Abstract
Background:
Gestational diabetes mellitus (GDM) is a metabolic disorder defined as glucose intolerance with the onset or first recognition during pregnancy. Women with GDM are at increased risk for adverse obstetric and perinatal outcome. The complications associated with GDM can be prevented by early recognition, intense monitoring and proper treatment.
Aims:
The present study was done to screen the high-risk pregnancy group for GDM, to find the incidence of abnormal results on screening and to correlate the abnormal results with the maternal and fetal outcomes. The study was done in a tertiary care hospital and teaching institute. It was a prospective cohort study.
Materials and Methods:
Selective screening for GDM was done in 150 pregnant women with high-risk factors. Screening was done with 50 g glucose challenge test (GCT) after 18 weeks, and if GCT was negative then the test was repeated after 28 weeks of pregnancy. The patients who were having an abnormal GCT were subjected to 100 g oral glucose tolerance test (OGTT). All GDM patients were followed up and treated with diet and/or insulin therapy till delivery to know maternal and fetal outcomes. The period of study was from April 2008 to March 2009.
Results:
7.3% of study population was OGCT positive. 6% of the study population was OGTT positive. Age >25 years, obesity, family history of DM, and past history of GDM were the risk factors significantly associated with GDM. One newborn had hypoglycemia and one had hyperbilirubinemia. The fetal and maternal outcome in GDM patients was good in our study due to early diagnosis and intervention.
Conclusion:
Women with GDM are at an increased risk for adverse obstetric and perinatal outcome. The increased morbidity in GDM is preventable by meticulous antenatal care.
Keywords: Hyperbilirubinemia, hypoglycemia, gestational diabetes mellitus, glucose challenge test, glucose tolerance test, obesity
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with the onset or first recognition during pregnancy, with or without remission after the end of pregnancy.[1] India leads the world with the largest number of diabetic subjects, earning the dubious distinction of “the diabetes capital of the world.”[2]
It was estimated to have had 31.7 million people having diabetes in the year 2000, which is projected to be 79.4 million by the year 2030.[3] GDM is important in that it poses a risk to the pregnant woman and her baby. GDM is associated with increased incidence of maternal hypertension, pre-eclampsia, obstetric intervention and risk of developing diabetes mellitus (DM) in later life.[4] The major morbidities associated with infants of diabetic mothers include respiratory distress, growth restrictions, polycythemia, hypoglycemia, congenital malformations, hypocalcemia and hypomagnesemia.[5] Perinatal outcome associated with poor glycemic control in mothers is associated with as high as 42.9% mortality.[6] Appropriate diagnosis and management of GDM can improve maternal and perinatal outcome.
Despite more than 40 years of research, there is no consensus regarding the optimal approach to screening for GDM. The major issues include whether universal or selective screening should be used and which plasma glucose level after a 50 g glucose test threshold is the best to identify women at risk of GDM.[7] The American Diabetes Association (ADA), Australasian Diabetes in Pregnancy Society (ADIPS) and the fifth International Workshop-Conference on Gestational Diabetes recommendations acknowledge that there are variable levels of risk for GDM and, consequently, selective rather than universal screening can be considered.[8–10] Selective screening both reduces costs and, for women deemed not to need screening, eliminates the minor physical inconvenience of the procedure and any anxiety raised by the possibility of suffering diabetes. The present study was undertaken to find the prevalence of GDM in the high-risk group attending a tertiary care teaching institute in Karnataka, India. Pregnant women were categorized as high risk according to the criteria shown in Table 1.[8,9] The maternal and fetal outcomes of mothers with GDM were also studied.
Table 1.
Inclusion criteria

MATERIALS AND METHODS
This study was carried out from April 2008 to March 2009 at a tertiary care teaching institute in Karnataka, India. A detailed history was obtained from each expectant mother attending the hospital. Out of 1210 women delivered during this period, 150 were categorized as high risk and included in our study according to the criteria shown in Table 1.[8,9] The exclusion criteria included history of pregestational diabetes (overt diabetes)/cardiac/respiratory/hepatic and other medical disease and history of intake of drugs that affect glucose metabolism like corticosteroids. The high-risk women were screened for gestational diabetes with 50 g glucose challenge test (GCT) after 18 weeks, and if GCT was negative, then the test was repeated after 28 weeks of pregnancy. The patients who were having an abnormal GCT were subjected to oral glucose tolerance test (OGTT). All GDM patients were followed up and treated with diet and/or insulin therapy till delivery to know maternal and fetal outcomes.
