Abstract
Glutamate contributes to the reinforcing and stimulant effects of methamphetamine, yet its potential role in the interoceptive stimulus properties of methamphetamine is unknown. In the current study, adult male Sprague-Dawley rats were trained to discriminate methamphetamine (1.0 mg/kg, i.p.) from saline in a standard operant discrimination task. The effects of methamphetamine (0.1-1.0 mg/kg, i.p.), the N-methyl-D-aspartate (NMDA) receptor channel blockers MK-801 (0.03-0.3 mg/kg, i.p.) and ketamine (1.0-10.0 mg/kg, i.p.), the low-affinity NMDA antagonist memantine (1.0-10 mg/kg, i.p.), the polyamine site NMDA receptor antagonist ifenprodil (1-10 mg/kg), the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 1-10 mg/kg, i.p.), and the metabotropic 5 (mGluR5) receptor antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP; 1-10 mg/kg) given alone were determined in substitution tests. The effects of MK-801 (0.03 and 0.1 mg/kg), ketamine (1.0 and 3.0 mg/kg), ifenprodil (5.6 mg/kg), CNQX (5.6 mg/kg) and MPEP (5.6 mg/kg) were also tested in combination with methamphetamine to assess for alterations in the methamphetamine cue. In substitution tests, none of the test drugs generalized to the methamphetamine cue. However, ketamine and ifenprodil produced significant leftward shifts in the methamphetamine dose-response curve; pretreatment with 3 mg/kg of ketamine, for example, decreased the ED50 value for methamphetamine by half. These results suggest that blockade of the NMDA receptor augments the interoceptive stimulus properties of methamphetamine.
Keywords: drug discrimination, methamphetamine, NMDA, AMPA, mGluR5, rat, MK-801
Introduction
Methamphetamine abuse is a worldwide public health concern (Gonzales et al., 2010; Watanabe-Galloway et al., 2009). A major impediment toward reducing the adverse impact of methamphetamine abuse has been the lack of an effective treatment medication (Dwoskin and Crooks 2002; Newton et al. 2006; Rothman et al. 2005). While much progress has been made in recent years (Carroll et al., 2009; Horton et al., 2010; Rothman et al., 2008; Vocci and Appel 2007), additional research into the underlying mechanisms of methamphetamine abuse will likely be an important aid to furthering medication development efforts..
Preclinical animal models, such as the drug discrimination procedure, are useful tools for identifying receptor mechanisms of drug action in vivo. Studies using this procedure to investigate the pharmacology of methamphetamine have identified that the monoamines, dopamine (Bergman, 2008; Czoty et al. 2004a, 2004b; Munzar and Goldberg 2000), norepinephrine (Czoty et al. 2004b; Munzar and Goldberg 1999) and serotonin (Czoty et al. 2004b; Munzar et al. 1999, 2002), play important roles in the methamphetamine cue. Other studies have revealed that acetylcholine (Desai and Bergman, 2010; Gatch et al., 2008), adenosine (Justinova et al. 2003; Munzar et al. 2002), histamine (Mori et al. 2002; Munzar et al. 1998) and γ-amino-butyric acid (GABA; Gasior et al. 2004; Gatch et al. 2005) neurotransmitter systems are also involved. These results illustrate the complex nature of the neurochemical events which mediate transduction of methamphetamine's interoceptive effects.
Despite this previous work, the specific involvement of glutamate in methamphetamine discrimination has not been addressed. Glutamate is the predominant excitatory neurotransmitter in mammalian brain, and exerts effects via actions at several distinct receptor subtypes. These include the ligand-gated N-methyl-D aspartate (NMDA) and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor channels, as well as a variety of G protein-coupled metabotropic receptor (mGluR1-8) subtypes. Previous behavioral studies using animal models of drug abuse have identified roles for several of the glutamate receptor subtypes. For instance, NMDA receptor antagonists reduce methamphetamine-induced hyperactivity (Witkin, 1993) and attenuate methamphetamine self-administration (Allen et al. 2005; Gass et al., 2009; Glick et al. 2001; Osborne and Olive, 2008)). The NMDA antagonist dextromethorphan and the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) also reduce cocaine self-administration and hyperactivity (Herzig and Schmidt 2004; Paterson and Markou 2005; Pulvirenti et al. 1997)). In drug discrimination studies using rats trained to discriminate d-amphetamine or cocaine, the NMDA receptor antagonists MK-801 and phencyclidine elicit partial substitution when tested alone, and enhance the effects of the training drugs when administered in combination (Gaiardi et al., 2001; Kantak et al. 1998). Conversely, the glycine site NMDA partial agonist D-cycloserine attenuates the effects of d-amphetamine (Gaiardi et al., 2001). There are two published investigations of the effects of drugs that act preferentially on glutamate using the methamphetamine discrimination procedure (Gatch and Pratt, 2006; Hart et al., 2002). In the first study, Hart et al. (2002), trained 6 human participants to discriminate methamphetamine (10 mg) from placebo using a standard two-choice procedure. Next, the effects of methamphetamine (5-20 mg) and the low-affinity noncompetitive NMDA antagonist memantine (40 mg), given alone or in combination, were then assessed under a three-choice procedure where a “novel” choice option was available. Although memantine alone did not substitute for methamphetamine, co-administration with methamphetamine resulted in increased “novel” choice responding, suggesting that the cue produced by the memantine/methamphetamine combination was distinct from the effects of placebo or methamphetamine alone. In the second study, Gatch and Pratt (2006) examined the effects of NMDA and MK-801 in rats trained to discriminate 1.0 mg/kg of methamphetamine from saline. Results indicated that NMDA (30 mg/kg) did not alter the effects of methamphetamine; however, MK-801 (0.1 mg/kg) produced a 2-fold leftward shift in the methamphetamine (0.1-1.0 mg/kg) dose-response curve, suggesting that blockade of NMDA receptors can augment the methamphetamine cue. Thus, evidence obtained in both rodents and humans indicates that NMDA receptor blockade alters the subjective properties of methamphetamine. Based on these findings, further testing of ligands that act on NMDA and other receptor subtypes in methamphetamine discrimination is warranted, especially given that these compounds may have some utility as treatments for stimulant abuse (Kalivas 2007; Markou 2007).
