Abstract
In general, infectious diseases are more frequent and/or serious in patients with diabetes mellitus, which potentially increases their morbimortality. The greater frequency of infections in diabetic patients is caused by the hyperglycemic environment that favors immune dysfunction (e.g., damage to the neutrophil function, depression of the antioxidant system, and humoral immunity), micro- and macro-angiopathies, neuropathy, decrease in the antibacterial activity of urine, gastrointestinal and urinary dysmotility, and greater number of medical interventions in these patients. The infections affect all organs and systems. Some of these problems are seen mostly in diabetic people, such as foot infections, malignant external otitis, rhinocerebral mucormycosis, and gangrenous cholecystitis. In addition to the increased morbidity, infectious processes may be the first manifestation of diabetes mellitus or the precipitating factors for complications inherent to the disease, such as diabetic ketoacidosis and hypoglycemia. Immunization with anti-pneumococcal and influenza vaccines is recommended to reduce hospitalizations, deaths, and medical expenses.
Keywords: Diabetes mellitus, immunization, infections, vaccines
INTRODUCTION
Diabetes mellitus (DM) is a clinical syndrome associated with deficiency of insulin secretion or action. It is considered one of the largest emerging threats to health in the 21st century. It is estimated that there will be 380 million persons with DM in 2025.[1] Besides the classical complications of the disease, DM has been associated with reduced response of T cells, neutrophil function, and disorders of humoral immunity.[2–4] Consequently, DM increases the susceptibility to infections, both the most common ones as well as those that almost always affect only people with DM (e.g. rhinocerebral mucormycosis).[4] Such infections, in addition to the repercussions associated with its infectivity, may trigger DM complications such as hypoglycemia and ketoacidosis.
This article aims to critically review the current knowledge on the mechanisms associated with the greater susceptibility of DM for developing infectious diseases and to describe the main infectious diseases associated with this metabolic disorder.
MATERIALS AND METHODS
The MEDLINE and LILACS databases were searched for articles published between 1999 and 2011, using the following keywords in various combinations: “diabetes mellitus,” “infections,” “immunization,” and “vaccines.” Bibliographic search included consensus papers, editorials, original articles, and review articles written in English, Spanish, and Portuguese. The articles were initially selected on the basis of their titles and abstracts. Articles published in languages other than the selected ones, articles without abstract, and those articles whose titles were not relevant to the purpose of this review were excluded.
DIABETES MELLITUS AND INFECTIONS: PHYSIOPATHOLOGY
The main mechanisms associated with the interface DM and infections are depicted in Figure 1.
Figure 1.
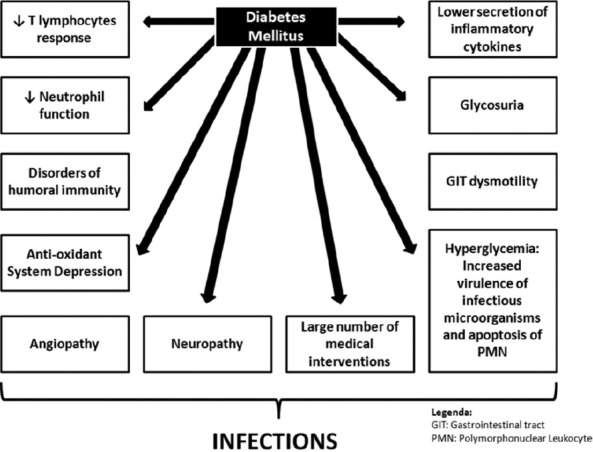
Pathophysiology of infections associated with diabetes millitus
Complement
The complement system is one of the main mechanisms responsible for the humoral immunity. It consists of serum and surface proteins whose main functions are to promote the opsonization and phagocytosis of microorganisms through macrophages and neutrophils and to induce the lysis of these microorganisms. Moreover, complement activation products provide the second signal for B-lymphocyte activation and antibody production.
Although some studies have detected a deficiency of the C4 component in DM,[5,6] this reduction of C4 is probably associated with polymorphonuclear dysfunction and reduced cytokine response.[2,5]
Inflammatory cytokines
Mononuclear cells and monocytes of persons with DM secrete less interleukin-1 (IL-1) and IL-6 in response to stimulation by lipopolysaccharides.[2,4] It appears that the low production of interleukins is a consequence of an intrinsic defect in the cells of individuals with DM.[2,7] However, other studies reported that the increased glycation could inhibit the production of IL-10 by myeloid cells, as well as that of interferon gamma (IFN-γ) and tumor necrosis factor (TNF)-α by T cells. Glycation would also reduce the expression of class I major histocompatibility complex (MHC) on the surface of myeloid cells, impairing cell immunity.[8]
Polymorphonuclear and mononuclear leukocytes
Decreased mobilization of polymorphonuclear leukocytes, chemotaxis, and phagocytic activity may occur during hyperglycemia.[4,9,10] The hyperglycemic environment also blocks the antimicrobial function by inhibiting glucose-6-phosphate dehydrogenase (G6PD), increasing apoptosis of polymorphonuclear leukocytes, and reducing polymorphonuclear leukocyte transmigration through the endothelium.[4] In tissues that do not need insulin for glucose transport, the hyperglycemic environment increases intracellular glucose levels, which are then metabolized, using NADPH as a cofactor. The decrease in the levels of NADPH prevents the regeneration of molecules that play a key role in antioxidant mechanisms of the cell, thereby increasing the susceptibility to oxidative stress.
