Abstract
Objectives
To determine the usefulness of urinary bromotyrosine, a noninvasive marker of eosinophil-catalyzed protein oxidation, in tracking with indexes of asthma control and in predicting future asthma exacerbations in children.
Study design
Children with asthma were recruited consecutively at the time of clinic visit. Urine was obtained, along with spirometry, exhaled nitric oxide, and Asthma Control Questionnaire data. Follow-up phone calls were made 6 weeks after enrollment.
Results
Fifty-seven participants were enrolled. Urinary bromotyrosine levels tracked significantly with indexes of asthma control as assessed by Asthma Control Questionnaire scores at baseline (R = 0.38, P = .004) and follow-up (R = 0.39, P = .008). Participants with high baseline levels of bromotyrosine were 18.1-fold (95% CI 2.1–153.1, P = .0004) more likely to have inadequately controlled asthma and 4.0-fold more likely (95% CI 1.1–14.7, P = .03) to have an asthma exacerbation (unexpected emergency department visit; doctor’s appointment or phone call; oral or parenteral corticosteroid burst; acute asthma-related respiratory symptoms) over the ensuing 6 weeks. Exhaled nitric oxide levels did not track with Asthma Control Questionnaire data; and immunoglobulin E, eosinophil count, spirometry, and exhaled nitric oxide levels failed to predict asthma exacerbations.
Conclusions
Urinary bromotyrosine tracks with asthma control and predicts the risk of future asthma exacerbations in children.
Current guidelines for asthma treatment from the National Institutes of Health (NIH) emphasize not only the need for asthma control, but also a reduction of airway inflammation. This is because of the wealth of data indicating airway inflammation directly contributes to airway hyperresponsiveness and obstruction, giving rise to clinical symptoms at baseline and, worse yet, during exacerbations.1,2 Mechanism-based noninvasive markers that concomitantly track with the extent of underlying airway inflammation, the degree of asthma control, and the risk of future exacerbations, could prove to be of tremendous clinical utility in achieving the goals set forth by NIH guidelines.
Sputum eosinophils have shown some promise in this regard. They serve as an indirect metric of the extent of eosinophilic airway inflammation that, according to early studies, tracks with indexes of asthma control and maybe even predicts exacerbations in children with asthma.3,4 Unfortunately, samples for sputum eosinophils are relatively difficult to collect, especially in children, because the hypertonic saline solution induction protocols are not well tolerated; moreover, the cytologic examination required is both relatively expensive and time-consuming.5 An alternative and perhaps better tolerated noninvasive diagnostic test for monitoring airway inflammation is exhaled nitric oxide (NO). Some studies have demonstrated a correlation between exhaled NO levels and eosinophilic airway inflammation. NO is produced abundantly in asthmatic airways as a result of the up-regulation of inducible NO synthase by proinflammatory mediators.7 However, exhaled NO, too, has its limitations, including an unclear relationship to risk for asthma exacerbation.8,9
Beyond NO, the inflammatory milieu of asthmatic airways gives rise to a diverse array of oxidant species.10 Although these oxidants are generally too labile to measure directly, the molecular footprints they leave behind can serve as quantitative indexes of the precise inflammatory processes at play. For example, we have shown that eosinophils generate potent brominating oxidants, such as hypobromous acid, when recruited to asthmatic airways and activated by proinflammatory mediators.11,12 On activation, eosinophils generate a respiratory burst and secrete granule proteins, including eosinophil peroxidase. We have shown that eosinophil peroxidase uses respiratory burst–generated hydrogen peroxide to form reactive brominating species such as hypobromous acid, a highly potent antimicrobial oxidant that can preferentially brominate protein tyrosine residues to form 3-bromotyrosine (BrTyr), a stable post-translational modification of proteins.11,12 BrTyr can be measured noninvasively in urine as a relatively specific molecular marker of eosinophil activation (respiratory burst and degranulation). After oxidative modification (eg, bromination) of proteins, as airway remodeling occurs and the modified proteins are digested/degraded, the oxidized amino acids are efficiently removed by excretion in urine. In fact, in a recent clinical study of patients with asthma and healthy control subjects, we showed that urinary BrTyr serves as a noninvasive marker that is higher in patients with asthma and associated with multiple spirometric parameters of airway obstruction.13
In this study, we sought to investigate the potential clinical utility of urinary BrTyr as a sensitive noninvasive marker for both assessing the degree of asthma control and the risk of subsequent acute decompensation of chronic asthma control in pediatric participants. In parallel, the ability of exhaled NO, a distinct noninvasive marker of airway inflammation, along with traditional blood and spirometric measures of asthma control, were examined for their ability to predict asthma exacerbations over a near term (6-week) follow-up period.
Methods
This prospective, observational study was approved by the Institutional Review Board at the Cleveland Clinic, Cleveland, Ohio. Informed consent was obtained in writing from all participants aged 18 years and older. For participants aged 8 to 17 years, informed consent was obtained in writing from each participant’s parent/guardian, and informed assent was obtained in writing from the participant him/herself. For participants aged 7 years and younger, informed consent was obtained in writing from the participant’s parent/guardian.