Consent and ethical approval
This study was approved by the institutional ethics committee. Informed consent was taken from the patient.
Method of performing glucose challenge test
This test was performed as a routine OPD procedure. Fifty grams of glucose was dissolved in 200 ml of water and the patient was asked to drink it within 5 minutes. The time was noted and the patient was asked to come back after an hour for the test. A capillary blood specimen was obtained and tested for blood sugar levels by glucometer. If the blood sugar levels were greater than 140 mg %, the screening test was considered positive and these patients were subjected to OGTT to confirm the diagnosis of gestational diabetes.
Method of performing oral glucose tolerance test
Initial blood sample was taken after 8–14 hours of fasting and the patient was asked to drink 100 g glucose dissolved in 200–400 ml water within 5 minutes. Blood samples were taken at 1, 2 and 3 hours. The plasma glucose concentration was considered normal if it was below 95 mg/dl – fasting, 180 mg/dl – 1 hour, 155 mg/dl – 2 hours and 140 mg/dl – 3 hours. A patient was considered to have gestational diabetes if two or more values were met or exceeded.
Statistical analysis
Results were expressed as number and percentages. Z test for proportions was used for comparing GDM and control. Two-tailed P values <0.05 were considered to be significant. The sensitivity, specificity and positive and negative predictive values for the risk factors significantly associated with GDM were calculated.
RESULTS
Out of 150 high-risk patients, 7.3% (n = 11) had positive GCT screening test and 6% (n = 9) had positive OGTT. Table 2 shows the comparison of age in the GDM and non-GDM population. The mean age of patients in the study population was 22.5 years. 88% of the population were in the age group 21–30 years. 31.3% of the study population belonged to the high-risk group of more than 25 years. Table 3 shows the comparison of prevalence of risk factors in GDM and non-GDM population. Past history of fetal loss was the most common high-risk factor observed in the non-GDM population, whereas obesity was the commonest risk factor in the GDM population. Family history of DM, past history of GDM, age >25 years and obesity were significantly associated with GDM. Table 4 shows the sensitivity, specificity, positive and negative predictive values of risk factors significantly associated with GDM. Family history of GDM, age >25 years and obesity had a high sensitivity and negative predictive value. Out of nine cases of GDM, only one baby had hyperbilirubinemia and one had hypoglycemia. Hyperbilirubinemia was defined as treatment with phototherapy after birth, or at least one laboratory report of bilirubin level ≥20 mg/dl, or readmission to the hospital for hyperbilirubinemia. Clinical hypoglycemia was diagnosed on the basis of treatment with intravenous glucose infusion or low levels of glucose, defined as <30.6 mg/dl in the first 24 hours after delivery or 45 mg/dl glucose after the first 24 hours. Two patients with GDM had lower segment caesarean section (LSCS) the indication being previous LSCS. Overall, the maternal and fetal outcomes in our study were excellent.
Table 2.
Comparison of age distribution of gestational diabetes mellitus and non-Gestational diabetes mellitus population
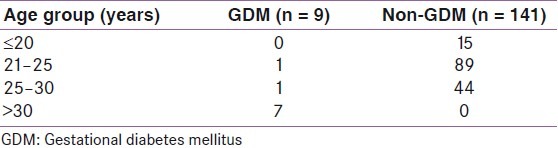
Table 3.
Comparison of prevalence of risk factors in GDM and non-GDM populations
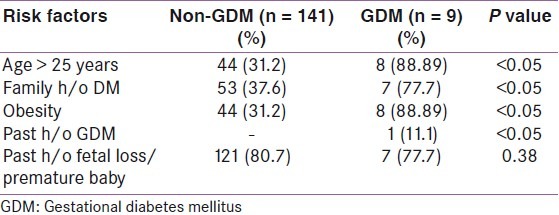
Table 4.