Regarding the mononuclear lymphocytes, some studies had demonstrated that when the glycated hemoglobin (HbA1c) is <8.0%, the proliferative function of CD4 T lymphocytes and their response to antigens is not impaired.[4]
Antibodies
Glycation of immunoglobulin occurs in patients with diabetes in proportion with the increase in HbA1c, and this may harm the biological function of the antibodies.[4] However, the clinical relevance of these observations is not clear, since the response of antibodies after vaccination and to common infections is adequate in persons with DM.[4]
MAJOR INFECTIONS ASSOCIATED WITH DIABETES MELLITUS
Some investigators claim that the differences in the risk factors for infection between diabetic and non-diabetic patients result either from non-controlled studies or biased studies (e.g. persons with DM have frequent medical appointments which may increase the probability of being diagnosed with other diseases). However, most researchers conclude that there is clinical evidence pointing to the higher prevalence of infectious diseases among individuals with DM.[2,3,10] Table 1summarizes the major infections associated with DM.
Table 1.
Major infections associated with diabetes mellitus

Respiratory infections
Respiratory tract infections are responsible for a significant number of medical appointments by persons with DM compared to those without DM.[4,11–16]
Streptococcus pneumoniae and influenza virus
The most frequent respiratory infections associated with DM are caused by Streptococcus pneumoniae and influenza virus.[9,15] Persons with DM six times more likely need hospitalization during influenza epidemics than non-diabetic patients.[4] Diabetes is also a common coexisting condition and a risk factor for complications in patients with H1N1 (pandemic influenza virus) infection.[17]
The American Diabetes Association (ADA)[11] and the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP)[13] recommend anti-pneumococcal and influenza vaccination for people with DM, as shown in Tables 2 and 3, respectively. The World Health Organization recommends vaccination against the H1N1 virus, which is a single-dose vaccine, to minimize the virus-related morbidity and mortality.[18]
Table 2.
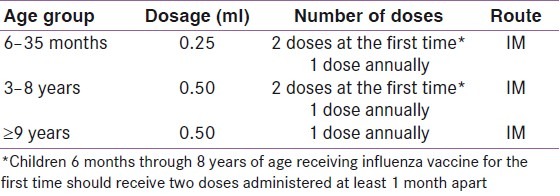
Table 3.
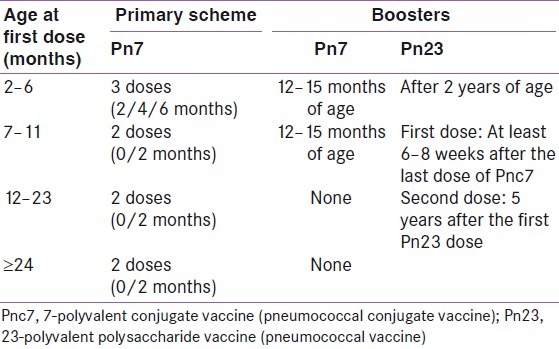
These vaccines reduce the number of respiratory infections, the number and length of hospitalizations, the deaths caused by respiratory tract infections, and the medical expenses related to influenza and pneumonia.[15] Despite these benefits, the vaccination coverage in persons with DM remains inadequate.[19,20]
Tuberculosis
In 2009, approximately 9 million new cases of tuberculosis were diagnosed and 1.7 million persons died from this infection.[21] Patients with diabetes are at higher risk of contracting tuberculosis than individuals without DM.[21,22] Some studies have reported that persons with DM are more likely to develop multi-resistant tuberculosis and that treatment failures and death are more frequent in these patients.[23] In addition, tuberculosis infection and treatment (rifampicin increasing the metabolism of oral antidiabetic drugs) may complicate the glycemic control.[24]
It is suggested that DM depresses the immune response (impairing chemotaxis, phagocytosis, and antigen presentation in response to Mycobacterium tuberculosis infection and affecting T-cell function and proliferation) facilitating infection and progression to symptomatic disease.[21–23]
The association of these two diseases poses a major burden to the health public system, especially in the developing countries where tuberculosis is one of the most important causes of bacterial infection and the prevalence of type 2 DM is increasing. Therefore, routine screening of patients with tuberculosis for DM and patients with DM for tuberculosis should be implemented.
Urinary infections
Urinary tract infections (UTIs) are more prevalent in individuals with DM and may evolve to complications and/or serious manifestations.[25–27] The main risk factors for UTI in DM are: inadequate glycemic control, duration of DM, diabetic microangiopathy, impaired leukocyte function, recurrent vaginitis, and anatomical and functional abnormalities of the urinary tract.[4,25–29]
Asymptomatic bacteriuria
Although women with DM have greater prevalence of asymptomatic bacteriuria,[25,27–30] the data on the natural history of this condition in women with DM are conflicting. Some studies reported progression to pyelonephritis,[28,30] whereas other suggested that this does not lead to serious complications.[11,25,31] Thus, the routine recommendation of antibiotic therapy for asymptomatic bacteriuria in diabetic women remains controversial.[16]
Bacterial pyelonephritis
Acute pyelonephritis is 4–5 times more common in individuals with DM.[26] Most infections are caused by Escherichia coli or Proteus sp.[9] The clinical presentation is similar to that of non-diabetic individuals, except for the bilateral renal involvement.[4,32] Additionally, persons with DM are at increased risk for complications such as perinephric and/or renal abscesses, emphysematous pyelonephritis (EP), and renal papillary necrosis.[4,9,32]
Emphysematous pyelonephritis
EP is characterized by necrosis of the renal parenchyma with the presence of gas in the collecting system or in the perinephric tissues.[30–36] It is most commonly observed in DM women.[16,25,32–36]
E. coli and Enterobacter aerogenes are the most frequent pathogens, followed by Klebsiella sp., Proteus sp., Candida and Streptococcus sp.[9,32,33]
Fever, chills, mass and flank pain, nausea, and vomiting are the first symptoms.[27] Crackles in the flank or thigh are less frequent.[16,32,35,37] Abdominal computerized tomography allows the identification of gas in the urinary tract.[9,16,32,33,38–40]
Fungal cystitis
Fungal infections are more common in DM, particularly those caused by Candida.[28,41] The distinction between infection and colonization can be difficult. The presence of urinary symptoms or pyuria suggests infection.[9] Fungal cystitis may result in the formation of “fungal balls” which may complicate as urinary tract obstruction.[28]