Participants were recruited consecutively from the Sections of Pediatric Pulmonology and Pediatric Allergy and Immunology at the Cleveland Clinic if they were aged 5 to 21 years and diagnosed with asthma by a pediatric pulmonologist or allergist. All subjects enrolled were considered to be at baseline status and were being seen as part of a regularly scheduled clinic visit. Subjects considered to be having an exacerbation at clinic visit were not included in this study. Additional exclusion criteria included comorbid lung disease or inflammatory conditions, cystic fibrosis, diabetes mellitus, any smoking history, and current immunosuppressive therapy other than corticosteroids for asthma. Only three of the participants were receiving oral corticosteroids at their initial baseline visit. Recruitment occurred between July and September 2006, and follow-up data were collected between August and November 2006.
Asthma control was assessed at the initial study encounter with the six-item Asthma Control Questionnaire (ACQ6), developed by Juniper et al,14 which is a validated metric of asthma control that has been administered to children.15,16 Six weeks after the initial study encounter, participants (with parent/guardians) were readministered the ACQ6 by telephone and asked about any asthma exacerbations since the initial study encounter, defined a priori as an acute de-compensation of asthma control resulting in a visit to the emergency department, unexpected doctor’s appointment or phone call, oral or parenteral corticosteroid burst, or an acute onset of asthma-related respiratory symptoms per self-report. All clinicians involved with the care of study participants were blinded to ACQ6 results, urinary BrTyr levels, and exhaled NO measurements.
Spirometry was performed as part of routine clinical care during the initial study encounter with an automated spirometer, as described elsewhere,17 consistent with American Thoracic Society standards.18 When clinically indicated, spirometry was repeated after the administration of two puffs (180 μg) of albuterol. Exhaled NO was measured during the initial study encounter by an online method at a constant flow rate of 50 mL/s, consistent with American Thoracic Society standards.19,20
Spot clean-catch urine samples were collected at the initial study encounter, from which levels of free BrTyr were determined by stable isotope dilution high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry using [13C6]-BrTyr as an internal standard, as described elsewhere.13 To adjust for variations in urinary dilution, BrTyr concentrations are reported as a ratio with urinary creatinine concentrations, which was determined by standard laboratory techniques.
Charts were reviewed for relevant medical history, current medications, and laboratory results on record, such as complete blood counts, serum IgE levels, and allergy skin tests that were performed as part of routine clinical care. Allergy skin test results were considered “positive” if the participant reacted to at least two of 16 allergens tested.
Statistical Analyses
Comparisons of continuous variables between the two groups were assessed with Wilcoxon rank-sum tests. Relationships between two continuous variables were assessed with Spearman rank correlation coefficients. Categorical variables were assessed with likelihood ratio χ2 tests (or two-tailed Fisher exact tests in the event that any of the expected frequencies was less than five). A P value ≤ .05 was defined as statistically significant. All statistical analyses were performed in JMP version 8 (SAS Institute, Cary, North Carolina).
Results
Fifty-seven children with asthma (19 female) participated in the study (Table I; available at www.jpeds.com), all of whom completed the ACQ6 and provided urine specimens at the initial study encounter, defined as baseline. Ages ranged between 6 and 20 years with a median age of 11 years. On average, children in the study sample fell in higher percentiles of body mass index (BMI) than children in the general population, with a median BMI percentile of 73.1 and IQR of 48.2% to 97.5%. Forty-five participants were able to perform spirometry with adequate technique, 22 of whom performed repeat spirometry after administration of albuterol. Median spirometric parameters fell within normal limits, as defined by NIH guidelines,2 specifically the percent of the predicted value of the forced expiratory volume in 1 second (FEV1 percent predicted, median 96.6% predicted), the ratio of the forced expiratory volume in 1 second to the forced vital capacity (FEV1/FVC, median 0.85), and the percent change in FEV1 after administration of bronchodilator (median 6.4%). Many participants (70%) were treated with inhaled corticosteroids at baseline. Three of the subjects were also concomitantly maintained on oral corticosteroids at baseline. A significant proportion of participants (64%) were atopic on the basis of skin testing results. The median (IQR) BrTyr level was 0.12 (0.00–0.31) ng/mg creatinine; the median (IQR) NO level was 9.1 (6.0–19.8) parts per billion (ppb). Median (IQR) ACQ6 scores at baseline and follow-up were 0.50 (0.17–1.08) and 0.33 (0.17–1.50), respectively, consistent with adequately controlled asthma.21 At follow-up 6 weeks after baseline, 36% of participants had experienced an asthma exacerbation since baseline, including all three of the participants maintained on oral corticosteroids. Features of the study participants and symptoms associated with asthma exacerbations are summarized in Table I.
Table I.
