Abstract
Objective:
Addition of vildagliptin to ongoing insulin therapy may help in terms of overall glycemic control as well as reduction in dose of insulin and weight. This study sought to evaluate the effect of vildagliptin and fixed dose combination (FDC) of vildagliptin and metformin in patients in ongoing insulin therapy for Type 2 diabetes mellitus.
Materials and Methods:
This was an open label, prospective, non-randomised, multicentric observational study. In this study 400 patients with T2DM on insulin were enrolled and allocated with the treatment of vildagliptin 50 mg in monotherapy and FDC of vildagliptin 50 mg and metformin strengths as 500/ 850 / 1000 mg. Baseline investigations included fasting blood glucose (FBG) and post prandial plasma glucose (PPPG) Estimation and glycosylated haemoglobin (HbA1c).
Results:
The combined analysis was carried out on 300 completed patients in this study, who were treated with vildagliptin or FDC of vildagliptin and metformin. The difference in mean value of insulin dose (MID) showed a highly significant decrease (P <0.0001) from baseline to end of the treatment i.e. from 36.26 ± 18.21 to 26.87 ± 16.49 IU. A highly significant decrease (P <0.0001) in FBG from 194.94 ± 56.19 to 124.93 ± 30.11 mg/dl was observed. Similarly PPPG showed a highly significant (P <0.0001) decrease from baseline to end of the treatment i.e. from 287.60 mg/dl to 172.05 mg/dl and there was highly significant (P <0.0001) decrease in HbA1c i.e. from 9.01% to 7.65% respectively. At the same time, highly significant decrease (P <0.0001) in mean weight also observed from baseline to end of the treatment i.e. from 71.23 ± 11.06 kg to 70.06 ± 10.62 Kg.
Conclusion:
Addition of vildagliptin and FDC of vildagliptin and metformin is an effective strategy in glycemic control, reduction in dose of insulin and weight of patients suffering with T2DM.
Keywords: Fixed dose combination of vildagliptin and metformin, glycemic control and insulin dose, type 2 diabetes mellitus, vildagliptin
INTRODUCTION
The pathophysiology of Type 2 Diabetes Mellitus (T2DM) is characterized by deficient insulin activity arising from decreased insulin secretion secondary to beta cell failure, and/or compromised insulin action in peripheral target tissues (insulin resistance). This abnormal metabolic state is exacerbated by excess hepatic glucose production and altered metabolism of proteins and lipids, which along with hyperglycemia, contribute to micro vascular and macro vascular complications. Therefore, achieving glycemic control is a prerequisite for prevention of cardiovascular and micro vascular complications in type 2 diabetes.
T2DM accounts for approximately 85% to 95% of diabetes patients in developed regions like the European Union (EU). Age and weight are established risk factors for T2DM. The majority of patients with T2DM are overweight or obese. Diet modification and exercise is the first line of treatment for T2DM. Pharmacologic intervention with one oral antidiabetic drug (OAD) is usually the next step in treatment. The recommended first line treatment is metformin which restrains hepatic glucose production and decreases peripheral insulin resistance. After 3 to 9 years of OAD monotherapy, patients typically require an additional intervention. Vildagliptin is one of a relatively new class of potential antidiabetic agents for use in the treatment of T2DM.
Vildagliptin is an inhibitor of dipeptidyl peptidase-IV (DPP-IV), an enzyme that inactivates inter alia the incretin peptides glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin tropic polypeptide (GIP).[1]
Vildagliptin acts by increasing GLP-1 levels and thereby stimulating insulin secretion and inhibiting glucagon secretion.[2]
Metformin has been shown to improve insulin sensitivity in liver and muscle.[3] Metformin has also been observed to increase GLP-1 levels which would be a potential for an additional synergistic action with vildagliptin.[4] The aim of the present study was to evaluate the effect of addition of vildagliptin and fixed dose combination (FDC) of vildagliptin and metformin in T2DM patient's on insulin therapy.
MATERIALS AND METHODS
Patients and study design
The study was conducted all over India in 22 centres viz. Madurai, Kolkata, Ahmedabad, Vijayawada, Delhi, Mumbai, Bangalore, Agra, Jaipur, Allepy, Lucknow, Madurai, Guwahati, Chandigarh, Aurangabad, Hubli, Jodhpur, Coimbatore, Trichy, Cochin, Cuttack and Rajkot. Patients aged between 18 to 70 years with body mass index (BMI) ≥ 23 kg/m2 and ≤ 35 kg/m2 were included in this study. This open label, prospective, non-randomized, multicentric observational study was carried out in patients with T2DM, not achieving glycemic control on current insulin therapy. The patients were on a steady dose of insulin for at least 3 months before enrollment in the study. The lack of glycemic control was defined by the following criteria:
HbA1c ≥ 7.0% or Fasting plasma glucose ≥ 140 mg/dl and/or post prandial plasma glucose ≥ 200 mg/dl.