Emphysematous cystitis
Emphysematous cystitis affects persons with DM more frequently than non-diabetics.[26,40,42,43] It is characterized by the presence of gas in the bladder cavity and infiltration of the bladder wall due to infection by bacteria that produce carbon dioxide.[44] The most frequent pathogen is E. coli, followed by Enterobacter, Proteus, Klebsiella, and Candida.[32] Women are more affected than men.[26,40,44] Computerized tomography is the standard diagnostic method.[42]
Perinephric abscess
The main etiologies of renal and perinephric abscesses are enteric gram-negative bacilli (predominantly E. coli) or polymicrobial infection.[45–47] Around one-third of perinephric abscesses occur in persons with DM.[16,46,47]
The initial clinical manifestations are fever, backache, dysuria, and/or polyuria.[47] A palpable mass may be present.[42] In case of perirenal suppuration, the overlying skin may show inflammatory reaction.[48] In individuals with DM, the clinical presentation is non-specific. Therefore, the diagnosis is usually late, contributing to worse prognosis. This diagnosis should be considered in patients with acute pyelonephritis that does not get better after antimicrobial therapy.[45]
Gastrointestinal and liver infections
The regularity of gastrointestinal motility and sensitivity are important mechanisms of defense against infections.[32] Chronic hyperglycemia contributes to increase the risk of gastrointestinal infectious processes.[32,49]
Gastritis caused by Helicobater pylori
The association of DM and infection by Helicobacter pylori is controversial. Although some studies showed that some virulent strains of H. pylori are related to macroangiopathy, neuropathy, and microalbuminuria in patients with DM2, there is apparently no relationship between H. pylori infection and these DM complications.[49–53]
Some data indicate a possible association of H. pylori infection with coronary insufficiency and/or cerebral occlusive vascular disease in adults with DM.[51] Another consequence of this infection is the increase in insulin requirements in children with DM1.[51]
The efficiency of H. pylori eradication is lower in persons with DM, whereas the re-infection rates are seen to be higher.[50,51,54]
Oral and esophageal candidiasis
The most common etiological agent is Candida albicans.[4,55] Its pathogenesis is related to a combination of factors that increase its virulence, with emphasis on the production of extracellular enzymes such as proteinase and phospholipase.[55] Candidiasis manifests in different ways: median rhomboid glossitis or central papillary atrophy, atrophic glossitis, denture stomatitis, pseudomembranous candidiasis, and angular cheilitis.[55] The diagnosis is eminently clinical. However, in case of esophageal candidiasis, endoscopy is needed.[55]
Emphysematous cholecystitis
The emphysematous cholecystitis is more frequent in males with DM.[32] The main pathogens are Salmonella enteritidis and Campylobacter.[32] The clinical presentation is not different from that of non-complicated cholecystitis (e.g. right upper quadrant abdominal pain, vomiting, and fever).[9] Clinical signs of peritonitis are usually not observed. Crackles can be felt on abdominal palpation, being associated with a worse prognosis.[9] The diagnosis is made by the detection of gas inside the gall bladder, demonstrated in radiograph or computerized tomography scan.[32]
Hepatitis C
Hepatitis C virus (HCV) is a major public health problem affecting more than 170 million people worldwide and this figure is expected to increase due the lack of a vaccine to prevent it.[56] Approximately 50–80% of these patients develop a chronic infection and have a greater chance to progress to cirrhosis.
Several studies, from different countries, have reported that 13–33% of patients with HCV infection have diabetes, mostly type 2 diabetes mellitus (T2DM),[57] compared with the prevalence of 4–10% for non-HCV control population.[58] These data suggest that patients with HCV are 3 times more likely to develop DM than individuals who are HCV negative. Therefore, T2DM is considered an extrahepatic manifestation of this infection.[57]
Patients with HCV who develop T2DM have a more severe liver disease and increased fibrosis compared to non-diabetic HCV patients.[59] Possible explanations are that in individuals with a genetic predisposition to T2DM, the HCV infection could cause (1) impairment of beta-cell responsiveness as a result of a direct viral effect, mostly the genotype 1 or 4,[60] and/or (2) liver damage leading to an abnormal glucose metabolism and insulin resistance.[57]
Early screening for T2DM should be instituted in all patients with HCV, especially if they have other risk factors for diabetes, and screening for T2DM should be performed in areas of high HCV prevalence.
Hepatitis B
About 350 million people are infected by the hepatitis B virus (HBV) worldwide.[60] This number is expected to decrease with the availability and widespread use of the anti-HBV vaccine.
The studies on the relationship between HBV and T2DM are not consistent. Some investigators have reported blood glucose abnormalities,[61] while others have not.[62] Lao et al.[63] reported an independent association between HBV infection and gestational diabetes. Further studies are needed to clarify this questionable association.
However, HBV infection outbreaks have been reported among diabetic patients who share a blood glucose meter without cleaning and disinfecting between uses, associated with limited awareness of the high risk for HBV transmission during fingerstick blood glucose monitoring.[64]
Enteroviruses
Enteroviruses are largely distributed in the world and are mainly transmitted by the fecal–oral route. They are classified into five species: poliovirus, human enterovirus A, human enterovirus B (including the six Coxsackie B virus serotypes), echovirus C, and echovirus D.
