Abstract
Background:
The objective of this study was to determine the prevalence of the metabolic syndrome and its components in people with thyroid disorders.
Materials and Methods:
112 subjects with a history of thyroid disorders were consecutively enrolled for the study. Clinical data were obtained by interviewing the patients and referring to their case folders and prescriptions. The subjects were categorized into three: thyrotoxic, those with hypothyroidism and those with nontoxic goiters, based on clinical parameters and or thyroid function tests. The study subjects were weighed and their anthropometric indices were documented. The laboratory parameters that were analyzed included total cholesterol, high-density and low-density cholesterol and triglyceride. Statistical analysis was performed using Student's t test, one-way analysis of variance (ANOVA) test and chi-square test.
Results:
The study subjects were aged between 14 and 76 years, with a mean age of 44.5 years, and the female:male ratio was 97:15. The mean age and anthropometric indices were comparable in subjects with thyrotoxicosis, hypothyroidism and euthyroidism. The overall prevalence of the metabolic syndrome was 28% and the frequency of occurrence of the metabolic syndrome in subjects with thyrotoxicosis, hypothyroidism and nontoxic goiter was 24%, 40% and 42%, respectively. The commonest occurring metabolic syndrome defining criterion was dysglycemia, while hypertension and elevated triglyceride were the least documented of the criteria.
Conclusion:
Metabolic syndrome occurs in 1 in every 4 persons with thyroid disorders, and as such, routine screening for this cardiovascular risk factor may be of benefit in this group of people, especially in those with hypothyroidism.
Keywords: Metabolic syndrome, Nigeria, thyroid
INTRODUCTION
Thyroid disorders are the second most common endocrine disorders in Nigeria and the disease burden associated with this group of disorders is often related to cardiovascular pathology.[1,2] Of the thyroid disorders, hyperfunctioning of the thyroid gland predominates and cardiac abnormalities of this component of thyroid dysfunction are well documented.[3,4] Epidemiologic data suggest not only that subclinical or treated thyroid disease is associated with increased vascular risk but also that the different forms of thyroid disease are associated with increased long-term vascular risk.[5] Metabolic syndrome (MetS) describes a constellation of interrelated metabolic risk factors, in which components coexist more frequently in a given individual than could be expected by chance alone. These risk factors include abdominal obesity, raised triglycerides (TG), low levels of high-density lipoprotein (HDL), elevated blood pressure and dysglycemia.[6] The underlying pathophysiology is as yet unclear, but has been closely linked to insulin resistance and obesity.
Thyroid disorders may be associated with cardiovascular disorders, especially since the thyroid hormones often affect the functions of the heart, and thus Thyroid dysfunction (TD) may be regarded as an independent cardiovascular risk factor. The MetS has been studied in people with various thyroid disorders and in euthyroid individuals.[7,8] In an Indian report,[7] hypothyroidism was found to be associated with the MetS, with females being more at risk than males. In another report[8] on euthyroid subjects, free thyroxine (FT4) was found to be significantly related to some components of the MetS. Overt hypothyroidism is reported to be a recognized risk factor for atherosclerotic cardiovascular disease, hyperlipidemia, low-grade inflammation and hypercoagulability.[9,10] The MetS, a well-recognized cardiovascular risk factor, is not widely studied in persons with thyroid dysfunction. The occurrence of the MetS in persons with thyroid dysfunction may connote a state of “double jeopardy” in persons with TD.
In this report, we aimed to document the frequency of occurrence of the MetS in various categories of thyroid disorders using a simple classification to categorize TD into thyrotoxicosis, hypothyroidism and nontoxic goiters.
MATERIALS AND METHODS
This was a cross-sectional study carried out at the Endocrine Clinic of the Lagos State University Teaching Hospital (LASUTH), Ikeja, Lagos State of Nigeria. Approval was given by the ethics committee of LASUTH. The research procedures were in accordance with the Helsinki Declaration.
A total of 112 subjects known to have thyroid disease were recruited consecutively for the study. The inclusion criteria for enrollment into the study included the following: (1) the presence of a goiter with or without abnormal thyroid function test results; (2) abnormal thyroid function test in the absence of goiter; and (3) patients already on treatment for at least a month for thyroid disorders. Exclusion criteria included a history of thyroid malignancy, patients with features suggestive of thyroid crisis and those that were ill enough to warrant hospitalization. Other exclusion criteria were patients with liver disorders, renal disorders, congestive cardiac failure, pregnant women and patients on oral contraceptive pills.
