a field worker, expected to have maximum sun exposure. More than half of the study population in both groups was above the expected normal BMI. Twenty-eight (31%) females in the breast cancer group versus eighteen (20%) females in the healthy group were obese (BMI >30), revealing a small but sinister causal link of low vitamin D levels with high BMI in breast cancer patients. Lower serum levels of vitamin D have been associated with obesity and lower physical activity in various epidemiological studies.[12,13]
Serum levels of vitamin D did not correlate inversely with the advance stage and grade of breast cancer in our study. Regarding the receptor types of breast cancer (luminal types A and B, triple negative or Her2 neu over-expressed) all had similar deficient vitamin D levels and there was no difference of statistical importance. Recently Kim et al., from Korea reported poorer outcomes of vitamin D deficient patients with luminal type breast cancer. Also there are data suggesting that triple negative breast cancer patients have the highest percentage of vitamin D deficiency.[16]
Studies with both pre- and postmenopausal female populations have shown high prevalence of vitamin D deficiency despite supplementation.[17,18] Premenopausal females had slightly more low mean serum levels of vitamin D compared to the postmenopausal females and the results yielded minimum significance. Vrieling et al. recruited 1295 postmenopausal females and showed that low levels of vitamin D correlated with increased risk of distant recurrence and poor overall survival.[19]
Examination of bone mineral density in breast cancer patients revealed that osteopenia and osteoporosis was common in postmenopausal female, as expected. More than half of vitamin D deficient breast cancer females had low BMD (osteopenia or osteoporosis) but the data did not show statistical significance and hence BMD cannot be considered as an indirect marker for evidence of vitamin D status for an individual.
Vitamin D concentration may be a risk and/or a valuable prognostic factor in breast cancer patients on the basis of various studies but data from large randomized trials are still sparse. The optimal circulating level of 25(OH)2D for reducing breast cancer risk or reducing the risk of recurrence of breast cancer has yet to be defined. It is also known that there are ethnic and racial variations in serum vitamin D levels.[20] Asians have low exposure to sunlight, there is high prevalence of various malabsorption syndromes, and use of vitamin D supplementation is rare. Few epidemiological efforts have investigated the association between vitamin D concentration and breast cancer risk in Asian women. To our knowledge ours is the first study from Pakistan to assess the association between breast cancer and serum vitamin D levels.
The relatively small size of the study population limited our ability to detect statistically significant trends of vitamin D deficiency with respect to histopathological characteristics of the breast cancer. Vitamin D deficiency is rampant in Pakistan and also serum 25(OH)2D levels are reflective of a recent and not life time vitamin D intake; hence one single measurement of vitamin D levels in our study may not be reflective of long term exposure. The relevant time period during which 25(OH)2D levels may affect breast cancer occurrence or survival is currently unknown.
Regardless of whether vitamin D helps prevent cancer development or its recurrence, the high frequency of vitamin D deficiency in the Pakistani population with its adverse impact on bone health and further intolerance to various systemic cancer treatments makes it important for the oncologists to recognize, treat, and prevent vitamin D deficiency. As more studies confirm similar results, dietary vitamin D and casual sunlight exposure will be among the modifiable risk factors for breast cancer.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Khan KA, Akram J, Fazal M. Hormonal actions of vitamin D and its role beyond just being a vitamin: A review article. Int J Med Sci. 2011;3:65–72. [Google Scholar]
- 2.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: Global perspective. Ann Epidemiol. 2009;19:468–83. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Viatmin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 4.De Lyra EC, da Silva IA, Katayama ML, Brentani MM, Nonogaki S, Góes JC, et al. 25(OH) D3 and 1, 25 (OH)2D3 serum concentration and breast tissue expression of 1@hydroxylas,24 hydroxylase and Vitamin D receptor in women with and without breast cancer. J Steroid Biochem Mol Biol. 2006;100:184–92. doi: 10.1016/j.jsbmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, et al. Plasma 25-hydroxyvitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–9. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen WY, Bertone-Johnson ER, Hunter DJ, Willet WC, Hankinson SE. Association between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2335–9. doi: 10.1158/1055-9965.EPI-05-0283. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 8.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, et al. Palsma 25 hydoxyvitamin D and 1,25 hydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxy vitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–63. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri C, MacGregor T, Girgis S, Vigushin D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J Clin Pathol. 2006;59:1334–6. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willet WC. Intake of dairy products, calcium and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 12.Napoli N, Vattikuti S, Ma C, Rastelli A, Rayani A, Donepudi R, et al. High prevalence of low vitamin D and musculoskeletal complaints in women with breast cancer. Breast J. 2010;16:609–16. doi: 10.1111/j.1524-4741.2010.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camacho PM, Dayal AS, Diaz JL, Nabhan FA, Agarwal M, Norton JG, et al. Prevalence of secondary causes of bone loss among breast cancer patients with osteopenia and osteoprosis. J Clin Oncol. 2008;26:5380–5. doi: 10.1200/JCO.2008.17.7451. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal R, Khan AH. Possible causes of vitamin D deficiency in Pakistani population residing in Pakistan. J Pak Med Assoc. 2010;60:1–2. [PubMed] [Google Scholar]
- 15.Khan QJ, Fabian CJ. How I treat vitamin D Deficiency. JOP. 2010;6:97–100. doi: 10.1200/JOP.091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Lee YM, Ko BS, Lee JW, Yu JH, Son BH, et al. Vitamin D deficiency is correlated with poor outcomes in patients with luminal type breast cancer. Ann Surg Oncol. 2011;18:1830–6. doi: 10.1245/s10434-010-1465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27:2151–6. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: The Iowa Women's Health Study. Cancer Causes Control. 2007;18:775–82. doi: 10.1007/s10552-007-9020-x. [DOI] [PubMed] [Google Scholar]
- 19.Vrieling A, Hein R, Abbas S, Schneeweiss A, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxy vitamin D and postmenopausal breast cancer survival: A prospective patient cohort study. Breast Cancer Res. 2011;13:R74. doi: 10.1186/bcr2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan QJ, Kimler BF, Fabian CJ. The relationship between vitamin D and breast cancer incidence and natural history. Curr Oncol Rep. 2010;12:136–42. doi: 10.1007/s11912-010-0081-8. [DOI] [PubMed] [Google Scholar]