Several epidemiological and clinical studies have supported the role of enterovirus, especially Coxsackie B4 and B3 virus, in the development of T1DM in genetically predisposed individuals.[6] Such association is supported by the description of a temporal relationship between the occurrence of T1DM and peaks of enterovirus infections and by the detection of anti-enterovius antibodies, enterovirus RNA, and capsid protein VP1 in blood, small intestine biopsies, and autopsy pancreas specimens of individuals with T1DM.[65]
Various mechanisms can explain the role of enterovirus in the pathogenesis of T1DM:[65,66] (1) persistent infection of pancreatic beta cells provoking cell damage and release of sequestered antigens inducing an autoimmune response; (2) molecular mimicry (partial sequence homology) between the 2C viral protease and the GAD65 (Glutamic Acid Descarboxilase) and between the VP1 viral capsid protein and the IA2 protein; (3) bystander activation of autoreactive T cells; (4) thymus infection; and (5) loss of regulatory T cells. However, a causative correlation still needs to be established.
Skin and soft tissue infection
Persons with DM are more predisposed to skin and soft tissue infections such as folliculitis, furunculosis, and subcutaneous abscesses. These infections may break out during the course of the disease or may be the first sign of DM presentation,[67,68] and can also be more severe in these populations.[4,32,67]
Foot infection
Foot infections are the most important chronic complications of DM, being one of the most common causes of hospitalization and often resulting in amputation, osteomyelitis, and death.[4,9,67,69,70]
The clinical signs of infections are very protean and poor, often leading to delayed diagnosis.[71] The diabetic foot infections are usually divided into moderate or “non-limb threatening” and serious or “limb-threatening.”[32,70,72] Moderate infections are defined as superficial, with cellulitis less than 2.0 cm in the largest diameter, without evidence of serious ischemia, systemic toxicity, or bone and/or articular involvement. Serious infections are defined as deep ulceration, with celullitis equal to or greater than 2.0 cm in the largest diameter, with evidence of serious ischemia, systemic toxicity, or bone and/or joint involvement.[72]
These infections can be monomicrobial or polymicrobial. Staphylococcus aureus and Staphylococcus epidermidis are isolated from around 60% of all the infected ulcers. Enterococci, streptococci, and enterobacteria are less frequent, and 15% of the infected ulcers have strict anaerobic bacteria.[70]
Infection in a very recently acquired superficial ulcer is likely to be monomicrobial due to aerobic Gram-positive cocci, such as staphylococci, while a long duration of ulceration and increased depth are likely to increase the chances of the wound, yielding both polymicrobial growth and resistant organisms.[70]
Simple clinical assessments are predictive of bone involvement, such as size and depth of ulcer. Ulcers larger or deeper than 2 cm2 are more likely to be associated with underlying bone infection.[16,32,71] It is worth stressing that imaging evaluation can be normal in the beginning of the infection, since radiological abnormalities are either observed 10–20 days after the beginning of the infectious process or when 40–70% of the bone is lost.[16,32] Thus, the most sensitive tests are scintigraphy and magnetic resonance imaging.[16]
Necrotizing fasciitis
Necrotizing fasciitis is characterized by fast and progressive necrosis of the fascia and subcutaneous tissue, causing fulminant local tissue destruction, microvascular thrombosis, and systemic signs of toxicity. Mortality occurs in approximately 40% of the cases.[9,73–76]
The initial symptoms are fever and intense local pain, followed by areas of skin necrosis with small ulcers that drain a colorless fluid and have unpleasant smell.[16,32] Air in the soft tissues can be better detected by radiograph.[32] The most affected sites are thorax, abdominal wall, extremities, perineum, and groin.[74–76]
In DM, fasciitis is typically polymicrobial, with one anaerobic and many aerobic microorganisms.[16] Type I fasciitis is caused by the combination of an anaerobic microorganism with one or more facultative aerobic microorganisms, and type II fasciitis is caused by a group A streptococcus with or without the involvement of staphylococci.[75,76]
Fournier gangrene
Fournier gangrene is a fasciitis that affects the male genitalia. The most common etiologic agents are E. coli, Klebsiella sp., Proteus sp., and Peptostreptococcus.[77,78] The etiology can also be polymicrobial, involving Clostridium, aerobic or anaerobic streptococci, and Bacteroides.[32,78]
Up to 70% of the patients with this infection have DM.[29,32,77] It usually involves the scrotum, but can be extend to the penis, perineum, and abdominal wall.[9,16,29,78] Contrary to the general belief, the testicles are usually spared.[77]
Head and neck infections
The two most serious head and neck infections in diabetic persons are invasive external otitis and rhinocerebral mucormycosis.[32]
Invasive external otitis
Invasive external otitis is an infection of the external auditory canal that can extend to the skull base and adjacent regions.[9,16,79] It often affects elderly diabetic individuals and the etiologic agent is usually Pseudomonas aeruginosa.[16,79]
Excruciating pain, otorrhea, and hearing loss are the characteristics.[9] Skull base osteomyelitis and cranial nerve involvement may occur. Facial paralysis occurs in 50% of the cases.[16] The best diagnostic method is the magnetic resonance imaging.[16]
Rhinocerebral mucormycosis
Mucormycosis is a rare opportunistic and invasive infection caused by fungi of the class Zygomycetes.[80,81] The genus most commonly associated with human infections is the Rhizopus, followed by Mucor and Cunninghamella.[82]
This infection occurs in approximately 50% of the cases in individuals with DM due to the greater availability of glucose to the pathogen that causes mucormycosis, the decrease in serum inhibitory activity against the Rhizopus in lower pH, and the increased expression of some host receptors that mediate the invasion and damage to human epithelial cells by Rhizopus.[32,79,83]
The mucormycosis can be acute and chronic.[80–82] The classical triad is characterized by paranasal sinusitis, ophthalmoplegia with blindness, and unilateral proptosis with cellulitis.[16,80] Facial or eye pain and necrotic wound of the palate of the nasal mucosa may occur. Black necrotic eschar in the nasal cornets is a characteristic sign.[16,85,82]
Periodontitis
Periodontitis is a chronic inflammatory disease characterized by the formation of a periodontal pocket, loss of connective tissue, and alveolar bone resorption, which may sometimes result in tooth loss. It is four times more common in persons with DM and is considered the sixth most common complication of DM.[4,84,85] Periodontitis starts or disseminates insulin resistance, thus worsening glycemic control.[4,16,84–86] Inversely, persistent poor glycemic control has been associated with a greater incidence and progression of gingivitis and periodontitis, producing a vicious circle.[4,85,86]
Many mechanisms have been proposed to explain the increased susceptibility to periodontal disease in these patients, such as alterations in immune response, subgingival microbiota aspects, altered collagen metabolism, alteration in oral vascularization, hereditary patterns, altered neutrophil function, reduced phagocytic capacity, and chemotaxis.[4–84]
Other infections
Human immunodeficiency virus
Approximately 33 million people were infected by the human immunodeficiency virus (HIV) in 2007.[87] The improvements in diagnosis and treatment have translated into an increasing number of patients developing chronic complications including DM.