Patients were excluded from this study, if they had evidence of any renal/hepatic de-compensation, positive test of pregnancy, failure to adhere to study regimen or significant comorbid conditions.
All patients were screened according to protocol by clinical evaluation, medication history, physical examination, and lab chemistry. The objective includes evaluation of overall glycemic control, change in dose of insulin and weight by addition of vildagliptin or FDC of vildagliptin and metformin to on-going insulin therapy.
Treatment
Patients either received 50mg dose of vildagliptin or FDC of vildagliptin 50mg and metformin in different strengths as 500/850/1000mg. Baseline investigations include fasting and post prandial plasma glucose estimation and glycosylated haemoglobin (HbA1c). At Visit 1 (Day 0) inclusion and exclusion criteria were reviewed and study medication was prescribed only to the eligible patients. Detailed instructions about the dose administration were given to each patient. The dose of the study medication was titrated as per the targeted glycemic levels. At visit 2 (4 weeks post treatment), visit 3 (8 weeks post treatment), visit 4 (12 weeks post treatment) dose of study medication was reviewed and titrated appropriately as per the results of fasting and post prandial plasma glucose. Adverse events were noted in the case record form.
Statistical snalysis
Statistical analysis was performed on the basis of primary as well as secondary efficacy parameters compared with the baseline value for the same patient. Data were reported as means and SD's. Student's t test (paired) and T-test (Wilcoxon Rank Sum Test) were used to assess the statistical significance. Value of P<0.0001 was considered to be highly significant and P<0.05 was considered to be significant. The SAS Version 9.1.3 statistical package was used for analysis of the study outcome data.
RESULTS
This was an observational study. Out of 400 (100%) enrolled patients, 100 (25.00%) patients were considered as drop out in this non-randomized trial. The analysis for vildagliptin and FDC of vildagliptin and metformin showed highly significant (P<0.0001) decrease in the mean insulin dose (288 patients on insulin BD therapy and 12 patients on insulin OD therapy) [Figure 1] from baseline i.e. 36.26 ± 18.21 to end of treatment i.e. 26.87 ± 16.49 IU. Mean value for FBG and PPPG [Figure 2] demonstrated highly significant (P <0.0001) decrease from baseline i.e. 194.94 ± 56.19 and 287.60 ± 78.15 to end of treatment i.e. 124.93 ± 30.11 mg/dl and 172.05 ± 37.41 mg/dl respectively. A highly significant (P <0.0001) decrease in mean value of HbA1c [Figure 3] from baseline i.e. 9.01 ± 1.29 to end of treatment i.e. 7.65 ± 1.02% was observed. And also highly significant (P <0.0001) decrease in mean weight was observed from baseline i.e. 71.23 ± 11.06 to 70.06 ± 10.62 Kg at the end of trial.
Figure 1.
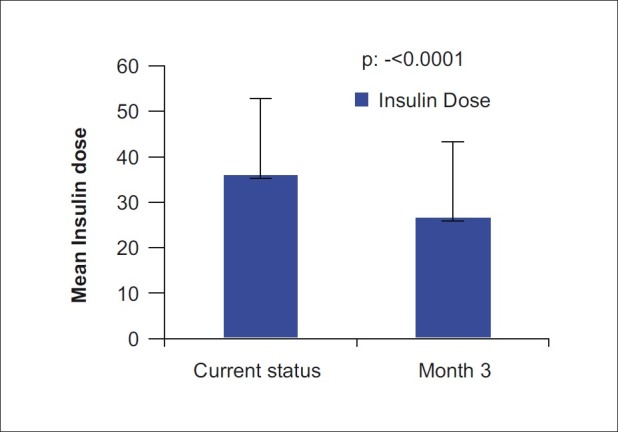
Comparison of insulin dose (288 patients on insulin BD therapy and 12 patients on insulin OD therapy) between baseline and end of treatment
Figure 2.
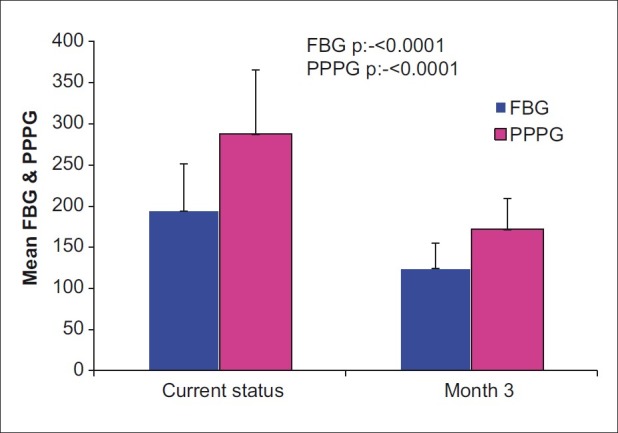
Comparison of post prandial plasma glucose and Fasting Blood Glucose between baseline and after treatment
Figure 3.