The study patients who met the inclusion criteria were categorized into three broad groups according to clinical parameters and biochemical features of thyroid disorders into those with nontoxic goiters, thyrotoxicosis and hypothyroidism. The clinical parameters that were obtained via interviewer-administered questionnaires included history pertaining to thyroid disease, management modalities and the presence of comorbidities which included diabetes mellitus (DM) and hypertension. The clinical features of thyroid disease were essentially those that were indicative of thyrotoxicosis, hypothyroidism and the presence of enlargement of the thyroid gland. Data extracted from the patients’ case folders which included treatment type also indicated the diagnosis of the underlying thyroid disorder.
Other findings documented from clinical examination included body mass indices obtained from the measurements of the weight and height and using the formula weight in kg/height in m2 types. The waist circumference (WC) was measured at a point midway between the lilac crest and the lower rib.
The laboratory parameters that were assessed included blood glucose, lipid parameters [total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C) and TG]. Venous blood samples were collected in a fasting state in the morning for analysis of the aforementioned biochemical parameters.
Lipid analyses: TC assay was done using the modified Liebermann-Burchard's method,[11] LDL-C was calculated using the Friedwalds's formula[12] and TG results were obtained using enzymatic colorimetric methods.[13]
Blood glucose analysis was done using the glucose oxidase method.[14]
Working diagnosis
Goiter: This referred to a thyroid gland which had lateral lobes with a volume greater than the terminal phalanges of the thumb of a person or an ultrasound scan evidence of an enlarged thyroid gland.[15,16]
The presence of the MetS was determined using the new definition.[6] The presence of three or more of any of the following is a pointer to the MetS: WC greater than 94 cm in men and 80 cm in women for people from sub-Saharan Africa; serum TG level of at least 150 mg/dL (1.69 mmol/L); HDL-C level of less than 40 mg/dL (1.04 mmol/L) in men and 50 mg/dL (1.29 mmol/L) in women; blood pressure of at least 130/85 mm Hg; or serum glucose level of at least 100 mg/dL (5.6 mmol/L).
Nontoxic goiters: Subjects with goiters who have never been treated for thyroid diseases and are clinically and biochemically euthyroid. Normal thyroid function tests referred to thyroid stimulating hormone (TSH) levels ranging from 0.3 to 4 mIU/L, free T4 levels ranging from 0.7 to 1.4 ng/dL, and free T3 levels ranging from 0.2 to 0.5 ng/dL.
Thyrotoxicosis: This was said to be present if the subject had clinical and/or biochemical evidence of thyrotoxicosis or was on antithyroid medications. The biochemical evidence of thyrotoxicosis referred to a TSH level of less than 0.3 mIU/L, with elevated T4 and/or elevated T3 levels.
Hypothyroidism: Subjects with biochemical or/and clinical evidence of hypothyroidism or on thyroxine replacement. The biochemical evidence of hypothyroidism referred to TSH levels greater than 4 mIU/L and low T4 or low T3 levels.
RESULTS
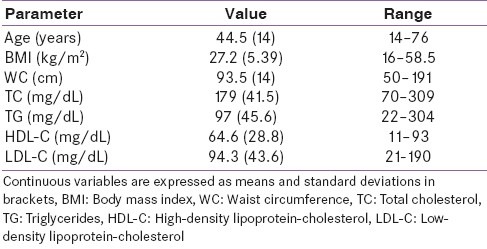
The ratio of females to males was 97:15. The mean age of the females was lower than that of the males, but there was no significant difference in their ages [44.2 (14) years vs. 50 (13) years, P-0.1]. A summary of the clinical and biochemical characteristics of the study subjects is shown in Table 1.
Table 1.
Baseline characteristics of the study subjects
The distribution of the three categories of thyroid disorders according to thyroid function is shown in Figure 1.
Figure 1.