Features of study participants (n = 57)
| Age (years) | 11.0 (8.0–15.0) |
| Sex (male/female) | 38:19 |
| BMI percentile | 73.1 (48.2–97.5) |
| FEV1 % predicted | 96.6 (89.6–106.5) |
| FEV1/FVC | 0.85 (0.79–0.89) |
| % Change FEV1 after bronchodilator | 6.4 (3.8–8.6) |
| % Corticosteroids* | 70 |
| % Inhaled corticosteroids | 70 |
| % Oral corticosteroids | 5 |
| % Parenteral corticosteroids | 0 |
| % Long-acting β-agonists | 32 |
| % Leukotriene receptor antagonist | 54 |
| % Omalizumab | 0 |
| Total WBC × 106 | 6.9 (6.5–8.1) |
| % Neutrophil | 53.1 (40.4–65.4) |
| % Eosinophil | 2.0 (1.0–3.7) |
| % Lymphocyte | 34.1 (26.3–46.5) |
| % Monocyte | 7.6 (5.6–9.8) |
| % Basophil | 0.4 (0.3–0.8) |
| IgE (IU/mL) | 105.7 (3.6–520.5) |
| Positive allergy skin test† | 64% |
| ACQ6score | |
| At baseline‡ | 0.50 (0.17–1.08) |
| At follow-up | 0.33 (0.17–1.50) |
| Asthma exacerbation by follow-up, %§ | 36 |
| ED visit, % | 5 |
| Unexpected clinician visit, % | 7 |
| Unexpected clinician phone call, % | 11 |
| Corticosteroid burst, % | 9 |
| None of the above, % | 23 |
| BrTyr (ng/mg creatinine) | 0.12 (0.00–0.31) |
| NO (ppb) | 9.1 (6.0–19.8) |
WBC, white blood cell; ED, emergency department.
Data are presented as median (interquartile range) unless otherwise specified.
Including inhaled, oral, and parenteral routes of administration.
Positive allergy skin test result is defined as an atopic response to at least two allergens assessed on allergy skin testing.
ACQ6 scores are calculated as the mean of six individual item scores, each graded on a Likert scale ranging from zero to six. Baseline refers to the time of marker collection; follow-up refers to 6 weeks after baseline.
Asthma exacerbation is defined as an acute decompensation of asthma control resulting in an emergency department visit, unexpected doctor’s appointment or phone call, or oral or parenteral corticosteroid burst, or as an acute onset of asthma-related respiratory symptoms per self-report.
Urinary BrTyr and exhaled NO levels did not differ between males and females, who had median (IQR) levels of BrTyr of 0.07 (0.00–0.21) ng/mg and 0.25 (0.00–0.53) ng/mg creatinine, respectively, P = .12, and median (IQR) levels of NO of 8.4 (5.4–16.4) ppb and 11.0 (6.7–31.9) ppb, respectively, P = .27. NO, but not BrTyr, correlated directly with age (R = 0.38, P = .007); neither correlated with BMI percentile. Urinary BrTyr levels demonstrated no significant correlation with spirometric parameters; NO correlated inversely with FEV1/FVC (R = −0.32, P = .04), but did not correlate with FEV1 percent predicted or the percent change in FEV1 after bronchodilator. Neither BrTyr nor NO correlated with any of the complete blood count parameters, including percent eosinophils, or IgE levels. BrTyr and NO did not correlate with one another (Table II).
Table II.
Correlations between markers and clinical parameters
| BrTyr (ng/mg Cr)
|
NO (ppb)
|
|||
|---|---|---|---|---|
| R | P value | R | P value | |
| Age (years) | 0.03 | 0.82 | 0.38 | .007 |
| BMI percentile | 0.14 | 0.31 | 0.01 | .94 |
| FEV1 % predicted | −0.19 | 0.22 | −0.02 | .90 |
| FEV1/FVC | 0.16 | 0.30 | −0.32 | .04 |
| % change FEV1 post-bronchodilator | −0.14 | 0.54 | 0.31 | .19 |
| Total WBC × 106 | −0.19 | 0.38 | 0.28 | .23 |
| % Neutrophil | −0.17 | 0.44 | 0.06 | .79 |
| % Eosinophil | 0.12 | 0.60 | −0.17 | .48 |
| % Lymphocyte | 0.10 | 0.66 | −0.06 | .79 |
| % Monocyte | 0.12 | 0.57 | −0.00 | .98 |
| % Basophil | 0.19 | 0.42 | 0.31 | .23 |
| IgE (IU/mL) | 0.34 | 0.25 | 0.50 | .12 |
| ACQ6 score at baseline* | 0.38 | 0.004 | 0.20 | .17 |
| Nocturnal waking† | 0.31 | 0.02 | 0.19 | .20 |
| Morning symptoms | 0.33 | 0.01 | 0.18 | .21 |
| Activity limitation | 0.37 | 0.005 | 0.17 | .26 |
| Short of breath | 0.29 | 0.03 | 0.14 | .33 |
| Wheeze | 0.32 | 0.01 | 0.18 | .21 |
| β2-agonist use | 0.49 | 0.0001 | 0.19 | .21 |
| ACQ6 score at follow-up | 0.39 | 0.008 | 0.27 | .10 |
| Nocturnal waking | 0.41 | 0.005 | 0.15 | .38 |
| Morning symptoms | 0.39 | 0.008 | 0.26 | .12 |
| Activity limitation | 0.43 | 0.003 | 0.41 | .01 |
| Short of breath | 0.35 | 0.02 | 0.24 | .15 |
| Wheeze | 0.36 | 0.02 | 0.22 | .18 |
| β2-agonist use | 0.14 | 0.37 | 0.19 | .25 |
| NO (ppb) | 0.21 | 0.15 | – | – |
CR, creatinine; WBC, white blood cell.
Data are presented as Spearman correlation coefficient (R) and corresponding P value.
ACQ6 scores are calculated as the mean of six individual item scores, each graded on a Likert scale ranging from zero to six. Baseline refers to the time of marker collection; follow-up refers to 6 weeks following baseline.
Individual ACQ6 item scores are graded on a Likert scale ranging from zero to six and treated as continuous variables for this analysis.