The purpose of the present study was to examine the potential roles of ionotropic (i.e., NMDA and AMPA) and metabotropic (i.e., mGluR5) glutamate receptor subtypes in methamphetamine discrimination in rats. The noncompetitive channel blockers MK-801 and ketamine, the low-affinity NMDA antagonist memantine, and the NR2B subunit polyamine site antagonist ifenprodil were tested. Although MK-801, ketamine and memantine have similar mechanisms of action, MK-801 has a greater affinity for NMDA receptors than both ketamine and memantine (Fujimoto et al., 2008; Wong et al. 1986); further, mutations of the M1, M3 and M4 loops of the NMDA receptor complex reduce channel blockade by MK-801 but not memantine (Kashiwagi et al., 2002), indicating that the binding sites of these drugs are not identical. The role of the NMDA polyamine recognition site was tested with ifenprodil, which does not block the ion pore channel (Carter et al. 1989). The role of AMPA receptors was investigated with the quinoxalinedione derivative, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), which has a Ki value of 300 nM at the AMPA receptor and 7-fold greater selectivity for AMPA than kainate receptors (Shimizu-Sasamata et al. 1996). The potential role of mGluR5 receptors was examined with the selective antagonist MPEP (Gasparini et al. 1999). Each drug was tested alone and in combination with methamphetamine.
Methods
Subjects
Adult male Sprague-Dawley rats (n=6) were obtained from Harlan Inc. (Indianapolis, IN) and housed individually in standard plastic cages in a temperature- and humidity-controlled facility set to a 14:10 hr light/dark cycle (lights on at 0600 hr). Rats were handled and acclimated to the colony for one week prior to the start of the experiment, which was conducted during the light phase. Rats were maintained at 85% of their free-feed weights, but had continuous access to water. Experimental protocols were in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
Experiments were conducted in operant conditioning chambers (ENV-008, MED Associates, St. Albans, VT, USA). Each chamber was housed in a sound-attenuating wooden enclosure (ENV-018M, Med Associates) and connected to a personal computer via an interface (SG-502, MED Associates). A 5 × 4.2 cm opening that allowed access to a recessed food tray was located on the response panel of the operant conditioning chamber. Two retractable metal response levers were mounted next to the food tray (one on each side) 7.3 cm above a metal-grid floor. A 28 V, 3-cm diameter white cue light was centered 6 cm above each response lever.
Procedure
Training phase
Rats were trained initially to lever press for food reinforcement (45 mg Precision Pellets, Bio-Serv, Frenchtown, NJ) under a fixed ratio (FR) 1 schedule during a single 60-min training session. The FR requirement for reinforcement was subsequently increased to a terminal FR10 over the next 12 daily sessions, which were 15 min in duration. In order to enhance discrimination acquisition, only one lever (the saline-appropriate lever; counter-balanced across rats) was presented during these sessions. The cue lights were illuminated when the lever was presented to signal the start of each session, and turned off once the lever retracted at the end of the session. After 2 sessions with the FR10 schedule, the methamphetamine discrimination training phase began. Here, rats received a single injection of either methamphetamine (1.0 mg/kg, i.p.) or saline 15 min prior to each session. Next, rats were placed in the operant conditioning chamber for daily 15-min sessions. When methamphetamine was administered, only the lever designated as the methamphetamine-appropriate lever was presented. When saline was administered, only the saline-appropriate lever was presented. The left lever was designated as the methamphetamine-appropriate lever and the right lever was designated the saline-appropriate lever for half of the rats, whereas lever designations were reversed for the other rats. Methamphetamine and saline were administered according to a double-alternation sequence (i.e., MMSSMMSS or SSMMSSMM) for 8 consecutive sessions. From that point on, and for the remainder of the experiment, both levers were presented each session. Responding on the injection-appropriate lever was reinforced according to the FR10 schedule, and responses on the incorrect lever were recorded but had no programmed consequence. Training continued until the following criteria were met on 7 of 8 consecutive sessions: 1) no more than 13 total responses were emitted prior to earning the first reinforcer; and 2) ≥85% of the total session responses occurred on the injection-appropriate lever.
Substitution and interaction tests
Once training criteria were met, test sessions were interspersed between methamphetamine and saline training sessions. Test sessions were similar to training sessions, with the exceptions that 1) they were 3 min in duration, and 2) completion of 10 responses on either lever was reinforced with food pellet delivery. Substitution tests were conducted to establish the dose-response curves for methamphetamine (0.1-1.0 mg/kg, i.p.), MK-801 (0.03 - 0.3 mg/kg, i.p.), ketamine (1-10 mg/kg, i.p.), ifenprodil (1-10 mg/kg, i.p.), CNQX (1-10 mg/kg, i.p.) and MPEP (1-10 mg/kg, i.p.). Methamphetamine was tested before any of the other drugs, followed by tests with MK-801 and ketamine (counterbalanced order). Tests with ifenprodil, CNQX and MPEP were then conducted in a random order across rats, although each dose of a particular drug was administered prior to initiating tests with another drug. Once substitution tests were completed, a second methamphetamine dose-response curve was determined following pretreatment with saline. Then, interaction tests were initiated in which the methamphetamine dose-response curve (0.1 - 1.0 mg/kg) was re-determined following pretreatment with saline, MK-801 (0.03 and 0.1 mg/kg), ketamine (1 and 3 mg/kg), ifenprodil (5.6 mg/kg), CNQX (5.6 mg/kg), and MPEP (5.6 mg/kg). Methamphetamine doses were given in a random order for each pretreatment drug. In substitution tests, all drugs were administered 15 min prior to the session. In interaction tests, all pretreatment drugs were given 15 min prior to methamphetamine, which was administered 15 min prior to the session (i.e., pretreatments were given 30 min prior to the session). At least two 15-min training session (one with 1.0 mg/kg of methamphetamine and one with saline) intervened each test session to ensure baseline stability. Any rat that did not meet criteria described above for acquisition was not given a dose of any test drug until stable performance re-emerged.