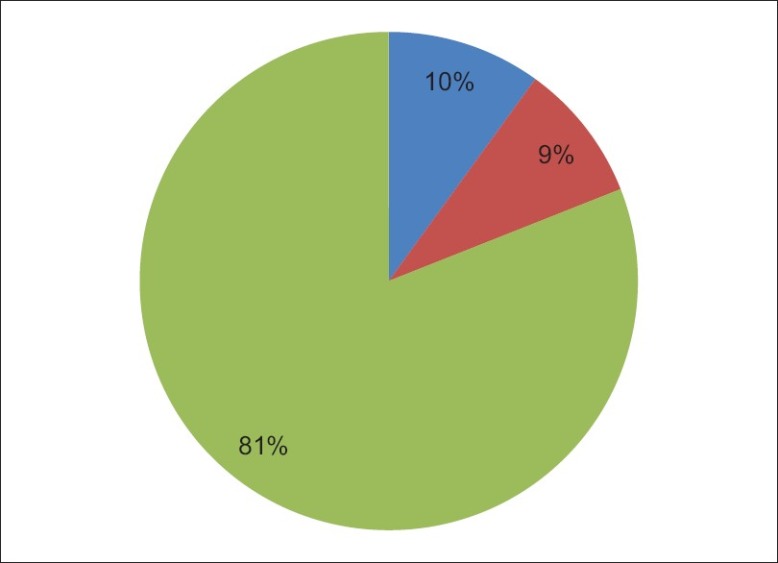
Distribution of the categories of thyroid disorder
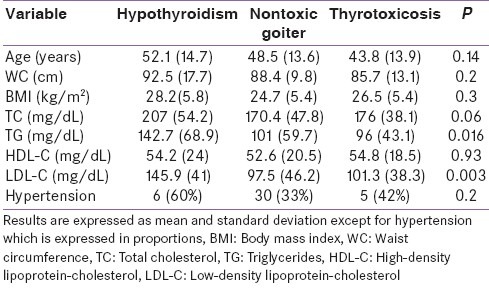
The male:female ratios of the subjects with hypothyroidism, nontoxic goiters and thyrotoxicosis were 5:5, 12:0 and 81:9, respectively. The mean TG and LDL-C levels were statistically significantly higher in subjects with hypothyroidism than in those belonging to the other two categories of thyroid disorders. The results of comparison of some clinical and biochemical data between the three categories of thyroid disorders using analysis of variance (ANOVA) are shown in Table 2.
Table 2.
Comparison of some biochemical and clinical variables in the three categories of thyroid disorders
The number of the study subjects with the MetS was 31, thus giving a frequency of occurrence of 28%. The proportion of subjects with hypothyroidism, euthyroidism and thyrotoxicosis who had the MetS was 4 (40%), 5 (42%) and 22 (24%), respectively. There was no significant difference between these proportions (P=0.3).
Dysglycemia was the commonly occurring MetS defining criterion and it was documented in 46% of the study subjects. Eighteen (16%) of the study subjects had a history of DM. The distribution of the components of the MetS in the study subjects is shown in Figure 2.
Figure 2.
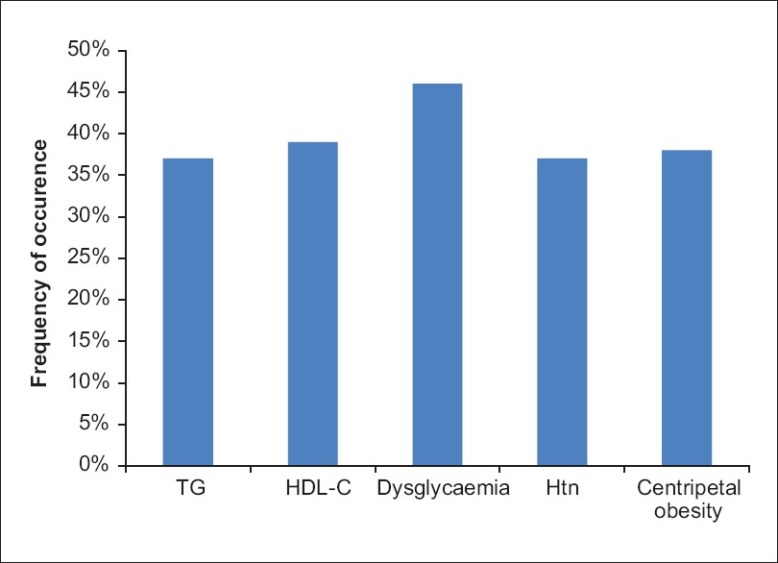
Distribution of the components of the MetS in the study objects
Of the three categories of the subjects with thyroid disorders, elevated TG was the only MetS defining criterion that differed significantly (P- 0.003). The distribution of the MetS criteria in the three categories of thyroid disorders is shown in Figure 3.