NO, but not BrTyr, was higher in participants with positive allergy skin test results (atopic) than those with negative allergy skin test results (nonatopic). The median (IQR) NO level among atopic participants was 11.1 (6.1–25.9) ppb versus 6.3 (3.9–11.5) ppb among nonatopic participants (P = .03. The median (IQR) BrTyr level among atopic participants was 0.14 (0.00–0.47) ng/mg creatinine versus 0.00 (0.00–0.47) ng/mg creatinine among nonatopics, P = .39.
Urinary BrTyr levels demonstrated a striking and consistent direct correlation with ACQ6 scores at baseline (R = 0.38, P = .004) and follow-up (R = 0.39, P = .008) (Table I). In contrast, NO did not (R = 0.20, P = .17 at baseline; R = 0.27, P = .10 at follow-up). BrTyr further correlated directly with each of the six individual items on the ACQ6 at baseline and all but one (β2-agonist use) of the items on the ACQ6 at follow-up. NO, on the other hand, did not correlate significantly with any of the individual items on the ACQ6 at baseline; at follow-up, NO correlated directly with only one item (activity limitation, R = 0.41, P = .01). Interestingly, eosinophil counts, in contrast to BrTyr levels, did not correlate with either baseline (R = 0.03, P = .88) or follow-up (R = 0.27, P = .26) ACQ6 scores.
We next examined whether baseline levels of either urinary BrTyr or exhaled breath NO were elevated among the subset of subjects who experienced an asthma exacerbation over the ensuing 6 weeks of follow-up versus those who did not experience an exacerbation. BrTyr levels were significantly higher among those destined to experience a near-term exacerbation (BrTyr median [IQR] 0.00 [0.00–0.18] vs 0.23 [0.02–0.68]; P = .02). In contrast, baseline levels of exhaled breath NO were comparable among those who experienced an exacerbation and those who did not (median [IQR] NO levels of 12.0 [5.7–16.9] vs 9.1 [6.7–22.9]; P = .71). To further assess the capacity of urinary BrTyr and exhaled NO to predict asthma exacerbations and indexes of asthma control, BrTyr and NO were dichotomized into high and low levels. High levels were defined as greater than their respective median levels; low levels were defined as less than or equal to their respective median levels. FEV1 percent predicted, FEV1/FVC, and percent change in FEV1 after bronchodilator were also dichotomized by their respective medians. ACQ6 scores at baseline and follow-up were dichotomized as ≥1.5 versus <1.5, a cut point that previous research has deemed optimal to detect inadequately controlled asthma (positive predictive value of 0.87).21 Individual ACQ6 item scores were dichotomized as at least one versus zero, indicating that the participant, in the week preceding questionnaire administration, has woken up at night because of asthma, experienced asthma symptoms on waking in the morning, was limited in his/her activities because of asthma, experienced shortness of breath, wheezed, or used a short-acting β2-agonist. Finally, the presence of an asthma exacerbation between baseline and follow-up was assessed as previously defined.
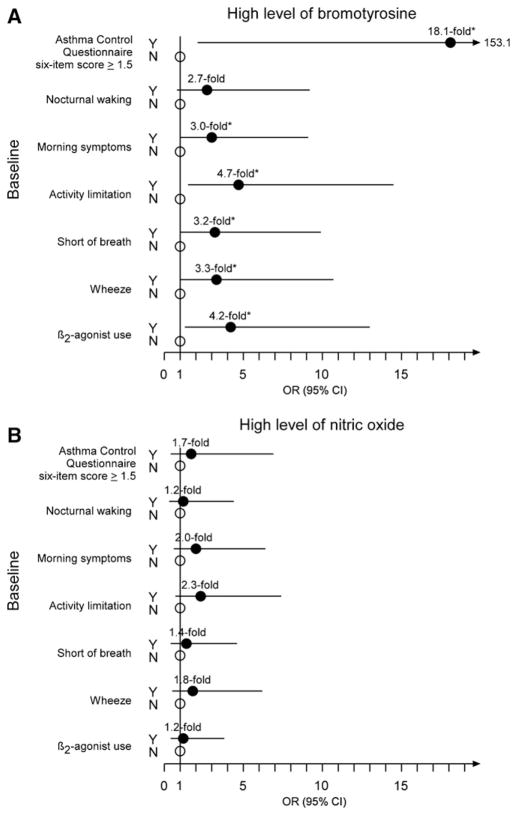
A high level of BrTyr at baseline conferred 18.1-fold the odds of inadequately controlled asthma at baseline, as defined by an ACQ6 score ≥1.5, than a low level of BrTyr (95% CI of 2.1–153.1, P = .0004). Moreover, participants with high levels of BrTyr were more likely to experience morning symptoms (OR, 95% CI of 3.0, 1.0–9.1; P = .04), activity limitation (4.7, 1.5–14.5; P = .005), shortness of breath (3.2, 1.0–9.9; P = .04), and wheezing (3.3, 1.0–10.7; P = .04) at baseline, as well as use a short-acting β2-agonist (4.2, 1.3–13.0; P = .01) at baseline. High levels of BrTyr showed a trend toward experiencing nocturnal waking at baseline, but this failed to reach statistical significance (2.7, 0.8–9.2; P = .11) (Figure 1, A). Unlike BrTyr, a high level of NO at baseline did not confer significantly greater odds of inadequately controlled asthma at baseline, neither with respect to the composite ACQ6 score nor to any of the individual asthma symptom items (Figure 1, B).