Two dependent measures were collected during each test session: 1) percentage of total responses occurring on the methamphetamine-appropriate lever (calculated as the number of responses on the methamphetamine-appropriate lever divided by the total number of responses on each lever); and 2) rate of responding in seconds (calculated as the total number of responses on each lever divided by 180). Although lever selection data from any rats that failed to complete at least 10 responses were excluded from the statistical analyses, data from all rats were included in analyses of response rates.
Drugs
(+)-Methamphetamine HCl, (±)-ketamine HCl, (+)-MK-801 hydrogen maleate, memantine and 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX) were obtained from Sigma (St. Louis, MO). 6-Methyl-2-(phenylethynyl)pyridine (MPEP) HCl and ifenprodil hemitartrate were obtained from Tocris Bioscience (Ellisville, MO). All drugs were prepared in 0.9% NaCl (saline) with the exception of ifenprodil, which was prepared in distilled water. All drugs were administered i.p. in a volume of 1 ml/kg. Doses indicate the salt weights.
Statistics
Dose-response curves were analyzed by one- or two-way repeated measures analysis of variance (ANOVA). Lever selection data were subjected to arcsine transformations prior to ANOVA to increase homogeneity of variance (Winer, 1971). Response rate data were calculated as the number of responses per second, which were then converted to % saline control data. Post-hoc comparisons were conducted in cases where ANOVA returned significant main effects or interactions. In interaction tests with glutamate antagonists, straight lines were also fit to the linear portion of each curve (i.e., no more than 1 point above or below the portion of the curve falling between 25% and 75% maximum effect) using the formula ‘effect = slope × log (dose) + intercept’ (Prism v. 5.0, GraphPad Sotware, Inc., San Diego, CA); for each line, the ED50 values and 95% confidence intervals were obtained. Statistical significance was declared at p ≤ .05.
Results
Training phase
Rats learned to discriminate methamphetamine from saline in an average of 47.6 (± 23.2) sessions. Once acquisition criteria were met, the mean (± SEM) levels of methamphetamine-appropriate responding produced by saline and the 1.0 mg/kg methamphetamine training dose were 3.22% (± 0.90) and 96.9% (± 1.45), respectively. Since the dose-effects of methamphetamine were consistent throughout the course of this study, the methamphetamine curve presented in the figures reflects the mean of each determination.
Substitution and interaction tests
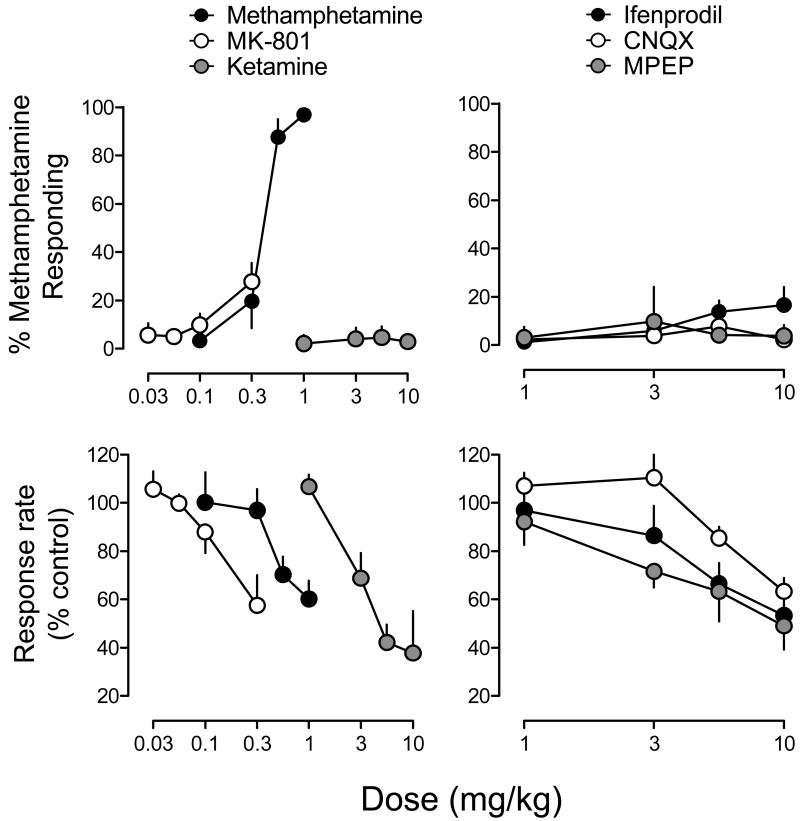
Figure 1 illustrates the dose effects of methamphetamine, MK-801 and ketamine (left panels), and ifenprodil, CNQX and MPEP (right panels) for percentage of responses on the methamphetamine-appropriate lever and response rate. For methamphetamine, ANOVA revealed a significant main effect of dose (F3,15=9.91, p<.001) on the percentage of responses on the methamphetamine-appropriate lever; post-hoc tests indicated that the 0.56 and 1.0 mg/kg doses of methamphetamine produced significantly greater levels of methamphetamine-appropriate responding than saline. In addition, ANOVA also revealed a significant main effect of methamphetamine dose on response rates (F3,15=6.12, p<.01); post-hoc tests indicated that 1.0 mg/kg of methamphetamine produced a significant reduction in response rate relative to saline. For MK-801, ketamine, ifenprodil, CNQX and MPEP, significant dose effects for the percentage of responses on the methamphetamine-appropriate lever were not observed; the only dose producing greater than 20% of responses on the methamphetamine lever was 0.3 mg/kg of MK-801 (27.8% methamphetamine-appropriate responding). In contrast, ANOVA revealed significant main effects of MK-801 (F3,15=4.96, p<.01), ketamine (F3,15=13.01, p<.001), ifenprodil (F3,15=7.59, p<.01), CNQX (F3,15=5.61, p<.01) and MPEP (F3,15=8.06, p<.001) dose on response rates. Significant reductions in response rate were produced by 0.3 mg/kg dose of MK-801, 5.6 and 10 mg/kg of ketamine, ifenprodil and MPEP, and the 10 mg/kg of CNQX, relative to saline.