Figure 3.
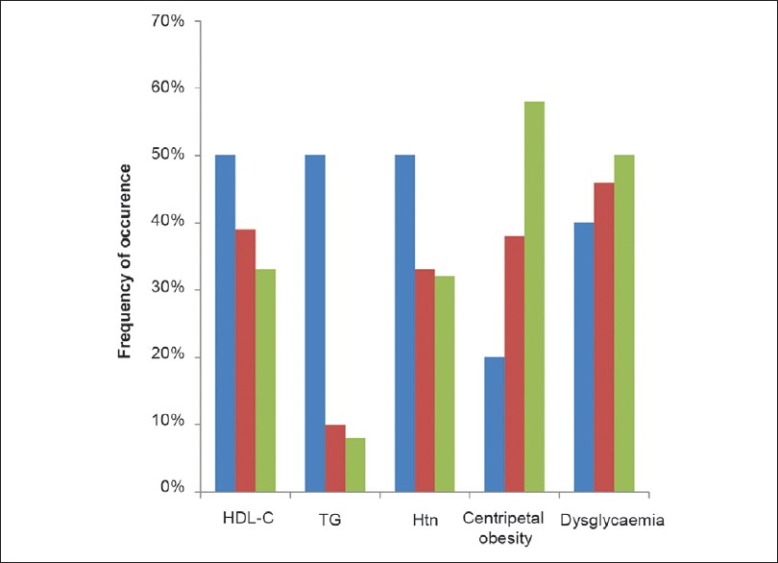
Distribution of the components of the MetS (Series 1 – Subjects with hypothyroidism; Series 2 – Subjects with nontoxic goiter; Series 3 – Subjects with thyrotoxicosis)
DISCUSSION
Thyroid diseases are often associated with cardiovascular morbidity, but the mechanisms that mediate this risk are unclear. Certain mechanisms have been proposed to be potentially responsible for this scenario and the MetS is one of these. The potential contributory role of the MetS to cardiovascular risk and its scope in subjects with TD was the focus of this study.
The main findings of this report are the documentation of the presence of the MetS in about a fourth of our patients with thyroid diseases. One limitation of this study lies in its nature which comprised a small study population of patients who were already on treatment for thyroid disorders.
In our report, we found the prevalence of the MetS to be 28%, with subjects with thyrotoxicosis having the least frequency of occurrence of the MetS compared with subjects with hypothyroidism and those with nontoxic goiters. The comparison of the MetS in this report showed that the occurrence of this cardiovascular risk factor was comparable in the three categories of study subjects. It is pertinent to note that the study subjects as classified in these three groups had comparable mean ages and anthropometric indices. Of the components of the MetS, dysglycemia was the prevalent MetS defining criterion documented in the subjects with TD. Reported prevalence rates of glucose intolerance in subjects with thyrotoxicosis and hypothyroidism are 40% and 50%, respectively.[17] In subjects with hypothyroidism, insulin resistance is suggested as the possible underlying pathophysiological basis for glucose intolerance when present.[18] The effects of excess thyroid hormone on carbohydrate metabolism are complex, with alterations in insulin secretion, insulin clearance, gluconeogenesis, glycogen synthesis, glucose oxidation, nonoxidative glucose metabolism, adipokine signaling, and lipid oxidation contributing to the state of hyperglycemia and insulin resistance observed in thyrotoxicosis.[19]
Abnormal lipid profile is an often documented abnormality in thyroid disorders, and some reports[20] have demonstrated that thyroid hormones influence LDL-C by various mechanisms which include catabolism of LDL-C–independent alterations in metabolism, stimulation of the synthesis of cholesterol as well as the influence on biliary lipid metabolism. Well-documented lipid abnormalities in hypothyroidism include hypercholesterolemia and elevated LDL levels, but HDL-C levels may be normal or elevated in severe hypothyroidism.[21] In this study, the mean levels of TG, TC and LDL-C were higher in persons with hypothyroidism than in those belonging to the other categories of TD, but statistically significant differences in the lipid parameters, TG and LDL-C, were noted between subjects with hypothyroidism and other subjects with TD. Thyroid hormones are known to affect the cardiovascular system both directly and indirectly and result in increased cardiac contractility and reduced systemic vascular resistance. Ogbera et al.[2] in a previous report had noted that thyrotoxicosis is a significant cause of cardiac mortality and morbidity in Nigerians and that systemic hypertension is a comorbidity that is found in half of the subjects with thyrotoxicosis. In this study, systemic hypertension occurred least in subjects with thyrotoxicosis, with a prevalence rate of 37%. Our findings on hypothyroidism and hypertension are similar to those by Saito et al.[22] who compared the occurrence of hypertension in subjects with hypothyroidism and those with euthyroidism. They found the rate of occurrence of hypertension to be 15.8% and this was significantly higher than that in euthyroid subjects in whom the rate was 5.5%.