Sensitivity, specificity, positive and negative predictive values of the risk factors significantly associated with gestational diabetes mellitus

DISCUSSION
DM in pregnancy has severe consequences for perinatal morbidity and mortality. GDM prevalence has been reported variably from 1.4 to 14% worldwide and differently among racial and ethnic groups.[11] Compared to European women, the prevalence of gestational diabetes has increased 11-fold in women from the Indian subcontinent.[1] Das et al., in their study of 300 women, found 61 with positive screening. Out of them, 12 were diagnosed as gestational diabetes. Among the 12 gestational diabetics, 10 (9.4%) belonged to high-risk group.[12] Bhattacharya et al., Maheshwari et al., and Kummar et al. found the incidence of gestational diabetes in high-risk group to be 8%, 4.9% and 5.5%, respectively.[13–15] Similarly, in our study, the incidence of gestational diabetes in high-risk group was found to be 6%. Various aspects of patient's medical history, family history and obstetric history have been advocated as a means of identifying population at risk for gestational diabetes. This group deserves diagnostic testing. 50 g GCT was found be very sensitive in the detection of gestational diabetes in high-risk group. Coustan et al. found that current American College of Obstetricians and Gynaecologists (ACOG) recommendations result in a sensitivity of 65%.[16] We found that 9 out of 11 (i.e. 81.8%) patients with positive GCT had GDM. Kini et al. opined that 50 g GCT should be repeated in 3rd trimester as it yields a large number of gestational diabetics.[17] Due to the simplicity, acceptibility, sensitivity and cost effectiveness of GCT, it is the best screening method to detect GDM in high-risk group.
Multiple studies support the idea that GDM appears more frequently in pregnancy after age 30 because of age-related metabolic changes and it is rare before age 20. The confluence of conditions more commonly seen at older ages – such as pregnancy-induced hypertension, increased body mass, and dyslipidemias – increases DM risk, as supported by Etchegoyen et al., and Cárdenas and Arroyo in Peru.[18,19] Similarly, in our study, seven patients with GDM were more than 30 years of age. Family history of DM was present in 77.7% of our cases, which concurs with the results from a study done by Gomez et al. in 180 pregnant diabetic women.[20] Das et al. found that 14.3% of women with GDM had family history of DM.[12] Obesity as a significant risk factor for GDM is supported by several studies finding that overweight or obesity at the start of pregnancy predispose to GDM. Das et al. and Gomez et al. found that 25% and 50% of women with GDM had obesity.[12,20] This may be due to increased demands on maternal metabolism during pregnancy from excess weight, resulting in imbalances in hormonal carbohydrate regulation mechanisms, and insulin sensitivity. Weight distribution also seems to play a role because Zhang et al. found that the risk of GDM was increased with truncal obesity.[21] We found obesity as a risk factor in 88.89% of GDM patients. Our study shows that 6 (66.67%) of the diabetic mothers had previous fetal or early neonatal deaths, this being one of the reasons (as documented in their case notes) for referral of some of them to our center. This re-emphasizes that diabetes still contributes significantly to perinatal and neonatal mortality in our environment. Neonatal hypoglycemia is much more common in infants born to GDM mothers (30–50%) as compared to the ones born to normal mothers (0.5–4%).[22] Maternal hyperglycemia with increased placental glucose transfer to the fetus and the resultant fetal hyperinsulinemia from fetal B-cell hyperplasia results in neonatal hypoglycemia after umbilical cord clamping. In addition, decreased hepatic glucose production and diminished ability to use glycogen in the first hour of life predisposes these infants to hypoglycemia.[23] We found hypoglycemia in only one baby (11.1%) born to mother with GDM. In contrast to our study, Opara et al. found hypoglycemia in 63.8% of babies born to mothers with diabetes.