Figure 1.
Discriminative stimulus and response rate effects of NMDA, AMPA and mGluR5 glutamate receptor antagonists. The effects of methamphetamine (0.1 – 1.0 mg/kg), MK-801 (0.03-0.3 mg/kg) and ketamine (1-10 mg/kg) are illustrated on the left, and the effects of ifenprodil (1-10 mg/kg), CNQX (1-10 mg/kg) and MPEP (1-10 mg/kg) are illustrated on the right. Data reflect the mean (±SEM) percentage of responses occurring on the methamphetamine-appropriate lever (upper panels), or the mean (±SEM) percentage of vehicle response rates (lower panels), as a function of dose. Saline alone elicited a mean level of 3.22% methamphetamine-appropriate responding at an average rate of 0.98 responses per second (results not shown). Data points reflect the average effects obtained in rats (n=6) trained to discriminate 1.0 mg/kg of methamphetamine from saline.
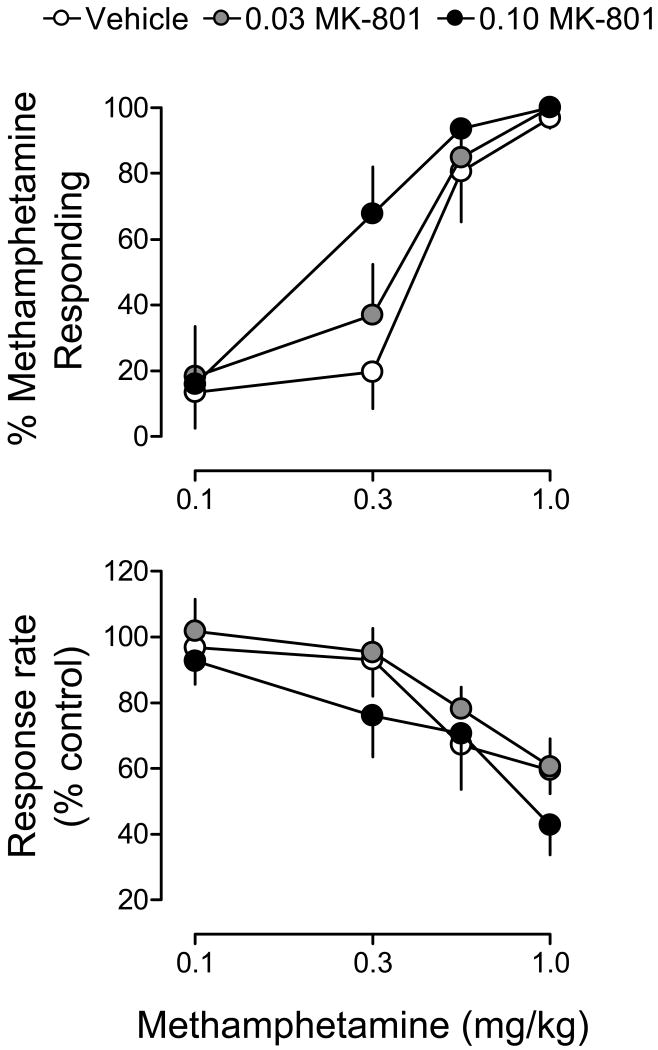
Figure 2 illustrates the dose effects of methamphetamine (0.1-1.0 mg/kg) in combination with MK-801 (0.03 or 0.1 mg/kg) or saline vehicle for percentage of responses on the methamphetamine-appropriate lever (upper panel) and response rate (lower panel). For percent methamphetamine-appropriate responding, ANOVA revealed a significant main effect of methamphetamine dose (F3,15=27.34, p<.001). Although the main effect of MK-801 pretreatment approached significance (p=.07), the MK-801 pretreatment × methamphetamine dose interaction did not attain significance. The ED50 values for methamphetamine administered with vehicle, 0.03 mg/kg and 0.1 mg/kg of MK-801 were 0.32 (0.11-1.10), 0.26 (0.17-0.75) and 0.19 (0.04-0.36) mg/kg, respectively (Table 1). Thus, MK-801 did not alter significantly the potency of methamphetamine. For response rate, ANOVA revealed a significant main effect of methamphetamine dose (F3,45=15.70, p<.001); however, no main effects or interactions for the MK-801 pretreatment factor on response rate were obtained.
Figure 2.
Effects of vehicle (white symbols) or MK-801 (0.03 and 0.1 mg/kg; gray and black symbols, respectively) on the discriminative stimulus and response rate effects of methamphetamine (0.1-1.0 mg/kg). Data reflect the mean (±SEM) percentage of responses occurring on the methamphetamine-appropriate lever (upper panels), or the mean (±SEM) percentage of vehicle response rates (lower panels), as a function of methamphetamine dose. Data points reflect the average effects obtained in rats (n=6) trained to discriminate 1.0 mg/kg of methamphetamine from saline.
Table 1.