Figure 1.
ORs and 95% CI for the associations between high levels of A, bromotyrosine and B, nitric oxide (B) and uncontrolled asthma at baseline. Results shown represent the ORs (filled circles) and 95% CI (lines) of having uncontrolled asthma versus controlled asthma (open circles) at baseline for participants with high levels of bromotyrosine or nitric oxide compared with participants with low levels of these markers. Asterisk indicates P < .05.
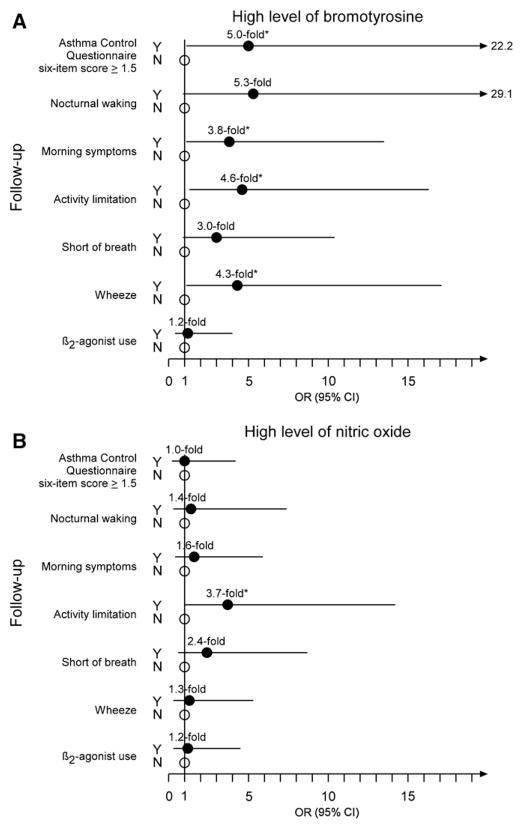
Similarly, a high level of BrTyr at baseline conferred 5.0-fold the odds of inadequately controlled asthma at follow-up than a low level of BrTyr (95% CI 1.1–22.2, P = .02). Participants with high levels of BrTyr were also more likely to experience morning symptoms (OR, 95% CI of 3.8, 1.1–13.5; P = .03), activity limitation (4.6, 1.3–16.3; P = .02), and wheezing (4.3, 1.1–17.1; P = .03) at follow-up. High levels of baseline BrTyr showed a trend toward experiencing nocturnal waking (5.3, 0.9–29.1; P = .06) and shortness of breath (3.0, 0.9–10.4; P = .07). They were not, however, more likely to use a short-acting β2-agonist at follow-up (Figure 2, A). Again, unlike BrTyr, participants with high levels of NO at baseline were not significantly more likely to have inadequately controlled asthma at follow-up, neither with respect to the composite ACQ6 score nor to any of the individual items (Figure 2, B).
Figure 2.
ORs and 95% CI for the associations between high levels of A, bromotyrosine and B, nitric oxide and uncontrolled asthma at follow-up. Results shown represent the ORs (filled circles) and 95% CI (lines) of having uncontrolled asthma versus controlled asthma (open circles). Asterisk indicates P < .05, as determined by likelihood-ratio χ2 test or two-tailed Fisher exact test in the event that any expected cell counts are < 5.
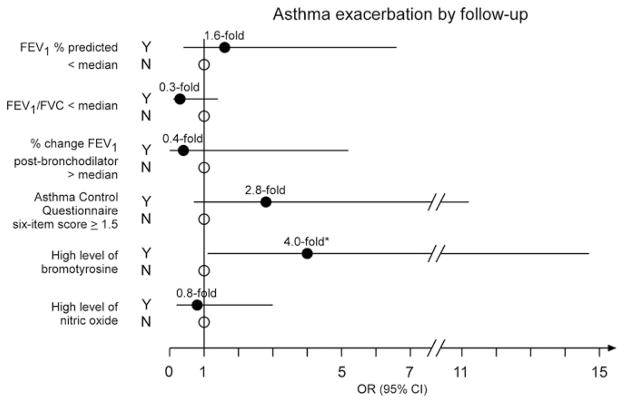
Urinary BrTyr, exhaled NO, asthma control as monitored by ACQ6, and traditional asthma laboratory diagnostics were next examined for their ability to predict asthma exacerbation over the ensuing 6-week interval. Remarkably, of all blood, urine, and spirometric measures examined, only a high level of BrTyr predicted the presence of an asthma exacerbation by follow-up; participants with elevated BrTyr levels demonstrated a 4.0-fold increased likelihood of experiencing an exacerbation by follow-up (95% CI 1.1–14.7, P = .03) compared with subjects with low BrTyr levels (Figure 3).
Figure 3.
ORs and 95% CI for the associations between the presence of an asthma exacerbation by follow-up and increased airway obstruction, greater airway reversibility, ACQ6 scores, high levels of bromotyrosine, and high levels of nitric oxide at baseline. Results shown represent the ORs (circles) and 95% CI (lines) of experiencing an asthma exacerbation by follow-up given a baseline FEV1 less than (median value, filled circle) versus ≥96.6% predicted (open circle), FEV1/FVC less than 0.85 (median value, filled circle) versus greater than or equal to 0.85 (open circle), change in FEV1 post-bronchodilator greater than 6.4% (median value, filled circle) versus ≤ 6.4%, ACQ6 score of at least 1.5 (calculated as the mean of six individual item scores, each graded on a Likert scale ranging from zero to six, a valid predictor of uncontrolled asthma; filled circle) versus less than 1.5 (open circle), high level of bromotyrosine (greater than median level of 0.12 ng/mg creatinine, filled circle) versus not a high level (open circle), and high level of nitric oxide (greater than median level of 9.1 ppb, filled circle) versus not a high level (open circle). Asterisk indicates P < .05.