[22] In our study, subjects with hypothyroidism had the greatest frequency of occurrence of hypertension. Hypothyroidism is a potentially important but overlooked cause of hypertension, and possible pathophysiological mechanisms responsible for the occurrence of hypertension in hypothyroidism include changes in circulating catecholamines, their receptors and renin–angiotensin–aldosterone.[23] Our results indicate that persons with hypothyroidism had higher mean levels and higher proportions of some of the MetS defining criteria compared with persons with nontoxic goiters and those with thyrotoxicosis. A Korean study[24] not only reported a relationship between thyroid function and cardiovascular risk factors, such as BP, TC, TG, HDL-C and fasting glucose, but also showed that higher levels of TSH indicative of hypofunctioning of the thyroid gland may predict the MetS in Koreans. A significant relationship between thyroid hormone and the components of the MetS has also been shown in the study by Kim et al.[25] in a report that sought to clarify the association of serum FT4 level with the presence of the MetS and its components in healthy euthyroid subjects. Although we did not set out to evaluate the insulin resistance which forms the basis of the MetS, one form of thyroid dysfunction, specifically hypothyroidism, has also been found to be associated with a syndrome – polycystic ovary disease – a condition with underlying insulin resistance.[26] Some other unusual association that has been found in relation to thyroid dysfunction is increased risk of dysglycemia – a MetS defining criterion – in TD subjects with impaired sleep disorder.[27]
Central obesity has a role in the development of the MetS and is reported to sometimes precede the appearance of other MetS components.[28] The occurrence of central obesity was comparable in the three categories of subjects with thyroid diseases and was noted in 38% of the study subjects.
The overall prevalence rate of the MetS in this study was much lower than the 86% rate reported in the diabetes population[29] and the 59% rate reported in the general population among Nigerians.[30]
The results of this report should be interpreted with caution, given the small number of the study population and also the fact that the patients were not thyroid treatment naive.
CONCLUSION
Routine screening for cardiovascular risk factors in patients with thyroid disorders, especially in those with hypothyroidism, may unmask the MetS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ogbera AO, Fasanmade O, Adediran O. Pattern of thyroid disorders in the SouthWestern region of Nigeria. Ethn Dis. 2007;17:327–30. [PubMed] [Google Scholar]
- 2.Ogbera AO, Fasanmade O, Isiba A. The scope of cardiac complications of thyrotoxicosis in Lagos Nigeria. Pak J Med Sci. 2007;23:671–5. [Google Scholar]
- 3.Klein I, Ojamaa K. Thyroid hormones and the cardiovascular system. N Engl J Med. 2001;15:501–9. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 4.Degroot LJ, Larsen PR, Refetoff S, Stanbury JB. The thyroid and its diseases. 5th ed. New York: Wiley Medical Publications; 1984. Clinical abnormalities of the heart; pp. 482–5. [Google Scholar]
- 5.Nyirenda MJ, Clark DN, Finlayson AR, Read J, Elders A, Bain M, et al. Thyroid disease and increased cardiovascular risk. Thyroid. 2005;15:718–24. doi: 10.1089/thy.2005.15.718. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome.A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Shantha GP, Kumar AA, Jeyachandran V, Rajamanickam D, Rajkumar K, Salim S, et al. Association between primary hypothyroidism and metabolic syndrome and the role of C reactive protein: A crosssectional study from South India. Thyroid Res. 2009;2:2. doi: 10.1186/1756-6614-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic svyndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–6. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 9.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457–69. [PubMed] [Google Scholar]