ED50 (±95% CI) values for methamphetamine (0.1 - 1.0 mg/kg) in combination with glutamate receptor antagonists in rats (n=6) trained to discriminate methamphetamine (1.0 mg/kg) from saline.
| Pretreatment | Dose (mg/kg) | ED50 (95% CI) |
|---|---|---|
| Vehicle | 0.32 (0.11-1.10) | |
| MK-801 | 0.03 | 0.26 (0.17-0.75) |
| 0.1 | 0.19 (0.04-0.36) | |
| Ketamine | 1 | 0.37 (0.28-0.46) |
| 3 | 0.16 (0.03-0.33) | |
| Ifenprodil | 5.6 | 0.21 (0.06-0.34) |
| CNQX | 5.6 | 0.23 (0.06-0.40) |
| MPEP | 5.6 | 0.37 (0.26-0.48) |
Figure 3 illustrates the dose effects of methamphetamine (0.1-1.0 mg/kg) in combination with ketamine (1 or 3 mg/kg) or saline vehicle for percentage of responses on the methamphetamine-appropriate lever (upper panel) and response rate (lower panel). For percent methamphetamine-appropriate responding, ANOVA revealed significant main effects of ketamine pretreatment (F2,10=4.61, p<.05) and methamphetamine dose (F3,15=24.16, p<.001); however, the ketamine pretreatment × methamphetamine dose interaction failed to attain significance. Compared to vehicle, pretreatment with 1 mg/kg of ketamine produced a slight rightward shift in the methamphetamine dose-response curve with an ED50 value of 0.37 (0.28-0.46) mg/kg, whereas 3 mg/kg of ketamine produced a robust leftward shift with an ED50 value of 0.16 (0.03-0.33) mg/kg (Table 1). For response rate, ANOVA revealed a significant main effect of methamphetamine dose (F3,45=13.49, p<.001); however, no main effects or interactions for the ketamine pretreatment factor on response rate were obtained.
Figure 3.
Effects of vehicle (white symbols) or ketamine (1 and 3 mg/kg; gray and black symbols, respectively) on the discriminative stimulus and response rate effects of methamphetamine (0.1-1.0 mg/kg). Data reflect the mean (±SEM) percentage of responses occurring on the methamphetamine-appropriate lever (upper panels), or the mean (±SEM) percentage of vehicle response rates (lower panels), as a function of methamphetamine dose. Data points reflect the average effects obtained in rats (n=6) trained to discriminate 1.0 mg/kg of methamphetamine from saline.
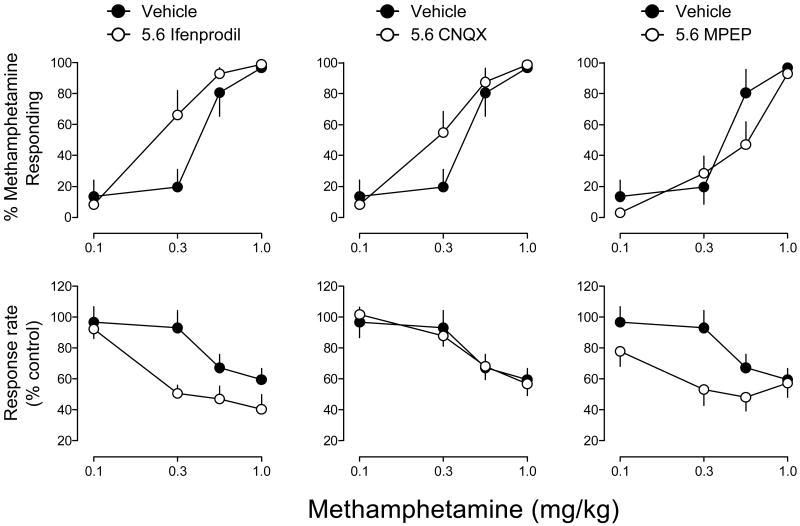
Figure 4 illustrates the dose effects of methamphetamine (0.1-1.0 mg/kg) in combination with 5.6 mg/kg of ifenprodil (left panels), CNQX (middle panels) and MPEP (right panels) for percentage of responses on the methamphetamine-appropriate lever (upper panels) and response rate (lower panels). With 5.6 mg/kg of ifenprodil, ANOVA revealed significant main effects of ifenprodil pretreatment (F1,10=10.19, p<.01) and methamphetamine dose (F4,40=19.84, p<.001) for percent methamphetamine-appropriate responding, although the methamphetamine dose × ifenprodil pretreatment interaction did not attain significance. The 0.3 mg/kg dose of methamphetamine following ifenprodil pretreatment elicited significantly greater levels of methamphetamine-appropriate responding than the same dose given after vehicle. The ED50 value for methamphetamine following ifenprodil pretreatment was 0.21 (0.06-0.34) mg/kg (Table 1). Further, ANOVA revealed significant main effects of ifenprodil pretreatment (F1,10=9.39, p<.05) and methamphetamine dose (F3,30=17.48, p<.001), as well as a significant ifenprodil pretreatment × methamphetamine dose interaction (F3,30=3..01, p<.05), for response rates. Post-hoc tests indicated that the effects of 0.3 mg/kg of methamphetamine differed significantly following ifenprodil pretreatment compared to vehicle pretreatment. With 5.6 mg/kg of CNQX, ANOVA revealed a significant main effect of methamphetamine dose (F3,30=20.33, p<.001), although there were no main effects or interactions for CNQX pretreatment. The ED50 value for methamphetamine following CNQX pretreatment was 0.23 (0.06-0.40) mg/kg (Table 1). There was a main effect of methamphetamine dose (F3,30=17.14, p<.001) on response rate, but CNQX pretreatment had no significant effect on responding. With 5.6 mg/kg of MPEP, ANOVA revealed a significant main effect of methamphetamine dose (F3,30=18.15, p<.001) for percent methamphetamine-appropriate responding, although there were no main effects or interactions for MPEP pretreatment. The ED50 value for methamphetamine following MPEP pretreatment was 0.37 (0.26-0.48) mg/kg (Table 1). In contrast, ANOVA revealed significant main effects of MPEP pretreatment (F1,10=5.22, p<.05) and methamphetamine dose (F3,30=11.74, p<.001) on response rate, as well as a significant MPEP pretreatment × methamphetamine dose interaction (F1,10=3.02, p<.05). Post-hoc tests indicated that the effects of 0.3 mg/kg of methamphetamine differed significantly between pretreatment conditions.
