Abstract
Enhanced understanding about the way human immunodeficiency virus (HIV) infects and causes infection in humans has led to invention and use of newer more effective antiretroviral drugs. As treatment for HIV is long term, side effects of the antiretrovirals become an important area of research focus. Antiretrovirals can cause severe metabolic abnormalities, collectively known as HIV lipodystrophy syndrome. If untreated, these metabolic abnormalities have the potential to increase stroke and cardiac ischemia. Management includes choice of nonoffending drugs, switch over to less toxic drugs, hypolipidemics, oral antidiabetics including thiazolidinediones, metformin and growth hormone analogs and finally facial surgeries. Updated knowledge about HIV lipodystrophy, and the hormone-related drugs used to treat it, is essential for physicians and endocrinologists to be able to diagnose the patients and effectively treat them.
Keywords: Antiretroviral, human immunodeficiency virus lipodystrophy, protease inhibitors, tesamorelin
INTRODUCTION
Advances in pathogenesis of HIV infection and its relentless progression have led to development and introduction of highly efficacious antiretroviral drugs and their systematic use in form of highly active antiretroviral regimens (HAART). HAART has reduced the rate of progression of HIV-related morbidities and improved patient survival.[1–3] Since antiretrovirals have to be taken long term, knowledge about their adverse effects and extend of impairment or improvement in quality of life of patients has become an area of intense research.
HIV-infected patients receiving long-term antiretroviral therapy often present with a number of metabolic complications, including altered glucose metabolism, body lipid abnormalities, body fat redistribution and mitochondrial abnormalities as well as the complications of these disorders; most prominent of these is the HIV lipodystrophy syndrome.[4] The exact etiology of these abnormalities remains largely unknown. A detailed knowledge about all these metabolic complications and drugs causing them is of immense importance to a treating general physician or endocrinologist. This would influence the choice of drug, timing of introduction, use of acceptable combination in terms of adverse effects, advising patients of possible switching therapy, and usage appropriate preventive or corrective measures including drugs.
HIV lipodystrophy is a disorder of fat metabolism which has diverse clinical and biochemical manifestations including lipoatrophy, lipohypertrophy and redistribution of fat.[5] Hypertrophic changes consist of excess fat deposition, most commonly around the abdominal viscera, but also around the neck, in the supraclavicular region, around the scapulae. Fat deposition may also take the form of bilateral symmetrical lipomatosis or breast enlargement. Atrophic changes consist of subcutaneous fat atrophy in the legs, buttocks, arms, and nasolabial and malar fat pads. Metabolic abnormalities include insulin resistance, raised triglycerides, and increased total cholesterol. Lipodystrophy-induced cosmetically disfiguring changes may have significant impact on quality of life.[6] The endocrinologist plays an important role in the management of HIV lipodystrophy in association with the primary care physician or HIV specialist.
Definition and diagnosis
What makes the matter grave is the lack of clear and objective definition of HIV lipodystrophy. This often leads to erroneous positive diagnosis in healthy patients, as there is high variability in body composition among normal subjects, and as metabolic abnormalities common to lipodystrophy are also common in the general population. On the other hand, patients with lipodystrophy may be missed because they did not fulfil the subjective diagnostic criteria of their particular physician.
There is an obvious need for objective diagnostic criteria to diagnose lipodystrophy. Such criteria would assist industry, regulators and researchers in the standardization of recruitment of patients to lipodystrophy studies and the reporting of incidence of lipodystrophy, in the assessment of drugs, drug combinations, drug classes as causative factor and of different patient populations, and in the identification of risk factors. Also, such criteria would assist less experienced clinicians/endocrinologist in making a correct diagnosis.[7]
More than five working case definitions or classification systems have been proposed [Table 1]. They have limited utility and applicability as they were generated from studies performed in relatively few sites in selected patients.[8–12] Consensus at the First International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV was that the presence of at least one patient-reported, examination-confirmed physical change may be useful. Preliminary data suggest that the HIV lipodystrophy case definition (LDCD) may be useful.
Table 1.