Interestingly, the median BrTyr level for females was more than triple the median BrTyr level for males. Among the 28 participants with high BrTyr levels, 12 (43%) were female and 16 (57%) were male; among the 29 participants with low BrTyr levels, 7 (24%) were female, and 22 (76%) were male in the low group (P = .13). To ensure that our findings were not influenced by a sex effect, we performed post-hoc logistic regression analyses to adjust for sex. After adjusting for sex, participants with high levels of BrTyr had 16.6-fold the odds of uncontrolled asthma at baseline (95% CI 2.8–319.7, P = .01), as defined by a baseline ACQ6 score > 1.5; 4.9-fold the odds of uncontrolled asthma at follow-up (95% CI 1.2–26.4, P = .04), as defined by a follow-up ACQ6 score > 1.5; and 4.0-fold the odds of an asthma exacerbation by follow-up (95% CI 1.1–16.6, P = .05). These analyses demonstrate that our findings are robust to sex variability.
Discussion
The management of children with asthma can be challenging, particularly because traditional laboratory- and spirometry-based testing does not reliably predict asthma control and near-term risk for acute decompensation of chronic asthma.8 The results of this study demonstrate that among children with asthma, baseline urinary BrTyr levels significantly correlate with measures of asthma control. Importantly, in contrast to traditional spirometry, laboratory, and exhaled NO measures, baseline urinary BrTyr levels predict incident risks for experiencing an asthma exacerbation over the ensuing 6-week interval. Although NO has been investigated considerably in pediatric patients with asthma,20,22,23 this is the first report to our knowledge that addresses the potential utility of BrTyr in this population.
Asthma is one of the most common chronic diseases in children, accounting for substantial morbidity rates and cost in terms of medical treatment and missed school days.24 Asthma exacerbations are particularly problematic because they herald declining lung function independent of other risk factors.25,26 Monitoring of asthma control is largely guided by clinical history, physical examination, and spirometry, not by direct assessment of underlying inflammatory processes. This poses an especially formidable problem in children with asthma, in which case the clinical history is often provided or supplemented by the caregiver, not the patient, and spirometric parameters frequently fall within normal limits.27,28 This latter observation is corroborated in our cohort, because FEV1 percent predicted, FEV1/FVC, and the percent change in FEV1 post-bronchodilator fell within normal limits in most of the participants in our study. Many children, especially younger ones, are unable to perform spirometry with adequate technique, substantially limiting the utility of spirometry in pediatric pulmonary practice.29
The direct correlation that we observed between NO levels and age, as well as the higher NO levels present in children with atopic versus nonatopic asthma (as determined by allergy skin testing) corroborates the findings of others.30,31 BrTyr levels, on the other hand, appeared robust to variations in age and degree of atopy, along with spirometric parameters, suggesting that its relationship to asthma control and capacity to predict exacerbations are independent of these factors. Furthermore, BrTyr and NO did not track with BMI percentile, which was higher, on average, in this cohort than in the general pediatric population. Adiposity has been associated with asthma in previous studies and has even been shown to track with asthma control (but not with exhaled NO levels) in select cohorts of children with asthma.32,33
Urinary BrTyr levels correlated directly with ACQ6 scores at baseline and follow-up, along with all six individual item scores at baseline and five of six individual item scores at follow-up. Consequently, high BrTyr levels were seen to confer significantly greater odds of inadequately controlled asthma, including increased symptoms, activity limitation, and short-acting bronchodilator use at baseline, plus significantly greater odds of inadequately controlled asthma, including all of the above (save short-acting bronchodilator use) at follow-up. We did not observe such relationships with respect to NO levels and traditional spirometry- and blood- (IgE, eosinophil count) based metrics of asthma control.
High levels of BrTyr conferred 4.0-fold the odds of having an asthma exacerbation in the subsequent 6 weeks. Alongside spirometric parameters, ACQ6 scores, and NO levels, BrTyr levels were the only variable to demonstrate this predictive capacity. These findings strongly support the potential clinical utility of BrTyr as both an aide in objectively monitoring asthma control and predicting risk of decompensation and subsequent exacerbation in a noninvasive manner. They thus suggest that urinary BrTyr levels may potentially serve as a quantitative index with which to guide asthma therapy.
Our findings that exhaled NO, contrary to BrTyr levels, neither track with asthma control nor predict asthma exacerbations are not unique to this study. Previous studies in children that have investigated putative relationships among NO levels, asthma control, and subsequent exacerbations, have reported weak associations, at best.8,34 Several studies looking at such relationships in adults, however, have found that NO does, indeed, track with asthma control and the risk of exacerbations.35–37 Taken together, these findings suggest that (1) the inflammatory milieu may be somewhat different in adults versus children with asthma; (2) for purposes of monitoring asthma control and predicting exacerbations, exhaled NO is more effective in adults than children; and (3) BrTyr serves as a potentially more useful noninvasive marker than NO for monitoring asthma control and predicting exacerbations, especially in pediatric populations.