The increased risk of developing diabetes is related to the HIV itself or its treatment.[88] Insulin resistance is the main mechanism implicated in the pathogenesis of diabetes in HIV patients.[87] Insulin resistance results from high levels of inflammatory cytokines that impair glucose tolerance, leading to the development of T2DM. More recently, some patients were reported to develop autoimmune T1DM after immune restoration during highly active antiretroviral therapy (HAART).[89]
Risk factors for DM in HIV patients are: high viral burden, low CD4 count, longer duration of HIV infection, advancing age, male gender, lower socioeconomic class, and accumulation of visceral fat.[87]
Diabetes is fourfold more frequent in HIV patients on HAART.[89] The protease inhibitors cause insulin resistance by interfering with the GLUT-4 mediated transport and by inhibiting the peroxisomal proliferator activated receptor which increases the release of fatty acids and induces insulin resistance. The nucleoside reverse transcriptase inhibitors also contribute to the insulin resistance.
Patients with HIV should be screened for diabetes at diagnosis, at the onset, and during HAART therapy. An oral glucose tolerance test is recommended to assess the insulin resistance. The treatment of DM in HIV poses some limitations. For example, although metformin is the drug of choice, its use may not be tolerated by cachexic patients or by those with lypoatrophy. The side effects of thiazolidinediones (e.g. higher cardiovascular morbidity, osteoporosis) may prevent their use in HIV patients with diabetes. Glinides and sulfonylureas may not be effective due to insulin resistance.[87] Therefore, insulin is the drug of choice for the HIV-associated diabetes.
Other viruses
T1DM has been associated with other viral infections including rubella, mumps, Epstein–Barr and cytomegalovirus. The viral infection usually precedes the clinical presentation of T1DM. The causality between enterovirus infections and the diabetogenic proocess are still unclear.[90]
CONCLUSIONS
Infectious diseases are more prevalent in individuals with DM. The main pathogenic mechanisms are: hyperglycemic environment increasing the virulence of some pathogens; lower production of interleukins in response to infection; reduced chemotaxis and phagocytic activity, immobilization of polymorphonuclear leukocytes; glycosuria, gastrointestinal and urinary dysmotility. Some infections almost always affect only diabetic persons, such as malignant external otitis, rhinocerebral mucormycosis, and gangrenous cholecystitis. In addition to being potentially more serious, infectious diseases in DM may result in metabolic complications such as hypoglycemia, ketoacidosis, and coma. The recommendation of compulsory immunization with anti-pneumococcal and influenza vaccines is essential because of their impact on the reduction of respiratory infections, the number and length of hospitalizations and the number of deaths related to respiratory tract diseases.
More research is needed for clarification of the immunopathogenic mechanisms linking DM and infections and to develop strategies to improve vaccination coverage for diabetic patients.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Atkins RC, Zimmet P. Diabetic kidney disease: Act now or pay later. Saudi J Kidney Dis Transpl. 2010;21:217–21. [PubMed] [Google Scholar]
- 2.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:256–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 3.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–8. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 4.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 5.Stoeckle M, Kaech C, Trampuz A, Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly. 2008;138:512–9. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 6.Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol. 2010;6:94–101. doi: 10.1038/nrendo.2009.266. [DOI] [PubMed] [Google Scholar]
- 7.Geerlings SE, Brouwer EC, Van Kessel KC, Gaastra W, Stolk RP, Hoepelman AI. Cytokine secretion is impaired in women with diabetes mellitus. Eur J Clin Invest. 2000;30:995–1001. doi: 10.1046/j.1365-2362.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 8.Price CL, Al Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2010;14:1806–15. doi: 10.1111/j.1582-4934.2009.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nirmal J, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 10.Vardakas KZ, Siempos II, Falagas ME. Diabetes mellitus as a risk factor for nosocomial pneumonia and associated mortality. Diabet Med. 2007;24:1168–71. doi: 10.1111/j.1464-5491.2007.02234.x. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association (ADA) Standards of medical care in diabetes-2011. Diabetes Care. 2011;33:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cano M, Iglesias P, Pérez G, Díez JJ. Influenza A virus (H1N1) infection as a cause of severe diabetic ketoacidosis in type 1 diabetes. Endocrinol Nutr. 2010;57:37–8. doi: 10.1016/S1575-0922(10)70008-5. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) The Pink Book: Chapters Epidemiology and Prevention of Vaccine Preventable Diseases. 12th Edition. 2011. Apr, [Last Accessed on 2011 Oct 12]. Available from: http://www.cdc.gov/vaccines/pubs/pinkbook/pink-chapters.htm .