Proposed definition of human immunodeficiency virus lipodystrophy[7]

Without a precise definition, the discussion about the prevalence of lipodystrophy becomes complicated. Nonetheless most studies report a prevalence rate of 40-50% in patients on long-term treatment for at least one physical manifestation, which slowly increases over the time.[4]
Body fat deposition and fat atrophy are diagnosed subjectively by physician examination for skin-fold thickness measurements, along with a waist-to-hip ratio measurement. This diagnosis is aided by dual-energy X-ray absorptiometry, CT and bioelectrical impendence analysis. But none of these can reliably diagnose lipodystrophy due to many factors, including the lack of baseline measures, significant natural variability in fat distribution, and the significant prevalence of central adiposity, dyslipidemia, and glucose intolerance in the general population. Moreover, there is significant variability in interpretation of “objective” tests.
PREDISPOSING FACTORS AND PATHOGENESIS
The pathogenesis of HIV lipodystrophy is largely unexplained, but understanding is rapidly improving with more and better planned, conducted and reported studies. Risk factors identified in cohort studies, and several in vitro studies of adipocytes, skeletal muscle and hepatocytes, suggest a complex mechanism which interplays in the pathogenesis of HIV lipodystrophy. Factors which are present consistently in lipodystrophy patients include use of antiretroviral therapy, HIV itself, host, genetic and environmental factors.[5,7]
Available evidence suggests that the nucleoside analog reverse transcriptase inhibitors (nRTIs) stavudine, didanosine and zidovudine may cause mitochondrial toxicity by inhibiting mitochondrial DNA polymerase-γ in fat and other tissues and thus interfering with respiratory chain complexes. The result is impaired fatty acid oxidation and intracellular accumulation of triglycerides and lactate, which can enter the systemic circulation [Figure 1]. The occurrence of fat accumulation or atrophy may depend on differences in nRTI tissue selectivity or cell function.[5]
Figure 1.
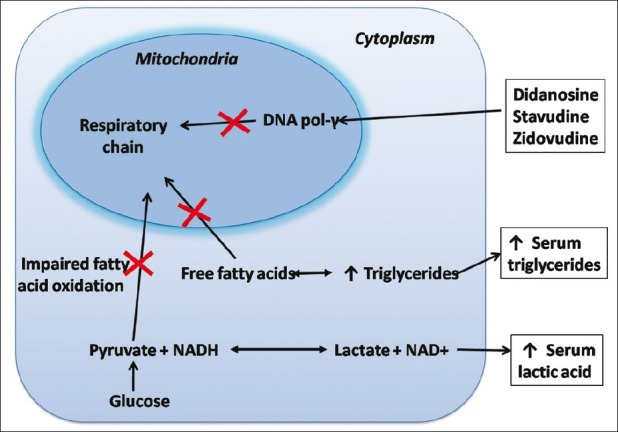
Intracellular pathways associated with mitochondrial toxicity due to nRTI which inhibit DNA polymerase. DNA polymerase is necessary for replication of mitochondrial DNA and normal function of respiratory chain
PIs inhibit maturation of sterol response element-binding proteins (SREBPs) which affect intracellular fatty acid and glucose metabolism and adipocyte differentiation (Mallon et al, J Infect Dis, 2005). The PIs also downregulate peroxisome proliferator-activated receptor-γ (PPAR-γ), an important nuclear transcription factor that is affected by SREBPs, and is necessary for adipocyte differentiation and function and fatty acid metabolism [Figure 2].[5] PIs have been shown to increase insulin resistance and reduce insulin secretion, by interfering with GLUT-4-mediated glucose transport.[13] However, it should be noted that individual PIs are often given in combination with low doses of ritonavir, a PI that inhibits cytochrome P450 3A4, which is the primary metabolic pathway of PIs. Coadministration with ritonavir increases PI serum concentrations and improves their pharmacokinetic profile. Although not active against HIV at these “boosting” doses, ritonavir may also worsen dyslipidemia.[14] Female patients sometimes describe an increase in breast size on PI-based therapy. This has been noted in male patients as well, which may occur without other findings of lipodystrophy. Mammary ultrasound reveals heterogenous echo-poor areas of nodularity as opposed to increased adipose tissue. Biopsy reveals proliferation of glandular tissue, which is consistent with results of ultrasound or mammography.
Figure 2.