Even though we did not look specifically at sputum eosinophils in this study, previous studies have demonstrated that sputum eosinophils also track with asthma control and possibly even the risk of exacerbations in children.3,4 Unfortunately, the potential utility of sputum eosinophils in everyday pediatric pulmonary practice is largely thwarted by cost and technical difficulties in both obtaining sufficient quantities of sputum in children and processing samples in the laboratory.5 Urinary BrTyr, on the other hand, can be obtained easily by spot (random) urine collection and quantified by a well-validated stable isotope dilution high performance liquid chromatography/tandem mass spectrometry assay. Furthermore, urinary BrTyr tracks with an even more specific inflammatory process than sputum eosinophils, that is, eosinophil activation per se. BrTyr production by eosinophils requires that the leukocytes are activated. This emphasizes the important distinction between eosinophil levels, as determined by blood or sputum eosinophil counts, and eosinophil activation, as determined by BrTyr levels. Detection of elevated urinary BrTyr requires that eosinophil activation with both respiratory burst and eosinophil degranulation has occurred, enabling significant protein oxidative injury within an inflamed asthmatic airway.11–13 Perhaps it is not surprising, then, that BrTyr levels appear more closely aligned with asthma symptoms and risk of exacerbation than eosinophil count. Indeed, BrTyr levels do not correlate with circulating eosinophil levels in this study, a finding also seen in our previous study in adults with severe asthma.13
Although our findings present compelling support for the potential utility of urinary BrTyr to assess asthma control and risk of subsequent exacerbations, several limitations should be noted. The study lacked the power necessary to examine how the reported findings may vary depending on particular asthma phenotypes, such as severe versus nonsevere and atopic versus nonatopic asthma.38 Most of the participants were being treated with corticosteroids, which may have attenuated the strength of the relationships observed. Exhaled NO levels, for example, are known to decrease with corticosteroid treatment.39 Moreover, complete blood counts with differentials, serum IgE levels, and allergy skin testing results were accrued from chart review only on participants for whom these data were available and not necessarily at the time of the initial study encounter. This may, at least in part, explain why exhaled NO did not correlate with serum IgE despite previous findings to the contrary.13 We did not confirm asthma diagnoses with provocative challenge testing in all subjects; however, all of the participants had been diagnosed with asthma by physicians specializing in pediatric pulmonology or allergy and immunology by clinical history, physical examination, and spirometry, and all diagnoses were made before exhaled and urinary marker measurements. Finally, because participants for the study were recruited from specialty pediatric pulmonary clinics and follow-up data collected during allergy season, participants may have been at higher risk of having an asthma exacerbation than the pediatric asthmatic population at large. The findings suggest that this high-risk population may benefit from a predictor of asthma exacerbations such as BrTyr; however, a broader sample of participants recruited from primary care clinics or at varying times of the year is necessary to validate that findings are generalizable to the pediatric asthmatic population at large and not exclusively to patients at high risk.
In conclusion, the findings identify that urinary BrTyr, a specific marker of reactive brominating oxidants formed by eosinophil-catalyzed oxidation, directly correlates with asthma control and predicts future exacerbations. This contrasts with exhaled NO, a marker of more general airway inflammation. Moreover, our findings suggest that the oxidative pathways invoked by eosinophil activation, as measured by urinary BrTyr, may contribute substantially to the clinical manifestations of asthma symptoms in children, emphasizing eosinophil peroxidase as a potential therapeutic target for asthma. Further research is necessary to better elucidate how eosinophil-driven oxidative pathways may translate to clinical symptomatology and herald asthma exacerbations, as well as the clinical implications of inhibiting these pathways. Measurement of urinary BrTyr levels may prove useful in such endeavors.
Acknowledgments
Supported by NIH (P01 HL081064, R01 HL69170, and 1UL1RR024989) and the Flight Attendant Medical Research Institute (FARMI). Cleveland Heart Lab provided assistance with bromotyrosine mass spectrometry analyses. S.H. is named as co-inventor on pending and approved patents filed by the Cleveland Clinic that refer to the use of biomarkers in inflammatory diseases, is the scientific founder of the Cleveland Heart Lab, has received research grant support from Abbott Diagnostics, Pfizer, Cleveland Heart Lab, Liposcience, and Esperion, and has received honoraria and consulting fees from Abbott Diagnostics, Merck, Lilly, Pfizer, Esperion, and Cleveland Heart Lab.
We graciously acknowledge and sincerely thank Elizabeth Juniper, MCSP, MSc, for providing the Asthma Control Questionnaire, Daniel Deane, MD, and James Tarbox, MD, for help in recruiting participants, and Marcelle Baaklini, MA, and Jacqueline Sharp, RN, CNP, for administrative assistance.