- 14.Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Type 2 diabetes and pneumonia outcomes: A population-based cohort study. Diabetes Care. 2007;30:2251–7. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 15.Barros MM, Cartagena SC, Bavestrello FL. Prevention of community-acquired pneumonia in adults. Rev Chilena Infectol. 2005;22:s67–74. [PubMed] [Google Scholar]
- 16.Rocha J, Baggio H, Cunha C, Niclewicz E, Leite S, Baptista M. Aspectos relevantes da interface entre diabetes mellitus e infecção. Arq Bras Endocrinol Metab. 2002;46:221–9. [Google Scholar]
- 17.Miller AC, Subranian RA, Safi F, Sinert R, Zehtabchi S, Elamin EM. Influenza A 2009 (H1N1) virus in admitted and critically ill patients. J Intensive Care Med. 2011;27:25–31. doi: 10.1177/0885066610393626. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 19.Aubert C, Vaudaux B, Bart PA. Immunization guidelines regarding patients with a chronic disease. Rev Med Suisse. 2010;6:798–803. [PubMed] [Google Scholar]
- 20.Giannattasio A, Squeglia V, Lo Vecchio A, Russo MT, Barbarino A, Carlomagno R, et al. Pneumococcal and influenza vaccination rates and their determinants in children with chronic medical conditions. Ital J Pediatr. 2010;36:28. doi: 10.1186/1824-7288-36-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Restrepo BI, Camerlin AJ, Rahbar MH, Wang W, Restrepo MA, Zarate I, et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull World Health Organ. 2011;89:352–9. doi: 10.2471/BLT.10.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harries AD, Lin Y, Satyanarayana S, Lönroth K, Li L, Wilson N, et al. The looming epidemic of diabetes-associated tuberculosis: learning lessons from the HIV-associated tuberculosis. Inter J Tuberc Lung Dis. 2011;15:1436–45. doi: 10.5588/ijtld.11.0503. [DOI] [PubMed] [Google Scholar]
- 23.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: Convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruslami R, Aarniutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 25.Geerlings SE. Urinary tract infections in patients with diabetes mellitus: Epidemiology, pathogenesis and treatment. Int J Antimicrob Agents. 2008;31S:S54–7. doi: 10.1016/j.ijantimicag.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig E. Urinary tract infections in diabetes mellitus. Orv Hetil. 2008;149:597–600. doi: 10.1556/OH.2008.28298. [DOI] [PubMed] [Google Scholar]
- 27.Hokkam EN. Assessment of risk factors in diabetic foot ulceration and their impact on the outcome of the disease. Prim Care Diab. 2009;3:219–24. doi: 10.1016/j.pcd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Stapleton A. Urinary tract infections in patients with diabetes. Am J Med. 2002;113:80S–4S. doi: 10.1016/s0002-9343(02)01062-8. [DOI] [PubMed] [Google Scholar]
- 29.Chen SL, Jackson SL, Boyko EJ. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009;182:S51–6. doi: 10.1016/j.juro.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 30.Papazafiropoulou A, Daniil I, Sotiropoulos A, Balampani E, Kokolaki A, Bousboulas S, et al. Prevalence of asymptomatic bacteriuria in type 2 diabetic subjects with and without microalbuminuria. BMC Res Notes. 2010;3:169. doi: 10.1186/1756-0500-3-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meiland R, Geerlings SE, Stolk RP, Netten PM, Schneeberger P, Hoepelman AI. Asymptomatic bacteriuria in women with diabetes mellitus: effect on renal function after 6 years of follow-up. Arch Intern Med. 2006;166:2222–7. doi: 10.1001/archinte.166.20.2222. [DOI] [PubMed] [Google Scholar]
- 32.Calvet HM, Yoshikawa TT. Infections in diabetes. Infect Dis Clin North Am. 2001;15:407–20. doi: 10.1016/s0891-5520(05)70153-7. [DOI] [PubMed] [Google Scholar]
- 33.Krishnasamy PV, Liby C. Emphysematous pyelonephritis caused by Candida tropicalis. Am J Med. 2010;123:e7–8. doi: 10.1016/j.amjmed.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Mokabberi R, Ravakhah K. Emphysematous urinary tract infections: diagnosis, treatment and survival (case review series) Am J Med Sci. 2007;333:111–6. doi: 10.1097/00000441-200702000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Mohsin N, Budruddin M, Lala S, Al-Taie S. Emphysematous pyelonephritis: a case report series of four patients with review of literature. Ren Fail. 2009;31:597–601. doi: 10.1080/08860220903003396. [DOI] [PubMed] [Google Scholar]
- 36.Pontin AR, Barnes RD. Current management of emphysematous pyelonephritis. Nat Rev Urol. 2009;6:272–9. doi: 10.1038/nrurol.2009.51. [DOI] [PubMed] [Google Scholar]
- 37.Yao J, Gutierrez OM, Reiser J. Emphysematous pyelonephritis. Kidney Int. 2007;71:462–5. doi: 10.1038/sj.ki.5002001. [DOI] [PubMed] [Google Scholar]
- 38.Falagas ME, Alexiou VG, Giannopoulou KP, Siempos II. Risk factors for mortality in patients with emphysematous pyelonephritis: a meta-analysis. J Urol. 2007;178:880–5. doi: 10.1016/j.juro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Solak Y, Turkmen K, Atalay H, Turk S. Culture-negative bilateral emphysematous pyelonephritis presented as acute renal failure and managed medically only. South Med J. 2010;103:154–5. doi: 10.1097/smj.0b013e3181bfd553. [DOI] [PubMed] [Google Scholar]
- 40.Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: A review of 135 cases. BJU Int. 2007;100:17–20. doi: 10.1111/j.1464-410X.2007.06930.x. [DOI] [PubMed] [Google Scholar]