[5] Hyperbilirubinemia is common in infants of women with GDM, complicating up to 20% of these births as compared with 10% of the general population. The exact cause of this is not well understood, but theories include excessive RBC breakdown in association with polycythemia and the result of an immature bilirubin conjugation by liver in the neonate.[22,24] Opara et al. found neonatal hyperbilirubinemia in 57.4% of cases in contrast to our study where only 1 (11.1%) baby born to GDM mother had jaundice.[5] Asymptomatic hypocalcemia was seen in one baby born to GDM mother. Several studies have reported hypocalcemia in babies born to diabetic mothers.[25,26] Tsang et al. advanced the hypothesis that hyperparathyroidism of diabetic mothers might suppress the fetal parathyroid function and lead to hypocalcemia of the newborn.[27]
None of our babies had other adverse outcomes related to diabetes, like macrosomia, respiratory distress syndrome, polycythemia, preterm delivery, congenital anomalies or stillbirth. The high rates of these neonatal complications have been identified as a marker of poor glycemic control in the mother. The major congenital malformations include caudal regression, situs inversus, spina bifida, hydrocephalus, anencephaly, cardiac anomalies and renal anomalies. The increased risk of severe malformations is the consequence of poorly controlled diabetes, both preconceptionally and early in pregnancy.[7] Higher perinatal mortality rate in uncontrolled gestational diabetes has been reported previously. However, among our diabetic patients, there was no perinatal mortality and no congenital malformation in the fetus. LSCS was done in only two cases of GDM population, the indication being previous LSCS. The excellent fetal and maternal outcome in our study was possible due to early diagnosis and intervention in our GDM patients.
CONCLUSION
Women with GDM are at an increased risk for adverse obstetric and perinatal outcomes. Age >25 years, obesity, family history of DM, and past history of GDM are significant risk factors in GDM population. Good maternal and fetal outcomes result from early and meticulous prenatal and intranatal care as seen in our study. Although eradication of GDM is impossible, we can definitely prevent its adverse effects on pregnancy outcome. Treatment of GDM prevents future DM in the mother and also acts as prevention for future DM in the unborn child.[28,29] Opening of maternal–infant centers with standard protocols for prevention and treatment of diabetes in pregnancy on a national scale will go a long way in reducing the scourge of this condition. With effective screening and management of GDM, from “the diabetes capital of the world,” we (INDIA) can lay claim to be the “diabetes care capital of world.”[2]
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S Diabetes in Pregnancy Study Group. Gestational diabetes mellitus--guidelines. J Assoc Physicians India. 2006;54:622–8. [PubMed] [Google Scholar]
- 2.Magon N. Gestational diabetes mellitus: Get, set, go From diabetes capital of the world to diabetes care capital of the world. Indian J Endocrinol Metab. 2011;15:161–9. doi: 10.4103/2230-8210.83398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Davey RX, Hamblin PS. Selective versus universal screening for gestational diabetes mellitus: An evaluation of predictive risk factors. Med J Aust. 2001;174:118–21. doi: 10.5694/j.1326-5377.2001.tb143181.x. [DOI] [PubMed] [Google Scholar]
- 5.Opara PI, Jaja T, Onubogu UC. Morbidity and mortality amongst infants of diabetic mothers admitted into a special care baby unit in Port Harcourt, Nigeria. Ital J Pediatr. 2010;36:77. doi: 10.1186/1824-7288-36-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otolorin EO, Famuyiwa OO, Bella AF, Dawodu AH, Adelusi B. Reproductive performance following active management of diabetic pregnancies at the University College Hospital, Ibadan, Nigeria. Afr J Med Med Sci. 1985;14:155–60. [PubMed] [Google Scholar]