Glossary
- ACQ6
Asthma Control Questionnaire
- BMI
Body mass index
- BrTyr
3-Bromotyrosine
- IgE
Immunoglobulin E
- IQR
Interquartile range
- NIH
National Institutes of Health
- NO
Nitric oxide
- ppb
Parts per billion
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2008. Vital Health Stat. 2009;10(244):1–81. [PubMed] [Google Scholar]
- 2.NHLBI. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma full report; 2007. Bethesda: National Heart Lung and Blood Institute; 2007. [Google Scholar]
- 3.Covar RA, Spahn JD, Martin RJ, Silkoff PE, Sundstrom DA, Murphy J, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. 2004;114:575–82. doi: 10.1016/j.jaci.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Gibson PG, Simpson JL, Hankin R, Powell H, Henry RL. Relationship between induced sputum eosinophils and the clinical pattern of childhood asthma. Thorax. 2003;58:116–21. doi: 10.1136/thorax.58.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Marcos L, Brand PL. The utility of sputum eosinophils and exhaled nitric oxide for monitoring asthma control with special attention to childhood asthma. Allergol Immunopathol (Madr) 2010;38:41–6. doi: 10.1016/j.aller.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61:817–27. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–65. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 8.Gruchalla RS, Sampson HA, Matsui E, David G, Gergen PJ, Calatroni A, et al. Asthma morbidity among inner-city adolescents receiving guidelines-based therapy: role of predictors in the setting of high adherence. J Allergy Clin Immunol. 2009;124:213–21. 21, e1. doi: 10.1016/j.jaci.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005;115:1130–6. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–25. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Chen Y, d’Avignon A, Hazen SL. 3-Bromotyrosine and 3,5-di-bromotyrosine are major products of protein oxidation by eosinophil peroxidase: potential markers for eosinophil-dependent tissue injury in vivo. Biochemistry. 1999;38:3538–48. doi: 10.1021/bi982401l. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, et al. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest. 2000;105:1455–63. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedes SH, Khatri SB, Zhang R, Wu W, Comhair SAA, Wenzel S, et al. Noninvasive markers of airway inflammation in asthma. Clinical and Translational Science. 2009;2:112–7. doi: 10.1111/j.1752-8062.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–8. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kaminsky DA, Rice AA, Bissonette M, Larose T, Phillips L, Cohen L, et al. Exhaled nitric oxide decreases in association with attendance at an asthma summer cAMP. J Asthma. 2008;45:415–9. doi: 10.1080/02770900801971842. [DOI] [PubMed] [Google Scholar]
- 16.Rosias PP, Dompeling E, Dentener MA, Pennings HJ, Hendriks HJ, Van Iersel MP, et al. Childhood asthma: exhaled markers of airway inflammation, asthma control score, and lung function tests. Pediatr Pulmonol. 2004;38:107–14. doi: 10.1002/ppul.20056. [DOI] [PubMed] [Google Scholar]
- 17.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.ATS Workshop Proceedings. Exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate: executive summary. Am J Respir Crit Care Med. 2006;173:811–3. doi: 10.1164/rccm.2601014. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying “’well-controlled” and “not well-controlled” asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–21. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Dinakar C. Exhaled nitric oxide in pediatric asthma. Curr Allergy Asthma Rep. 2009;9:30–7. doi: 10.1007/s11882-009-0005-6. [DOI] [PubMed] [Google Scholar]
- 23.Nordvall SL, Janson C, Kalm-Stephens P, Foucard T, Toren K, Alving K. Exhaled nitric oxide in a population-based study of asthma and allergy in schoolchildren. Allergy. 2005;60:469–75. doi: 10.1111/j.1398-9995.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- 24.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 25.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–6. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 26.O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 27.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–32. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 28.Paull K, Covar R, Jain N, Gelfand EW, Spahn JD. Do NHLBI lung function criteria apply to children? A cross-sectional evaluation of childhood asthma at National Jewish Medical and Research Center, 1999–2002. Pediatr Pulmonol. 2005;39:311–7. doi: 10.1002/ppul.20161. [DOI] [PubMed] [Google Scholar]
- 29.Hammer J, Eber E. Pediatric pulmonary function testing. Basel, Switzerland: Karger Medical and Scientific Publishers; 2005. [Google Scholar]
- 30.Avital A, Uwyyed K, Berkman N, Bar-Yishay E, Godfrey S, Springer C. Exhaled nitric oxide is age-dependent in asthma. Pediatr Pulmonol. 2003;36:433–8. doi: 10.1002/ppul.10377. [DOI] [PubMed] [Google Scholar]
- 31.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 65:258–62. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–92. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol. 2010;108:735–43. doi: 10.1152/japplphysiol.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sardon-Prado O, Korta-Murua J, Valverde-Molina J, Fernandez-Paredes JJ, Mintegui J, Corcuera-Elosegui P, et al. Association among lung function, exhaled nitric oxide, and the CAN questionnaire to assess asthma control in children. Pediatr Pulmonol. 45:434–39. doi: 10.1002/ppul.21144. [DOI] [PubMed] [Google Scholar]
- 35.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 36.Harkins MS, Fiato KL, Iwamoto GK. Exhaled nitric oxide predicts asthma exacerbation. J Asthma. 2004;41:471–6. doi: 10.1081/jas-120033990. [DOI] [PubMed] [Google Scholar]
- 37.Malerba M, Ragnoli B, Radaeli A, Tantucci C. Usefulness of exhaled nitric oxide and sputum eosinophils in the long-term control of eosinophilic asthma. Chest. 2008;134:733–9. doi: 10.1378/chest.08-0763. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 39.Kharitonov SA, Barnes PJ. Exhaled biomarkers. Chest. 2006;130:1541–6. doi: 10.1378/chest.130.5.1541. [DOI] [PubMed] [Google Scholar]