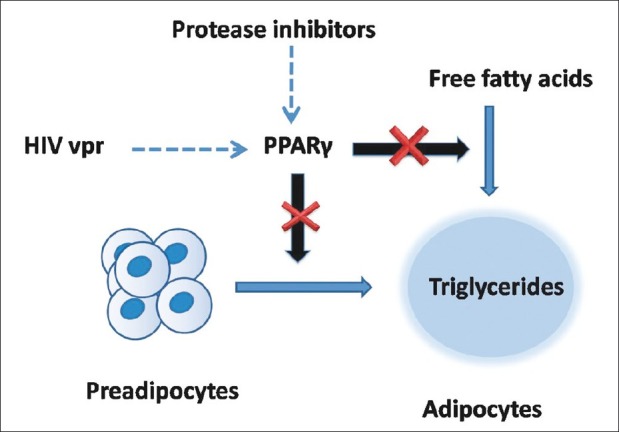
Human immunodeficiency virus protease inhibitors and HIV viral proteins accessory protein inhibit intracellular peroxisome proliferator-activated receptor-γ, which is necessary for differentiation of preadipocytes to mature adipocytes
Combination of these drugs increases the prevalence of metabolical abnormalities. Lipodystrophy in patients taking PI-based combinations was found to be 4% in patients who were also taking zidovudine and lamivudine, 52% in patients taking stavudine and lamivudine, and 89% in patients taking stavudine and didanosine.[15] Fabre et al. found that the fatty changes of lipodystrophy are more associated with stavudine and/or lamivudine use than PI use, although the latter were strongly associated with metabolical complications.
Role of HIV vpr is also being unmasked as it inhibits PPAR-γ and PPAR-γ-deleted mice exhibit lipoatrophy, hepatic steatosis, and increased triglyceride levels. In addition, the duration of HIV infection and its treatment as well as nadir,
CD4+ cell count has been implicated in lipodystrophy. Lipodystrophy is also observed in acute HIV infection, lending support to a direct viral role as well.[5]
Lipodystrophy is more common in older patients; fat accumulation is more common in women and lipoatrophy in men. A genetic component is indicated by a recent analysis in AIDS Clinical Trials Group (ACTG) study 5005s, suggesting either predisposition or protection associated with mitochondrial DNA polymorphisms. (Hulgan et al, J Infect Dis, 2008).[5]
Growth hormone (GH) levels are significantly lower in lipodystrophic HIV-infected patients with visceral adiposity, as is seen in GH deficiency. Paradoxically, insulin-like growth factor type 1 levels were not found to be increased, as would be expected with true GH deficiency.[7]
The development of cervicodorsal and supraclavicular fat pads led early investigators to speculate that hypercortisolism was responsible for the development of HIV lipodystrophy. Although there was evidence of altered steroid synthesis in HIV, results of both dexamethasone suppression tests and 24-hour urine collections for free cortisol had ruled out a simple hypercortisol state. Also, levels of testosterone, progesterone, estradiol, thyroid hormones, prolactin, β-hCG, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) have not been found to be abnormal or to differ between lipodystrophic and normal individuals.
PATHOLOGICAL CHANGES
The Fat Redistribution and Metabolic Change in HIV (FRAM) study (Bacchetti et al, JAIDS, 2005) showed that HIV-infected men with lipoatrophy had less peripheral and central subcutaneous adipose tissue (SAT) and less visceral adipose tissue (VAT), contrary to the early impression that peripheral lipoatrophy was frequently accompanied by central fat accumulation.[5]
In a study in the Women's Interagency Health Study (WIHS) population, dual-energy X-ray absorptiometry (DEXA) scans showed that overall, HIV patients receiving either type of antiretroviral therapy had greater decreases in leg fat than in trunk fat relative to non–HIV-infected women or those not receiving antiretroviral therapy. Trunk fat appeared to be retained in women receiving PI-containing antiretroviral therapy but significantly reduced in those not receiving a PI (P<0.05).[5]
There is evidence that women experience more fat deposition and men experience more fat atrophy, whereas the metabolic perturbations are much more common in men.[15]
Metabolic abnormalities
Metabolic abnormalities may occur separate from or in conjunction with the physical manifestations explained above.
Numerous studies have shown that fat loss in the extremities is accompanied by insulin resistance. In the FRAM study (Grunfeld et al, JAIDS, 2007), increased values of VAT and upper trunk SAT were independently associated with insulin resistance as assessed by the homeostasis model assessment of insulin resistance (HOMAIR).