- 41.Etienne M, Caron F. Management of fungal urinary tract infections. Presse Med. 2007;36(12 Pt 3):1899–906. doi: 10.1016/j.lpm.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine. 2007;86:47–53. doi: 10.1097/MD.0b013e3180307c3a. [DOI] [PubMed] [Google Scholar]
- 43.Kelesidis T, Osman S, Tsiodras S. Emphysematous cystitis in the absence of known risk factors: An unusual clinical entity. South Med J. 2009;102:942–6. doi: 10.1097/SMJ.0b013e3181adf18c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baena JF, Romeu JP, Llopis JA, Tamayo AL, Encinas JJ. Emphysematous cystitis.Case report and review of literature. Actas Urol Esp. 2008;32:948–50. doi: 10.1016/s0210-4806(08)73967-6. [DOI] [PubMed] [Google Scholar]
- 45.Fullá J, Storme O, Fica A, Varas MA, Flores J, Marchant F, et al. Renal and perinephric abscesses: A series of 44 cases. Rev Chilena Infectol. 2009;26:445–51. [PubMed] [Google Scholar]
- 46.Coelho RF, Schneider-Monteiro ED, Mesquita JL, Mazzucchi E, Marmo Lucon A, Srougi M. Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg. 2007;31:431–6. doi: 10.1007/s00268-006-0162-x. [DOI] [PubMed] [Google Scholar]
- 47.Maldonado-Alcaraz E, Ixquiac-Pineda G, López-Sámano V, Serrano-Brambila E. Perinephric abscess: Associated factors and mortality. Arch Esp Urol. 2008;61:7–12. doi: 10.4321/s0004-06142008000100002. [DOI] [PubMed] [Google Scholar]
- 48.Tsukagoshi D, Dinkovski B, Dasan S, Jethwa J. Perinephric abscess secondary to a staghorn calculus presenting as a subcutaneous abscess. CJEM. 2006;8:285–8. doi: 10.1017/s1481803500013889. [DOI] [PubMed] [Google Scholar]
- 49.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–81. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 50.Schimke K, Chubb SA, Davis WA, Philipst P, Davis TM. Antiplatelet therapy, Helicobacter pylori infection and complicated peptic ulcer disease in diabetes: The fremantle diabetes study. Diabet Med. 2009;26:70–5. doi: 10.1111/j.1464-5491.2008.02637.x. [DOI] [PubMed] [Google Scholar]
- 51.Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ. Helicobacter pylori infection and endocrine disorders: is there a link.? World J Gastroenterol. 2009;15:2701–7. doi: 10.3748/wjg.15.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14:144–50. doi: 10.1111/j.1523-5378.2009.00705.x. [DOI] [PubMed] [Google Scholar]
- 53.Candelli M, Rigante D, Marietti G, Nista EC, Crea F, Bartolozzi F, et al. Helicobacter pylori, gastrointestinal symptoms, and metabolic control in young type 1 diabetes mellitus patients. Pediatrics. 2003;111(4 Pt 1):800–3. doi: 10.1542/peds.111.4.800. [DOI] [PubMed] [Google Scholar]
- 54.Ojetti V, Pellicano R, Fagoonee S, Migneco A, Berrutti M, Gasbarrini A. Helicobacter pylori infection and diabetes. Minerva Med. 2010;101:115–9. [PubMed] [Google Scholar]
- 55.Menezes EA, Augusto KL, Freire CC, Cunha FA, Montenegro RM, Montenegro-Júnior RM. Frequency and enzymatic activity of Candida spp.oral cavity of diabetic patients of the service of endocrinology of a hospital of Fortaleza-CE. J Bras Patol Med Lab. 2007;43:241–4. [Google Scholar]
- 56.Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. Seroprevalence of hepatitis C in type 2 diabetes: Evidence for a positive association. Virol J. 2010;7:304. doi: 10.1186/1743-422X-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elhawary EI, Mahmoud GF, El-Daly MA, Mekky FA, Esmat GG, Abdel-Hamid M. Association of UVV with diabetes mellitus: an Egyptian case-control study. Virol J. 2011;8:367. doi: 10.1186/1743-422X-8-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Negro F, Alaei M. Hepatitis C virus and type2 diabetes. World J Gastroenterol. 2009;15:1357–47. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M. Risk factors for diabetes mellitus and insulin resistance in chronic hepatitis C. J Hepatology. 2001;35:279–83. doi: 10.1016/s0168-8278(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 60.Gutiérrez-Grobe Y, Ponciano-Rodriguez G, Méndez-Sanchez N. Viral hepatitis infection and insulin resistance: A review of pathophysiological mechanisms. Salud Pub Mex. 2010;53:S46–51. [PubMed] [Google Scholar]
- 61.Demir M, Serin E, Göktürk S, Ozturk NA, Kulaksizoglu S, Ylmaz U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eu J Gastroenterol Hepatol. 2008;20:668–73. doi: 10.1097/MEG.0b013e3282f55e1e. [DOI] [PubMed] [Google Scholar]
- 62.Huang ZS, Huang TS, Wu TH, Chen MF, Hsu CS, Kai JH. Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: A ten-year observation. J Gastroenterol Hepatol. 2010;25:1420–5. doi: 10.1111/j.1440-1746.2010.06268.x. [DOI] [PubMed] [Google Scholar]
- 63.Lao TT, Chan BC, Leung WC, Ho LF, Tse KY. Maternal hepatitis B infection and gestational diabetes mellitus. J Hepatol. 2007;47:46–50. doi: 10.1016/j.jhep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Thomson ND, Perz JF. Eliminating the blood: ongoing outbreaks of hepatitis B virus infection and the need for innovative glucose monitoring technologies. J Diabetes Sci Technol. 2009;3:283–8. doi: 10.1177/193229680900300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hober D. Enteroviral pathogenesis of type 1 diabetes. Discov Med. 2010;10:151–60. [PubMed] [Google Scholar]