- 10.Dillmann WH. Mechanism of action of thyroid hormones. Med Clin North Am. 1985;69:849–61. doi: 10.1016/s0025-7125(16)30993-2. [DOI] [PubMed] [Google Scholar]
- 11.Abell LL, Levy BB, Brodie BB, Kendall FE. Simplified methods for the estimation of the total cholesterol in serum and demonstration of specificity. J Biol Chem. 1952;195:357–66. [PubMed] [Google Scholar]
- 12.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultra centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.McGowan MW, Artiss JD, Strandergh DR. Peroxidase-Coupled method for the calometric determination of serum triglycerides. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Cantrill JA. Diabetes Mellitus. In: Walker R, Edwards C, editors. Clinical Pharmacy and Therapeutics. 2nd ed. London: Churchill Livingstone; 1999. pp. 633–52. [Google Scholar]
- 15.Berhama N, Michael KF, Bezabih M. Endemic goiter in school children in school children in SouthWestern Ethiopia. Ethiop J Health Dev. 2004;18:175–8. [Google Scholar]
- 16.Hetzel BS. Iodine deficiency disorders and their eradication. Lancet. 1983;2:1126–9. doi: 10.1016/s0140-6736(83)90636-0. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsen PM, Danowski TS, Vidalon C, Ahmad U, Nolan S, Stephen T. Glucose tolerance, insulin, and growth hormone in thyrotoxicosis and in myxedema. Endocr Res Commun. 1974;1:435–48. doi: 10.3109/07435807409088999. [DOI] [PubMed] [Google Scholar]
- 18.Shah JH, Motto GS, Papagiannes E, Williams GA. Insulin metabolism in hypothyroidism. Diabetes. 1975;24:922–5. doi: 10.2337/diab.24.10.922. [DOI] [PubMed] [Google Scholar]
- 19.Potenza M, Via MA, Yanagisawa RT. Excess thyroid hormone and carbohydrate metabolism. Endocr Pract. 2009;15:254–62. doi: 10.4158/EP.15.3.254. [DOI] [PubMed] [Google Scholar]
- 20.Abrams JJ, Grundy SM. Cholesterol metabolism in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:323–38. [PubMed] [Google Scholar]
- 21.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–93. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 22.Saito I, Ito K, Saruta K. Hypothyroidism as a cause of hypertension. Hypertension. 1983;5:112–5. doi: 10.1161/01.hyp.5.1.112. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher AK, Weetman AP. Hypertension and hypothyroidism. J Hum Hypertens. 1998;12:79–82. doi: 10.1038/sj.jhh.1000574. [DOI] [PubMed] [Google Scholar]
- 24.Park SB, Choi HC, Joo NS. The relation of thyroid function to components of the metabolic syndrome in korean men and women. J Korean Med Sci. 2011;26:540–5. doi: 10.3346/jkms.2011.26.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, et al. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf) 2009;70:152–60. doi: 10.1111/j.1365-2265.2008.03304.x. [DOI] [PubMed] [Google Scholar]
- 26.Sridhar GR, Nagamani G. Hypothyroidism presenting with polycystic ovary syndrome. J Assoc Physicians India. 1993;41:88–90. [PubMed] [Google Scholar]
- 27.Sridhar GR, Putcha V, Lakshmi G. Sleep in thyrotoxicosis. Indian J Endocrinol Metab. 2011;15:23–6. doi: 10.4103/2230-8210.77578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron AJ, Boyko EJ, Sicree RA, Zimmet PZ, Söderberg S, Alberti KG, et al. Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring) 2008;16:2707–16. doi: 10.1038/oby.2008.412. [DOI] [PubMed] [Google Scholar]
- 29.Ogbera AO. Prevalence and gender distribution of the metabolic syndrome. Diabetol Metab Syndr. 2010;2:1. doi: 10.1186/1758-5996-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahab KW, Sani M, Gbadamosi M, Yandutse M. Frequency and determinants of the metabolic syndrome in apparently healthy adult Nigerians. Trop Doct. 2008;38:224–6. doi: 10.1258/td.2007.070335. [DOI] [PubMed] [Google Scholar]