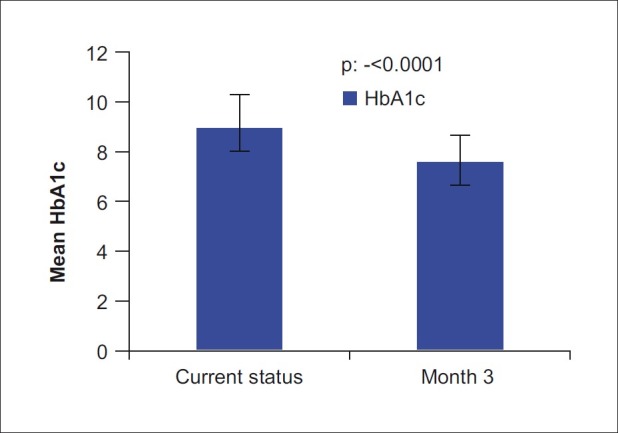
Comparison of HbA1c (Glycosylated haemoglobin) between baseline and end of treatment
DISCUSSION
Tight glucose control is difficult to establish.[5] If long-acting insulin or a twice daily injection of intermediate-acting insulin is not adequate to control blood sugar levels, a more intensive insulin treatment regimen may be recommended.[6] By increasing the dose of insulin, there is a risk of hypoglycemia, rapid heart rate, seizures and also weight gain .[7] During recent years there has been a discussion of introducing initial combination therapy when pharmacological treatment is required for T2DM, in order to reach therapeutic goal at an earlier stage and to avoid or delay subsequent changes in therapy for the maintenance of therapeutic goal. When combining the data on efficacy and safety, it is clear that most T2DM patients with sufficient beta-cell reserve will benefit from therapy with vildagliptin. Especially patients where hypoglycemia needs to be avoided at all cost, like elderly patients or patients with active professional lives, or where weight gain is a major concern, will benefit from this treatment.[8] In a study vildagliptin/metformin hydrochloride was indicated in the treatment of T2DM patients who were unable to achieve sufficient glycemic control at their maximally tolerated dose of oral metformin alone or who were already treated with the combination of vildagliptin and metformin as separate tablets.[7] Studies also suggest that vildagliptin is useful in the management of hyperglycemia in patients with T2DM as a mono therapeutic agent and when used in conjunction with metformin, pioglitazone or insulin.[9]
In a 12-week dose-ranging study, vildagliptin (25–100 mg/day) reduced HbA1C by about 0.4% at the higher doses (50 and 100 mg/day) and increased postprandial insulin concentrations at the 100 mg/day dose.[10] A higher dose of vildagliptin (100 mg twice daily) for four weeks improved the day glucose profile while lowering concentrations of insulin and glucagon.[11] Intact GLP-1 and GIP concentrations were increased by the vildagliptin therapy. The acute insulin response to an intravenous glucose challenge was increased after 12 weeks treatment with vildagliptin at 100 mg/day.[12] There was a preliminary evidence that vildagliptin (100 mg/day) reduced the post-prandial triglyceride excursion after a fat-rich meal.[13] During longer-term studies vildagliptin has shown greater and sustained reductions of HbA1C. A 52-week comparator study between vildagliptin (100 mg/day) and metformin (2,000 mg/day) in diet-treated patients with type 2 diabetes found a 1.0% reduction in HbA1C with vildagliptin. However, this was less than the 1.4% reduction in HbA1C observed with metformin.[14] In a 24-week comparator study vildagliptin (100 mg/day) produced a similar reduction in HbA1C (by 1.1%) to rosiglitazone (8 mg/day).[15]
Vildagliptin belongs to a new class of oral anti-diabetic drugs and is a selective and reversible inhibitor of dipeptidyl peptidase 4 (DPP-4), the enzyme which inactivates the incretin hormones, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP), hormones which significantly contribute to the maintenance of glucose homeostasis.[8] GLP-1 receptor agonists and DPP-IV inhibitors are premised on highly divergent mechanisms of action. GLP-1 receptor agonists provide pharmacologic levels of GLP-1 receptor stimulation, whereas DPP-4 inhibitor appear to increase circulating levels of GLP-1 to within the physiologic range.[9] Incretin-based therapies, both GLP-1 receptor agonists and DPP-IV inhibitors, unlike many other antidiabetic therapies (such as sulphonylureas and insulin in particular), they do not induce weight gain. Because DPP-IV inhibitor with GLP-1 receptor agonists results in progressive and sustained significant weight loss in most patients[16,17] and also in an open labeled clinical study DPP-IV inhibitors showed weight loss,[18] in the present study has evaluated the effect of vildagliptin and FDC of vildagliptin and metformin in patients in ongoing insulin therapy for Type 2 diabetes mellitus. In the present study reduction in weight and insulin dose was reported in the analysis of patients treated with vildagliptin and FDC of vildagliptin and metformin to treat T2DM. Vildagliptin and FDC of vildagliptin and metformin are expected to be of increasing value in the future treatment of T2DM. The present study hence proves the efficacy in terms of overall glycemic control, weight control and reduction in insulin dose, safety as well as tolerability of adding vildagliptin / FDC of vildagliptin and metformin to insulin.[4] Also the addition of vildagliptin shows a reduction in insulin dose which may be beneficial in the economical aspect.