- 66.Stene LC, Oikarinen S, Hyöty H, Barriga KJ, Norris JM, Klingensmith G, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes.The Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–80. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, Weigelt JA. Skin and soft tissue infections in hospitalized patients with diabetes: Culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia. 2010;53:914–23. doi: 10.1007/s00125-010-1672-5. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed I, Goldstein B. Diabetes mellitus. Clin Dermatol. 2006;24:237–46. doi: 10.1016/j.clindermatol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Fincke BG, Miller DR, Christiansen CL, Turpin RS. Variation in antibiotic treatment for diabetic patients with serious foot infections: A retrospective observational study. BMC Health Serv Res. 2010;10:193. doi: 10.1186/1472-6963-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicolau DP, Stein GE. Therapeutic options for diabetic foot infections: a review with an emphasis on tissue penetration characteristics. J Am Podiatr Med Assoc. 2010;100:52–63. doi: 10.7547/1000052. [DOI] [PubMed] [Google Scholar]
- 71.Powlson AS, Coll AP. The treatment of diabetic foot infections. J Antimicrob Chemother. 2010;65(Suppl 3):iii3–9. doi: 10.1093/jac/dkq299. [DOI] [PubMed] [Google Scholar]
- 72.Joseph WS, Lipsky BA. Medical therapy of diabetic foot infections. J Vasc Surg. 2010;52(Suppl 3):67S–71. doi: 10.1016/j.jvs.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Zhang WJ, Cai XY, Yang C, Zhou LN, Cai M, Lu XF, et al. Cervical necrotizing fasciitis due to methicillin-resistant Staphylococcus aureus: A case report. Int J Oral Maxillofac Surg. 2010;39:830–4. doi: 10.1016/j.ijom.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 74.Oguz H, Demirci M, Arslan N, Safak MA, Paksoy G. Necrotizing fasciitis of the head and neck: Report of two cases and literature review. Ear Nose Throat J. 2010;89:E7–10. [PubMed] [Google Scholar]
- 75.Shaikh N, Ummunissa F, Hanssen Y, Al Makki H, Shokr HM. Hospital epidemiology of emergent cervical necrotizing fasciitis. J Emerg Trauma Shock. 2010;3:123–5. doi: 10.4103/0974-2700.62108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med J. 2010;49:1051–7. doi: 10.2169/internalmedicine.49.2964. [DOI] [PubMed] [Google Scholar]
- 77.Tran HA, Hart AM. Fournier's gangrene. Intern Med J. 2006;36:200–1. doi: 10.1111/j.1445-5994.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- 78.Montoya R Chinchilla, Izquierdo E Morejon, Nicolae B Pietricicâ, Pellicer E Franco, Aguayo JL Albasini, Miñana B López. Fournier's gangrene. Descriptive analysis of 20 cases and literature review. Actas Urol Esp. 2009;33:873–80. doi: 10.1016/s0210-4806(09)72875-x. [DOI] [PubMed] [Google Scholar]
- 79.Carfrae MJ, Kesser BW. Malignant otitis externa. Otolaryngol Clin North Am. 2008;41:537–49. doi: 10.1016/j.otc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Artal R, Agreda B, Serrano E, Alfonso JI, Vallés H. Rhinocerebral mucormycosis: Report on eight cases. Acta Otorrinolaringol Esp. 2010;61:301–5. doi: 10.1016/j.otorri.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Giuliani A, Mettimano M, Viviani D, Scagliusi A, Bruno A, Russo A, et al. An uncommon case of systemic mucormycosis associated with spinal cord infarction in a recently diagnosed diabetic. Int J Immunopathol Pharmacol. 2010;23:355–8. doi: 10.1177/039463201002300135. [DOI] [PubMed] [Google Scholar]
- 82.Severo CB, Guazzelli LS, Severo LC. Zigomicose. J Bras Pneumol. 2010;36:134–41. doi: 10.1590/s1806-37132010000100018. [DOI] [PubMed] [Google Scholar]
- 83.Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–24. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alves C, Andion J, Brandão M, Menezes R. Pathogenic aspects of the periodontal disease associated to diabetes mellitus. Arq Bras Endocrinol Metab. 2007;51:1050–7. doi: 10.1590/s0004-27302007000700005. [DOI] [PubMed] [Google Scholar]
- 85.Nagasawa T, Noda M, Katagiri S, Takaichi M, Takahashi Y, Wara-Aswapati N, et al. Relationship between periodontitis and diabetes - importance of a clinical study to prove the vicious cycle. Intern Med. 2010;49:881–5. doi: 10.2169/internalmedicine.49.3351. [DOI] [PubMed] [Google Scholar]
- 86.Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev. 2010;5:CD004714. doi: 10.1002/14651858.CD004714.pub2. [DOI] [PubMed] [Google Scholar]
- 87.Kalra S, Kalra B, Agrawal N, Unnikrishnan AG. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3:2. doi: 10.1186/1758-5996-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alves C, Oliveira AC, Brites C. Lipodystrophic syndrome in children and adolescents infected with the human immunodeficiency virus. Braz J Infect Dis. 2008;12:342–8. doi: 10.1590/s1413-86702008000400018. [DOI] [PubMed] [Google Scholar]
- 89.Takarabe D, Rokukawa Y, Takahashi Y, Goto A, Talkaichi M, Okamoto M, et al. Autoimmune diabetes in HIV-infected patients on highly active antiretroviral therapy. J Clin Endocrinol Metab. 2010;95:4056–60. doi: 10.1210/jc.2010-0055. [DOI] [PubMed] [Google Scholar]
- 90.Van der Werf N, Kroese FG, Rozing J, Hillebrands JL. Viral infections as potential trigger of type 1 diabetes. Diabetes Metab Res Rev. 2007;23:169–83. doi: 10.1002/dmrr.695. [DOI] [PubMed] [Google Scholar]