Figure 4.
Effects of 5.6 mg/kg of infenprodil (left panels), 5.6 mg/kg of CNQX (middle panels) and 5.6 mg/kg of MPEP (right panels) on the discriminative stimulus and response rate effects of methamphetamine (0.1-1.0 mg/kg). Data reflect the mean (±SEM) percentage of responses occurring on the methamphetamine-appropriate lever (upper panels), or the mean (±SEM) percentage of vehicle response rates (lower panels), as a function of methamphetamine dose. Data points reflect the average effects obtained in rats (n=6) trained to discriminate 1.0 mg/kg of methamphetamine from saline.
Discussion
The purpose of the present study was to probe for potential glutamatergic mechanisms in the discriminative stimulus effects of methamphetamine. A variety of receptor subtype-selective ligands, including the NMDA receptor channel blockers MK-801 and ketamine, the polyamine site NMDA receptor antagonist ifenprodil, the AMPA receptor antagonist CNQX, and the mGluR5 receptor antagonist MPEP were tested for both production of methamphetamine-like effects and the ability to alter the effects of methamphetamine. Overall, with the exception of evidence of partial substitution obtained with 0.3 mg/kg of MK-801 based on commonly-used criteria (i.e., > 20% drug-appropriate responding), none of the compounds tested elicited methamphetamine-like interoceptive effects when administered alone. In interaction tests, ketamine and ifenprodil each produced leftward shifts in the methamphetamine dose-response curve (and the effect of MK-801 nearly attained significance). Collectively, these results suggest that inhibition of NMDA glutamate receptors may contribute to the methamphetamine cue, whereas the AMPA and mGluR5 subtypes do not appear to play a role.
There have been at least two prior studies of the discriminative effects of MK-801 in methamphetamine-trained rats (Gatch and Pratt, 2006; Liang and Zheng, 2000). The present findings are generally concordant with the results of the former study, as Gatch and Pratt (2000) reported that 0.1 mg/kg of MK-801 produced no more than ∼15% methamphetamine-appropriate responding given alone, yet shifted the methamphetamine dose-response curve leftward when administered prior to methamphetamine (i.e., the ED50 value for methamphetamine decreased from 0.33 mg/kg to 0.15 mg/kg). In contrast, Liang and Zheng (2000) reported that 0.1 mg/kg of MK-801 resulted in partial to full substitution for methamphetamine when given alone, yet pretreatment with 0.1 mg/kg of MK-801 reduced the level of methamphetamine-appropriate responding elicited by the 1.0 mg/kg methamphetamine training dose from 97% to 43%, indicative of antagonism of the methamphetamine cue. The basis of the discrepant results of Liang and Zheng (2000) relative to Gatch and Pratt (2006) and the current data is unknown, although methodological differences between these studies may have played a role. For example, Liang and Zheng (2000) used s.c. administration of methamphetamine vs. the i.p. administration used here and by Gatch and Pratt (2006). In addition, the effects of MK-801 pretreatment were examined in combination with the methamphetamine training dose only in the Liang and Zheng (2000) study, whereas MK-801 was tested in combination with a range of methamphetamine doses in the Gatch and Pratt (2006) study. Thus, it is possible that the MK-801 enhancement is observed only with methamphetamine doses lower than the training dose. Together with evidence that MK-801 also potentiates d-amphetamine discrimination (Gaiardi et al., 2001), the preponderance of data support a facilitatory role of NMDA channel blockade for discrimination of amphetamine-type stimulus effects.
The ability of MK-801 to enhance methamphetamine discrimination highlights the potential involvement of the NMDA glutamate receptor. Accordingly, results obtained with the NMDA channel blocker ketamine and the polyamine site NMDA antagonist ifenprodil support this notion. Each of these drugs produced effects similar to MK-801 in interaction tests, as pretreatment with 3 mg/kg of ketamine or 5.6 mg/kg of ifenprodil resulted in a leftward shift in the methamphetamine dose-response curve. The similar pattern of results obtained with these compounds is likely due to their common discriminative stimulus effects. Both MK-801 and ketamine substitute fully in ketamine- or phencyclidine-trained rats (Benvenga et al. 1991; Koek et al. 1990; Narita et al. 2001), and MK-801 and ketamine cross-substitute in rhesus monkeys (France et al. 1989, 1991; Koek et al. 1988). Similarly, ifenprodil has been shown to elicit at least partial substitution for the discriminative stimulus effects of ketamine and phencyclidine (De Vry and Jentzsch 2003; Koek et al. 1990). The similar effects produced by the NMDA antagonists MK-801, ketamine and ifenprodil support the notion that NMDA blockade may be a component of the methamphetamine cue. In contrast to the NMDA receptor, less information is available regarding the role of AMPA receptors in the abuse-related effects of methamphetamine. In fact, there are no published studies of the discriminative stimulus effects of the AMPA antagonist CNQX in methamphetamine-trained subjects. The current findings indicate that CNQX does not itself produce a methamphetamine-like discriminative cue, nor does CNQX alter significantly the methamphetamine dose-response curve. These discrepant results (relative to the NMDA receptor antagonists) are consistent with other reports in the literature showing that NBQX, a structurally-related AMPA receptor antagonist, does not produce MK-801- or ketamine-like discriminative stimulus effects (Jackson et al. 1996; Geter-Douglass and Witkin 1997).