With insulin resistance and overt type 2 diabetes mellitus may occur and diabetic ketoacidosis has been reported.[16,17] Hyperinsulinemia may increase soluble leptin receptor levels, leading to increased food intake and reduced energy expenditure.
Insulin resistance is part of the metabolic syndrome, which leads to enhanced risk of cardiovascular disease complications (e.g., ischemic heart disease, stroke, peripheral vascular disorders). It is very common to find other features of the metabolic syndrome including central obesity, hypertension and dyslipidemia present concurrently in HIV patients with lipodystrophy.[5] Hypercholesterolemia tends to develop early, followed by later development of hypertriglyceridemia. Hypercholesterolemia is often marked by an atherogenic profile, with elevated very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and total level and reduced high-density lipoprotein (HDL). The type of hyperlipidemia present is reported variably by different authors. The association between hypercholesterolemia and the development of coronary artery disease in the general population is unequivocal. Also, hypertriglyceridemia is associated to a lesser extent with increased atherosclerosis.[18,19] PI-induced hypertriglyceridemia may lead to pancreatitis,[20] which may also occur as a direct toxic effect of some PIs. There is some evidence that blood pressure may be elevated in patients on PI therapy compared to untreated HIV-positive controls.
Osteopenia, osteoporosis, and avascular necrosis have been reported in patients with HIV infection. Various risk factors may contribute to these abnormalities, including prior steroid treatment for avascular necrosis, cigarette smoking, and hormone therapy for osteoporosis.
Management
As discussed above HIV lipodystrophy significantly hampers quality of life, and if untreated, can lead to severe sequelae. Therefore, timely diagnosis and corrective measures forms the cornerstone of treatment. Spontaneous resolution of lipodystrophic changes is uncommon, hence mandating active management.
Few options that are available to a clinician include exercise and therapeutic lifestyle changes, using lipid-friendly drugs, switching to less dystrophy-prone drugs, corrective drugs and cosmetic surgery. Treatment of the fat changes occurring in HIV lipodystrophy is marginally and inconsistently effective, at best. Lifestyle changes, in general, have not been found to be effective at preventing or treating somatic fat changes.[5] Central adiposity may subside with increased exercise, but the results are inconsistent and this approach may worsen peripheral fat thinning. The effect of dietary alterations on lipohypertrophy has not been well studied, but the effect would likely be marginal.[21]
Initial regimen
A successful strategy in antiretroviral therapy–naive patients is selection of initial regimens less likely to be associated with lipoatrophy.[5] For example, two studies have shown benefit in this regard with use of initial regimens including tenofovir/emtricitabine versus zidovudine/lamivudine; the results suggested that tenofovir causes less lipoatrophy than zidovudine. In one study (Gallant et al, JAMA, 2004) use of tenofovir/emtricitabine statistically significantly reduced the rate of clinical lipodystrophy at 3 years to 3% versus 19% with zidovudine/lamivudine (P<0.001). In another study (Gallant et al, N Engl J Med, 2006) weight gain was similar with tenofovir/emtricitabine and zidovudine/ lamivudine (2.1 vs 1.1 kg, respectively; P=0.14), and limb fat was statistically significantly greater.
Drug switch
In patients who are already on an antiretroviral regimen and develop lipodystrophy are the candidates for switch to “lipoatrophy friendly” drugs. The benefits of switching antiretroviral therapy were shown in the Mitochondrial Toxicity (MITOX) study (Martin et al, AIDS, 2004). In that study, 111 patients who developed lipodystrophy while receiving stavudine or zidovudine underwent randomization to continue treatment or switch to abacavir. At week 24, control patients were permitted to switch to abacavir. At week 104, results of DEXA measurements showed that the abacavir group gained 1.26 kg of limb fat versus 0.49 kg in control subjects (P=0.008). No statistically significant differences were found in change in VAT, nor was there clinical evidence of lipoatrophy. In the Randomized Abacavir Versus Viread Evaluation (RAVE) study (Moyle et al, AIDS, 2006), thymidine nRTIs were replaced with tenofovir or abacavir in 105 patients with lipoatrophy. At the end there were statistically significant within group reductions in lipoatrophy, with limb fat gains of 0.33 kg in patients taking tenofovir (P=0.01) and 0.48 kg in those taking abacavir (P=0.0001). There were no changes in trunk fat or VAT measures. Tenofovir treatment was associated with modest reductions in levels of total cholesterol, LDL cholesterol, and triglycerides, whereas there were no lipid changes with abacavir treatment.