- 7.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, editors. Williams Obstetrics. 23rd ed. United States of America: McGraw-Hill Companies; 2010. Diabetes; pp. 1104–25. [Google Scholar]
- 8.The Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1998;21(Suppl 1):S5–19. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D. Gestational diabetes mellitus--management guidelines.Australasian Diabetes in Pregnancy Society. Med J Aust. 1998;169:93–7. doi: 10.5694/j.1326-5377.1998.tb140192.x. [DOI] [PubMed] [Google Scholar]
- 10.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on gestational diabetes mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 11.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 12.Das V, Kamra S, Mishra A. Screening for gestational diabetes and maternal and fetal outcome. J Obstet Gynaecol India. 2004;54:449–51. [Google Scholar]
- 13.Bhattacharya C, Awasthi RT, Kumar S, Lamba PS. Routine screening for gestational diabetes mellitus with glucose challenge test in antenatal patients. J Obstet Gynaecol India. 2001;51:75. [Google Scholar]
- 14.Maheshwari JR, Mataliya MY, Patil DR. Screening for glucose intolerance in pregnancy utilising random plasma glucose assay. J Obstet Gynaecol India. 1989;39:351. [Google Scholar]
- 15.Kumar A, Takkar D, Kumar S. Implications of diagnosis of glucose intolerance during pregnancy; Perinatal mortality and morbidity. J Obstet Gynaecol India. 1993;43:759. [Google Scholar]
- 16.Coustan DR, Nelson C, Carpenrer MW. Maternal age and screening for gestational diabetes.A Population Based Study. Obstet Gynaecol. 1989;73:557. [PubMed] [Google Scholar]
- 17.Kini S, Partap K, Kurup M. Screening for gestational diabetes in 3rd Trimester. J Obstet Gynaecol India. 1996;6:46. [Google Scholar]
- 18.Etchegoyen GS, De Martín ER, Parral Longobardi C, Cedola N, Alvariñas J, González C, et al. [Determinación del peso relativo de sus factores de riesgo] Medicina. 2001;61:161–6. [PubMed] [Google Scholar]
- 19.Cárdenas Goicochea SJ, Arroyo L. [Prueba de tolerancia oral a la glucosa modi cada en puérperas como diagnóstico retrospectivo de diabetes gestacional] An Fac Med (Peru) 2004;65:7–13. [Google Scholar]
- 20.Gómez HL, Martínez ML, Rodríguez ZM. Clinical and epidemiological profile of diabetes mellitus in pregnancy, Isle of Youth, 2008. MEDICC Rev. 2011;13:29–34. doi: 10.37757/MR2011V13.N1.8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Folsom AR, Flack JM, Liu K. Body fat distribution before pregnancy and gestational diabetes: Findings from coronary artery risk development in young adults (CARDIA) study. BMJ. 1995;311:1139–40. doi: 10.1136/bmj.311.7013.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uvena-Celebrezze J, Catalano P. The infant of the woman with Gestational Diabetes Mellitus. Clin Obstet Gynecol. 2000;43:127–39. doi: 10.1097/00003081-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Marshall RE. Infant of the diabetic mother: Aneonatologist's view. Clin Diabetes. 1990;8:49–57. [Google Scholar]
- 24.Wildness JA, Cowett RM, Coustan DR, Carpenter MW, Oh W. Neonatal morbidities in infants of mother with glucose intolerance in pregnancy. Diabetes. 1985;34(Suppl 2):S61–5. doi: 10.2337/diab.34.2.s61. [DOI] [PubMed] [Google Scholar]
- 25.Cordero L, Landon MB. Infant of diabetic mother. Clin Perinatol. 1993;20:635–48. [PubMed] [Google Scholar]
- 26.Nasrat HA, Salleh M, Ardawi M, Ghafouri H. Outcome of pregnancy in diabetic mothers. Int J Gynaecol Obstet. 1993;43:29–34. doi: 10.1016/0020-7292(93)90270-7. [DOI] [PubMed] [Google Scholar]
- 27.Tsang RC, Kleinman LI, Sutherland JM, Light IJ. Hypocalcaemia in infants of diabetic mothers: Studies in calcium, phosphorus and magnesium metabolism and parathormone reponsiveness. J Pediatr. 1972;80:384–95. doi: 10.1016/s0022-3476(72)80494-3. [DOI] [PubMed] [Google Scholar]
- 28.Kalra S, Malik S, John M. Gestational diabetes mellitus: A window of opportunity. Indian J Endocrinol Metab. 2011;15:149–51. doi: 10.4103/2230-8210.83395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]