Although the present study is the first to investigate the stimulus properties of the mGluR5 receptor antagonist MPEP in animals trained with methamphetamine, several previous studies have examined interactions between MPEP and the discriminative stimulus effects of other stimulant drugs (Lee et al., 2005; Murray and Bevins, 2007; Zakharova et al. 2005). In a study using squirrel monkeys trained to discriminate cocaine, Lee et al. (2005) reported that MPEP produced a dose-dependent attenuation of cocaine discrimination, whereas MK-801 shifted the cocaine dose-response curve leftward in those animals. In other studies using rats trained to discriminate nicotine, MPEP did not substitute for nicotine, but produced a slight rightward shift in the nicotine dose-response curve in an operant discrimination task (Zakharova et al., 2005). Using a Pavlovian task where nicotine served as a conditional stimulus for sucrose availability, Murray and Bevins (2007) reported that MPEP attenuated the effects of nicotine, but only at doses that also reduced motor activity.
Based on the current results, there appears to be some potential for glutamate compounds as treatments for methamphetamine addiction. However, the relation between discriminative stimulus and reinforcing effects has not been studied extensively. In an intriguing study conducted in rhesus monkeys, Martelle and Nader (2009) used a within-subjects design to characterize cocaine discrimination and self-administration using a chained schedule in the same animals. Results of that study revealed that, in all monkeys tested, at least one dose of cocaine functioned as a reinforcer without eliciting cocaine-appropriate responding, indicating that these effects are to some extent dissociable. In another study, Childs et al. (2006) examined acquisition rates of cocaine self-administration in rats that were trained previously to discriminate cocaine, and in rats that were also treated with cocaine prior to pressing for food rewards, but were not subject to discrimination training. Those investigators reported that cocaine-exposed rats tended to acquire self-administration more readily than naïve rats, but that this was true regardless of whether they had been trained to discriminate cocaine, or simply treated with cocaine. Thus, the available preclinical literature has yet to provide compelling evidence that alterations in discriminative stimulus effects translate directly to alterations in reinforcing effects, especially for stimulant drugs. One possibility for this is that such a relation may be more straightforward for other drug classes (e.g., opiates) whose activity is mediated primarily by signaling at specific receptors (e.g., μ-opioid). Since methamphetamine promotes release of all monoamines (Rothman and Baumann, 2003), it is possible that the downstream activation of multiple receptors contribute to the abuse liability of this drug. Alternatively, targeting specific consequences of methamphetamine abuse in animal models should also yield valuable information; for instance, a recent study demonstrated that the mGluR5 allosteric modulator CDPPB alleviated deficient object recognition memory resulting from extended methamphetamine self-administration in rats (Reichel et al., 2011). Given the current unmet need for methamphetamine abuse treatments, the development and use of multiple animal models may be an important avenue toward the eventual realization of that goal.
Acknowledgments
We gratefully acknowledge the technical assistance provided by Jason T. Ross, Joshua Cutshall and Ross Weido. During preparation of the manuscript, T.E.W. was supported by USPHS grants T32 DA007304 and F31 DA023853. This work was supported by USPHS grant DA013519.
References
- Allen RM, Carelli RM, Dykstra LA, Suchey TL, Everett CV. Effects of the competitive N-methyl-D-aspartate receptor antagonist LY235959 [(-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid] on responding for cocaine under both fixed and progressive ratio schedules of reinforcement. J Pharmacol Exp Ther. 2005;315:449–457. doi: 10.1124/jpet.105.086355. [DOI] [PubMed] [Google Scholar]
- Benvenga MJ, Wing AV, Del Vecchio RA. The discriminative stimulus effect of MK-801 in ketamine-trained rats. Pharmacol Biochem Behav. 1991;38:211–213. doi: 10.1016/0091-3057(91)90613-7. [DOI] [PubMed] [Google Scholar]
- Bergman J. Medications for stimulant abuse: agonist-based strategies and preclinical evaluation of the mixed-action D2 partial agonist aripiprazole (Abilify) Exp Clin Psychopharmacology. 2008;16:475–483. doi: 10.1037/a0014398. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Abraham P, Gong PK, Pidaparthi RR, Blough BE, Che Y, Hampton A, Gunnell M, Lay JO, Jr, Peterson EC, Owens SM. The synthesis of haptens and their use for the development of monoclonal antibodies for treating methamphetamine abuse. J Med Chem. 2009;52:7301–7309. doi: 10.1021/jm901134w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Shoaib M, Stolerman IP. Cocaine self-administration in rats with histories of cocaine exposure and discrimination. Psychopharmacology. 2006;186:168–176. doi: 10.1007/s00213-006-0364-9. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ramanathan CR, Mutschler NH, Makriyannis A, Bergman J. Drug discrimination in methamphetamine-trained monkeys: effects of monoamine transporter inhibitors. J Pharmacol Exp Ther. 2004;311:720–727. doi: 10.1124/jpet.104.071035. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology. 2004;175:170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010;335:807–816. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Role of the NMDA receptor NR2B subunit in the discriminative stimulus effects of ketamine. Behavioural pharmacology. 2003;14:229–235. doi: 10.1097/00008877-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- France CP, Woods JH, Ornstein P. The competitive N-methyl-D-aspartate (NMDA) antagonist CGS 19755 attenuates the rate-decreasing effects of NMDA in rhesus monkeys without producing ketamine-like discriminative stimulus effects. Eur J Pharmacol. 1989;159:133–139. doi: 10.1016/0014-2999(89)90697-3. [DOI] [PubMed] [Google Scholar]