In brief, findings in few other studies indicate that substituting a PI with a non-nucleoside reverse transcriptase inhibitor (NNRTI) appears safe, decreases insulin resistance, usually reduces triglyceride levels, has inconsistent effects on total cholesterol and HDL cholesterol levels, and has no consistent effects on fat gain or loss. Other findings indicated that abacavir substitution for stavudine results in increased SAT but no change in VAT.
Androgen
Testosterone replacement to physiological levels reduces VAT, total fat, and abdominal fat and improves insulin sensitivity and lipid profile in older, non–HIV-infected men with upper body obesity and low testosterone levels.[5] In a recent study, 88 HIV-infected men with central obesity (waist circumference>100 cm) and low testosterone levels (<400 ng/dL) underwent randomization to testosterone as a transdermal gel at a dose of 10 g daily or placebo for 24 weeks (Bhasin et al, J Clin Endocrinol Metab, 2007). The testosterone group had statistically significant reductions in abdominal fat, abdominal SAT, trunk fat and limb fat; the latter finding is of potential concern in a population predisposed to lipoatrophy. No statistically significant difference in change in VAT was observed, and no statistically significant differences were observed in changes in lipid levels, fasting blood glucose levels, insulin levels, or insulin resistance.
Growth hormone
A substantial proportion of HIV patients with central obesity (approximately 30%-40%) have impaired GH biology, including reduced GH mass secretion, reduced response to GH-releasing hormone (GHRH) and free fatty acids, and increased somatostatin tone, which suppresses GH. A number of recent studies have assessed GH treatment in HIV patients with fat accumulation. Like testosterone, GH has fat-oxidizing and lipolytic properties.[5]
A number of recent studies have assessed GH treatment in HIV patients with fat accumulation. In one study, 325 HIV patients with increased waist:hip ratios and increased VAT measurements received supraphysiological doses of recombinanthuman GH (rhGH). There were statistically significant reductions in VAT and abdominal SAT, and a statistically significant decrease in limb fat. However, there was also increase in fasting blood glucose levels and the insulin area under the concentration curve (indicating increased insulin resistance).
A second study evaluated the effects physiological doses of rhGH in 56 HIV patients with lipodystrophy and low GH levels after a GHRH/arginine stimulation test (Lo et al, CROI, 2008). Patients receiving rhGH had large and statistically significant reductions in VAT, smaller but statistically significant reductions in trunk fat, no loss of extremity fat, and statistically significant reductions in diastolic blood pressure. Unfortunately, there was no overall reduction in carotid intima-media thickness, and rhGH treatment was again associated with evidence of insulin resistance.
In a potentially more promising approach to GH modulation, another study evaluated treatment with 2 mg daily of an investigational GHRH agent, tesamorelin, versus placebo in 412 HIV patients with high waist circumferences (Falutz et al, N Engl J Med, 2007). GHRH treatment was associated with a large and statistically significant reduction in VAT with only a marginal reduction in abdominal SAT, and a statistically significant reduction in triglyceride levels and improvement in adiponectin; there was no apparent adverse effect on glucose metabolism.
In vitro, tesamorelin binds and stimulates human GRF receptors with similar potency as the endogenous GRF which acts on the pituitary somatotroph cells to stimulate the synthesis and pulsatile release of endogenous GH, which is both anabolic and lipolytic. GH exerts its effects by interacting with specific receptors on a variety of target cells, including chondrocytes, osteoblasts, myocytes, hepatocytes, and adipocytes, resulting in a host of pharmacodynamical effects. Some, but not all these effects, are primarily mediated by IGF-1 produced in the liver and in peripheral tissues.
Tesamorelin has been approved by US-FDA on Nov. 10, 2010.[22] A recently carried out study showed that tesamorelin reduces visceral fat by approximately 18% and improves body image distress in HIV-infected patients with central fat accumulation. These changes are achieved without significant side effects or perturbation of glucose (Falutz et al, JAIDS 2010).