CONCLUSION
It is concluded from the present study that both the treatments i.e. vildagliptin and FDC of vildagliptin and metformin were effective in achieving glycemic control while reducing the dose requirement of insulin, and causing weight loss, in patients of T2DM who were poorly controlled on insulin. For future studies, the durability of effects of the combination of vildagliptin with metformin needs to be explored as well as more detailed mechanistic studies need to be undertaken, with particular to studies on glucagon secretion, prandial lipid levels and insulin sensitivity.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Australian Public Assessment Report for Vildagliptin/Metformin HCl, Department of Health and Ageing, Therapeutic Goods Administration. 2011 Jan [Google Scholar]
- 2.Ahrén B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, et al. Inhibition of dipeptidyl peptidase IV im proves metabolic control over a 4 week study period in type 2 diabetes. Diabetes Care. 2002;25:869–75. doi: 10.2337/diacare.25.5.869. [DOI] [PubMed] [Google Scholar]
- 3.Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: The evidence today. Diabetes Metab. 2003;29:6S28–35. doi: 10.1016/s1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, et al. Effect of metformin on glucagon-like pep tide 1 (GLP-1) and leptin levels in obese non diabetic subjects. Diabetes Care. 2001;24:489–94. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- 5.Metchick LN, Petit WA, Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med. 2002;113:317–23. doi: 10.1016/s0002-9343(02)01213-5. [DOI] [PubMed] [Google Scholar]
- 6. [Last accessed on 2012 Jan 25]. Available from: http://www.uptodate.com/contents/patientinformation-diabetes-mellitus-type-2-insulin-treatment .
- 7.Novartis pharmaceuticals UK Ltd. Galvus 50 mg tablets. 2008. [Last accessed in 2008]. Available from: http://emc.medicines.org.uk/
- 8.Mathieu C, Degrande E. Vildagliptin: A new oral treatment for type 2 diabetes mellitus. Vasc Health Risk Manag. 2008;4:1349–60. doi: 10.2147/vhrm.s3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John RW. Dipeptidyl peptidase – IV inhibitors: Pharmacological profile and clinical use. Clin Diabetes. 2008;26:53–7. [Google Scholar]
- 10.Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: Vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7:692–8. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 11.Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–94. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- 12.D’Alessio DA, Watson CE, He YL, Ligueros-Saylan M, Wang Y, Dunning BE, et al. Restoration of an acute insulin response to glucose in drug-naive patients with type 2 diabetes by 3-month treatment with vildagliptin. Diabetes. 2006;55:A454. [Google Scholar]
- 13.Matikainen N, Mänttäri S, Schweizer A, Ulvestad A, Mills D, Dunning BE, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–57. doi: 10.1007/s00125-006-0340-2. [DOI] [PubMed] [Google Scholar]
- 14.Dejagers S, Lebeaut A, Couturier A, Schwizer A. Sustained reduction in HbA1c during one-year treatment with vildagliptin in patients with type 2 diabetes. Diabetes. 2006;55(Suppl 1):A120. [Google Scholar]
- 15.Rosenstock J, Baron MA, Schweizer A, Mills D, Dejagers S. Vildagliptin is as effective as rosiglitazone in lowering HbA1c but without weight gain in drug-naïve patients with type 2 diabetes (T2DM) Diabetes. 2006;55(Suppl 1):557. [Google Scholar]
- 16.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: Properties, functions, and clinical implications. Am J Med. 2011;124:S3–18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Varanasi A, Chaudhuri A, Dhindsa S, Arora A, Lohano T, Vora MR, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, Crp and triglyceride concentrations. Endocr Pract. 2010;17:192–200. doi: 10.4158/EP10199.OR. [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ, Kela R, Khunti K. Over view of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab. 2011;13:207–20. doi: 10.1111/j.1463-1326.2010.01330.x. [DOI] [PubMed] [Google Scholar]