- France CP, Moerschbaecher JM, Woods JH. MK-801 and related compounds in monkeys: discriminative stimulus effects and effects on a conditional discrimination. J Pharmacol Exp Ther. 1991;257:727–734. [PubMed] [Google Scholar]
- Gaiardi M, Gubellini C, Dall'Olio R, Gandolfi O, Bartoletti M. Effects of N-methyl-D-aspartate agonists and antagonists in rats discriminating amphetamine. Behav Pharmacol. 2001;12:317–324. doi: 10.1097/00008877-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Gasior M, Witkin JM, Goldberg SR, Munzar P. Chlormethiazole potentiates the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;494:183–189. doi: 10.1016/j.ejphar.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Selvig M, Forster MJ. GABAergic modulation of the discriminative stimulus effects of methamphetamine. Behav Pharmacol. 2005;16:261–266. doi: 10.1097/01.fbp.0000166464.68186.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Pratt R. Ethanol modulates the discriminative stimulus effects of methamphetamine. In: Toolaney GH, editor. New Research on Methamphetamine Abuse. New York: Nova Science Publishers Inc.; 2006. pp. 69–98. [Google Scholar]
- Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug Alc Depend. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geter-Douglass B, Witkin JM. Dizocilpine-like discriminative stimulus effects of competitive NMDA receptor antagonists in mice. Psychopharmacology. 1997;133:43–50. doi: 10.1007/s002130050369. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. Comparative effects of dextromethorphan and dextrorphan on morphine methamphetamine and nicotine self-administration in rats. Eur J Pharmacol. 2001;422:87–90. doi: 10.1016/s0014-2999(01)01066-4. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Ann Rev Pub Health. 2010;21:385–98. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Effects of the NMDA antagonist memantine on human methamphetamine discrimination. Psychopharmacology. 2002;164:376–384. doi: 10.1007/s00213-002-1225-9. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Horton DB, Siripurapu KB, Norrholm SD, Culver JP, Hojahmat M, Beckmann JS, Harrod SB, Deaciuc AG, Bardo MT, Crooks PA, Dwoskin LP. meso-Transdiene Analogs Inhibit Vesicular Monoamine Transporter-2 Function and Methamphetamine-Evoked Dopamine Release. J Pharmacol Exp Ther. 2011;336:940–951. doi: 10.1124/jpet.110.175117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Brown G, Stephens DN. N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4- isoxazoleproprionate (AMPA) glutamate-receptor antagonists have different interactions with the discriminative stimuli of abused drugs. Psychopharmacology. 1996;128:320–327. doi: 10.1007/s002130050140. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, Highkin JL, Hockemeyer J, Munzar P, Goldberg SR. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Edwards MA, O'Connor TP. Modulation of the discriminative stimulus and rate-altering effects of cocaine by competitive and noncompetitive N-methyl-D-aspartate antagonists. Pharmacol Biochem Behav. 1998;59:159–169. doi: 10.1016/s0091-3057(97)00379-1. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Winger GD. MK-801 a proposed noncompetitive antagonist of excitatory amino acid neurotransmission produces phencyclidine-like behavioral effects in pigeons rats and rhesus monkeys. J Pharmacol Exp Ther. 1988;245:969–974. [PubMed] [Google Scholar]
- Koek W, Woods JH, Colpaert FC. N-methyl-D-aspartate antagonism and phencyclidine-like activity: a drug discrimination analysis. J Pharmacol Exp Ther. 1990;253:1017–1025. [PubMed] [Google Scholar]
- Liang JC, Zheng IW. Effect of dizocilpine maleate on discriminative properties of methamphetamine in rats. Acta Pharmacol Sin. 2000;21:605–608. [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Nader MA. A within-subject assessment of the discriminative stimulus and reinforcing effects of self-administered cocaine in rhesus monkeys. Psychopharmacology. 2009;203:343–353. doi: 10.1007/s00213-008-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Narita M, Onodera K, Suzuki T. Modulation of the discriminative stimulus effects of cocaine and methamphetamine by the histaminergic system. Jap J Psychopharmacology. 2002;22:73–78. [PubMed] [Google Scholar]
- Munzar P, Nosal R, Goldberg SR. Potentiation of the discriminative-stimulus effects of methamphetamine by the histamine H3 receptor antagonist thioperamide in rats. Eur J Pharmacology. 1998;363:93–101. doi: 10.1016/s0014-2999(98)00789-4. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Noradrenergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 1999;143:293–301. doi: 10.1007/s002130050950. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 2000;148:209–216. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, Ferre S, Goldberg SR. Adenosinergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 2002;161:348–355. doi: 10.1007/s00213-002-1075-5. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Yoshizawa K, Nomura M, Aoki K, Suzuki T. Role of the NMDA receptor subunit in the expression of the discriminative stimulus effect induced by ketamine. Eur J Pharmacol. 2001;423:41–46. doi: 10.1016/s0014-2999(01)01089-5. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Osborne MP, Olive MF. A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann New York Acad Sci. 2008;1139:206–211. doi: 10.1196/annals.1432.034. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Koob GF. Dextromethorphan reduces intravenous cocaine self-administration in the rat. Eur J Pharmacol. 1997;321:279–283. doi: 10.1016/s0014-2999(96)00970-3. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Watanabe-Galloway S, Ryan S, Hansen K, Hullsiek B, Muli V, Malone AC. Effects of methamphetamine abuse beyond individual users. J Psychoactive Drugs. 2009;41:241–248. doi: 10.1080/02791072.2009.10400534. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2nd. New York: McGraw-Hill; 1971. [Google Scholar]
- Witkin JM. Blockade of the locomotor stimulant effects of cocaine and methamphetamine by glutamate antagonists. Life Sci. 1993;53:PL405–410. doi: 10.1016/0024-3205(93)90496-p. [DOI] [PubMed] [Google Scholar]
- Zakharova ES, Danysz W, Bespalov AY. Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology. 2005;179:128–135. doi: 10.1007/s00213-004-2067-4. [DOI] [PubMed] [Google Scholar]