Although the change in limb fat was statistically different from that in the placebo group, the absolute change (0.02 kg) was quite small and unlikely to be of clinical importance. With rhGH, 24 weeks after discontinuation of treatment, improvements in VAT dissipated, indicating that long-term suppressive therapy will be necessary to sustain these improvements (Falutz et al, CROI, 2008).
Although the GH and GHRH therapies show some promise, there are limitations to their use. They are parenteral therapies and either expensive (rhGH).
The long-term safety of such treatments also has not been established, and it is uncertain whether there is increased risk of cancer if IGF-1 levels are excessively elevated.[5]
Oral antidiabetics
As PPAR-γ is one of the major target in pathogenesis of lipodystrophy, drugs affecting it i.e., thiazolidinediones have been tried. Results of seven initial studies of thiazolidinediones (Walli et al, Res Exp Med (Berl), 2000; Sutinen et al, Antivir Ther, 2003; Hadigan et al, Ann Intern Med, 2004; van Wijk et al, Ann Intern Med, 2005; Gavrila et al, Clin Infect Dis, 2005; Feldt et al, Infection, 2006; Mulligan et al, AIDS, 2007) have shown no change in VAT. While others have shown partial result (Gelato et al, JAIDS, 2002), increased abdominal SAT and VAT (van Wijk et al, Ann Intern Med, 2005), and increased limb fat (Hadigan et al, Ann Intern Med, 2004; Mulligan et al, AIDS, 2007). Promising results have been observed recently with pioglitazone in a study (Slama etal, Antivir Ther, 2008).
Metformin improves visceral fat accumulation, fasting lipid profile and endothelial function, reduced body weight, improved waist:hip ratio.[23] While other studies do not support this claim, nevertheless, metformin particularly in combination with exercise training, may be useful in HIV-infected patients with significant lipohypertrophy with minimal lipoatrophy.
Surgery
Given the negative psychological effects and stigmatization of facial lipoatrophy, facial fillers, generally administered by a plastic surgeon or dermatologist, have gained popularity. Both permanent and absorbable compounds have been successful in improving lipoatrophy grading, improving quality of life, and decreasing anxiety and depression symptoms.[24–26] For facial dystrophy, FDA approved use of Sculptra, an injectable form of poly-L-lactic acid, a biodegradable, biocompatible synthetic polymer from the α-hydroxy-acid family in 2004[27] and Radiesse, a sterile, semi-solid cohesive implant consisting of synthetic calcium hydroxylapatite suspended in a gel carrier in 2006.[28]
SUMMARY
The causes of the manifestations of HIV lipodystrophy remain uncertain, although significant progress has been made in this area of research in the past several years. Treatments remain imperfect; prevention through careful choice of antiretroviral therapy for treatment-naive individuals or a switch to less-offending agents for those with suppressed viral loads on first-line therapy seems promising. Moreover, newer antiretrovirals may have fewer lipodystrophic adverse effects. The long-term impact of the metabolic complications of antiretrovirals are unclear but are concerning. With approval of GHRH agent, tesamorelin, hopes of successful treatment have increased, but still there is room for more research into the exact mechanism of lipodystrophy and thus revealing more targets for drugs.
Footnotes
Source of Support: Nil,
Conflict of Interest: Nil.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Katzenstein DA, Hughes MD, Gundacker H, Schooley RT, Haubrich RH, et al. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. N Engl J Med. 1996;335:1081–90. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 3.Cameron DW, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, et al. Randomised, placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351:543–9. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 4.Sweet DE. Metabolic complications of antiretroviral therapy. Top HIV Med. 2005;13:70–4. [PubMed] [Google Scholar]
- 5.Sattler FR. Pathogenesis and treatment of lipodystrophy: What clinicians need to know. Top HIV Med. 2008;16:127–33. [PubMed] [Google Scholar]
- 6.Kravcik S. HIV Lipodystrophy: A Review. HIV Clin Trials. 2000;1:37–50. doi: 10.1310/3hhb-59up-93mm-aaxy. [DOI] [PubMed] [Google Scholar]
- 7.Carr A. HIV lipodystrophy: Risk factors, pathogenesis, diagnosis and management. AIDS. 2003;17(Suppl 1):S141–8. [PubMed] [Google Scholar]
- 8.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction and natural course of HIV protease inhibitor-associated lipodystrophy, hyperlipidaemia and diabetes mellitus: A cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, Jr, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–98. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley L, Smit E, Riddler S, Li R, Chmiel J, Palella F, et al. Program and Abstracts of the 8th Conference on Retroviruses and Opportunistic Infections. Chicago, IL: 2001. Feb 4-8, Prevalence of lipodystrophy and metabolic abnormalities in the Multicenter AIDS Cohort Study (MACS) Abstract 538. [Google Scholar]
- 11.Saint-Marc T, Partisani M, Poizot-Martin I, Rouviere O, Bruno F, Avellaneda R, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: Preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 12.Belloso W, Ivalo S, Perman M, Tessler J, Galich A, Gonzalez Toledo E, et al. Associated features of lipodystrophy may change significantly with definition criteria. 1st IAS Conference on HIV Pathogenesis and Treatment. Int Conf AIDS. 2000;14:13. [Google Scholar]
- 13.Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3:2. doi: 10.1186/1758-5996-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–5. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 15.Polo R, Verdejo M, Gonzalez-Munoz M, et al. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: 1999. Lipodystrophy related to NRT inhibitors in HAART therapy [abstract] Abstract 1302. [Google Scholar]
- 16.Muurahainen N, Santos G, Kleintop M, Kleintop M, Pettit R, Balser J, et al. Seventh Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2000. Gender differences in HIV associated adipose redistribution syndrome (HARS): An update. Abstract 26. [Google Scholar]
- 17.Visnegarwala F, Krause KL, Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00016. [DOI] [PubMed] [Google Scholar]
- 18.Austin MA, McKnight B, Edwards KL, Bradley CM, McNeely MJ, Psaty BM, et al. Cardiovascular disease mortality in familial forms of hypertriglyceridemia: A 20-year prospective study. Circulation. 2000;101:2777–82. doi: 10.1161/01.cir.101.24.2777. [DOI] [PubMed] [Google Scholar]
- 19.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 20.Mirete G, Masia M, Gutierrez F, Mora A, Escolano C, Maestre A. Acute pancreatitis as a complication of ritonavir therapy in a patient with AIDS. Eur J Clin Microbiol Infect Dis. 1998;17:810–1. doi: 10.1007/s100960050194. [DOI] [PubMed] [Google Scholar]
- 21.Roubenoff R, Weiss L, McDermott A, Heflin T, Cloutier GJ, Wood M, et al. A pilot study of exercise training to reduce trunk fat in adults with HIVassociated fat redistribution. AIDS. 1999;13:1373–5. doi: 10.1097/00002030-199907300-00015. [DOI] [PubMed] [Google Scholar]
- 22.Aschenbrenner DS. First drug approved for hiv therapy-related lipodystrophy. Am J Nurs. 2011;111(4):68–9. [Google Scholar]
- 23.van Wijk JP, de Koning EJ, Cabezas MC, op’t Roodt J, Joven J, Rabelink TJ, et al. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: A randomized trial. Ann Intern Med. 2005;143:337–46. doi: 10.7326/0003-4819-143-5-200509060-00009. [DOI] [PubMed] [Google Scholar]
- 24.Moyle GJ, Brown S, Lysakova L, Barton SE. Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006;7:181–5. doi: 10.1111/j.1468-1293.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 25.Carey DL, Baker D, Rogers GD, Petoumenos K, Chuah J, Easey N, et al. A randomized, multicenter, open-label study of poly-L-lactic acid for HIV-1 facial lipoatrophy. J Acquir Immune Defic Syndr. 2007;46:581–9. doi: 10.1097/qai.0b013e318158bec9. [DOI] [PubMed] [Google Scholar]
- 26.Loutfy MR, Raboud JM, Antoniou T, Kovacs C, Shen S, Halpenny R, et al. Immediate versus delayed polyalkylimide gel injections to correct facial lipoatrophy in HIV-positive patients. AIDS. 2007;21:1147–55. doi: 10.1097/QAD.0b013e3281c6148d. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. FDA approval of Sculptra for treating facial lipoatrophy. [Last cited on 2011 Jun 12]. Available from: http://www.fda.gov/ForConsumers/ ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm125007.Htm .
- 28.U.S. Food and Drug Administration. FDA approves Radiesse for treating facial lipoatrophy. [Last cited on 2011 Jun 12]. Available from: http://www.fda.gov/ForConsumers/ ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124413.htm .


