Abstract
The mammalian Golgi apparatus is composed of multiple stacks of cisternal membranes organized laterally into a polarized ribbon. Furthermore, trans-Golgi membranes come in close apposition with endoplasmic reticulum (ER) membranes to form ER-trans-Golgi contact sites, which may facilitate transfer of newly synthesized ceramide from the ER to sphingomyelin (SM) synthase at the trans-Golgi via ceramide transfer protein (CERT). CERT interacts with both ER and Golgi membranes, and together with Golgi morphology contributes to efficient SM synthesis. Here, we show that Golgi disassembly during proapoptotic stress induced by tumor necrosis factor (TNFα) and anisomycin results in decreased levels of CERT at the Golgi region. This is accompanied by a caspase-dependent loss of full-length CERT and reduction in de novo SM synthesis. In vitro, CERT is cleaved by caspases-2, -3 and -9. Truncated versions of CERT corresponding to fragments generated by caspase-2 cleavage at Asp213 were mislocalized and did not promote efficient de novo SM synthesis. Thus, it is likely that during cellular stress, disassembly of Golgi structure together with inactivation of CERT by caspases causes a reduction in ceramide trafficking and SM synthesis, and could contribute to the cellular response to proapoptotic stress.
Keywords: Golgi complex, caspase, CERT, sphingomyelin synthesis, apoptosis
INTRODUCTION
Apoptosis is a highly regulated form of cell death that serves to eliminate damaged cells without causing inflammation, and is important for maintaining normal growth and development [1]. Signals that induce cell death can come from different organelles within the cell such as the nucleus after DNA damage, or extrinsically after death receptor ligation [1]. During this evolutionarily conserved process, damaged cells are disassembled and packaged into membrane-bound blebs, which are then phagocytosed by adjacent cells.
Some of the apoptotic machinery that mediates cleavage of cellular targets is found at the Golgi complex, suggesting that this organelle may be able to sense specific stress signals and regulate downstream signaling pathways [1, 2]. While most procaspases are localized in the cytoplasm, procaspase-2 is also localized in the nucleus and on the cytoplasmic face of the Golgi apparatus [3, 4]. The localization pattern of procaspase-2 suggests that the nuclear pool of caspase-2 mediates apoptosis after DNA damage while the Golgi pool mediates apoptosis in response to stress signals sensed by the secretory pathway, particularly the Golgi apparatus itself [4]. Several known substrates of caspase-2 are localized at the Golgi, including golgin-160 [4] and giantin [5]. Both of these proteins are also substrates for effector caspases-3 and -7 [4]. Cleavage of golgin-160 by caspase-2 is rapid and precedes cleavage by caspase-3, suggesting that caspase-2 activation at the Golgi is an early event [4]. Many Golgi structural proteins, including GM130 and p115, are cleaved by other caspases such as caspase-3 [6, 7]. Cleavage of Golgi structural proteins promotes Golgi disassembly. First, the ribbon structure that is characteristic of mammalian cells is lost, followed by further disassembly. With disassembly of the Golgi ribbon structure, it is expected that Golgi function is also affected [8–11].
Stress signals sensed by the secretory pathway not only include disassembly of Golgi structure but also lipid perturbations [12]. Ceramide, which serves as the backbone for sphingolipid biosynthesis, is also an important second messenger in cells [13]. Therefore, ceramide synthesis and distribution within the cell must be tightly regulated. Ceramide is synthesized de novo in the endoplasmic reticulum (ER) and transported to various cellular locations by either vesicular or non-vesicular routes, where it is converted to sphingolipids, including SM by SM synthase I at trans-Golgi membranes [14, 15]. Ceramide can also be generated at various locations in the cell by sphingomyelinase mediated hydrolysis of SM [16, 17]. Ceramide and its derivatives including sphingosine, sphingosine-1-phosphate, and ceramide-1-phosphate are important signaling lipids and are involved in regulating various aspects of cell growth, differentiation, proliferation, necrosis and apoptosis [13, 16, 18–21].
Here, we analyzed how cellular stress affects de novo SM synthesis. Transfer of ceramide from the ER to the trans-Golgi mainly occurs by the activity of a soluble protein called CERT [22]. CERT interacts with phosphatidylinositol-4-phosphate (PI4P)-enriched Golgi membranes through its N-terminal pleckstrin homology (PH) domain, and with the ER through a sequence in its middle region that specifically interacts with the ER resident vesicle associated membrane protein associated protein [22]. The C-terminal domain of CERT, called the steroidogenic acute response protein related lipid transfer (START) domain, is responsible for extracting ceramide from the ER [22]. It has been previously suggested that both the Golgi and ER interacting domains of CERT are required for its function [22–24]. Since CERT localizes mainly at the Golgi, it may act at ER-trans-Golgi contact sites [25, 26]. Proper localization of CERT and its function depends on the morphology of the Golgi apparatus, with certain structural perturbations disrupting SM synthesis [23, 27]. In this study, we determined how cellular stress affects Golgi morphology and CERT function. Our data suggest a link between apoptotic signaling pathways and the Golgi as a platform for sensing and mediating cellular stress through CERT.
EXPERIMENTAL PROCEDURES
Cells and plasmids
HeLa cells were grown and maintained as described previously [23]. Plasmids encoding Myc-tagged CERT and galactosyl transferase fused to the coral Discosoma striata red fluorescent protein (galT-DsRed) were described previously [23]. The plasmid encoding CERT with an N-terminal V5 tag was constructed by inserting synthetic oligonucleotides encoding the tag upstream of the CERT sequence in pcDNA3.1 between the HindIII and EcoRI sites. Myc-tagged CERT FFAT-mut (CERT lacking its ER interacting motif) was constructed as described previously [23]. Myc-tagged D197A and D213A CERT mutants were generated by site directed mutagenesis using QuikChange (Stratagene, La Jolla, CA). The Myc-tagged N-terminal fragment of CERT was generated by amplifying the sequence corresponding to amino acids 1-213 of full length CERT by polymerase chain reaction and inserting into pcDNA 3.1/Myc-His (Invitrogen) at the EcoRI and NotI restriction sites, resulting in a C-terminal Myc tag. The sequence was confirmed by dideoxy sequencing. Similarly, the Myc-tagged C-terminal fragment of CERT was generated by amplifying the sequence corresponding to amino acids 214–598 of the full length CERT (with an N-terminal methionine preceding amino acid 214) and inserted into pcDNA 3.1/Myc-His.
Antibodies
Affinity purified anti-golgin-160 antibodies recognizing residues 60–139 and 140–311 (described in [23]) were used in a ratio of 1:1. Mouse anti-GM130 was obtained from BD Transduction (San Diego, CA), monoclonal anti-Myc antibody (clone 9E10) was from Roche Molecular Biochemicals (Indianapolis, IN), and mouse anti-V5 was from AbD Serotec (Raleigh, NC). Rabbit anti-CERT IgG (recognizing an epitope between amino acids 300–350) was from Bethyl Labortories, Inc (Montgomery, TX). Alexa-488 conjugated goat anti-rabbit IgG, Alexa-488 conjugated donkey anti-mouse IgG, Alexa-568 conjugated goat anti-rabbit IgG, and Alexa-568 conjugated donkey anti-mouse IgG were from Molecular Probes, Inc (Eugene OR). Horseradish peroxidase conjugated donkey anti-mouse IgG and horseradish peroxidase conjugated donkey anti-rabbit IgG were obtained from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ).
Labeling of endogenous sphingolipids with 3H-serine
HeLa cells were grown on 6 cm dishes as described previously [23]. The cells were treated with 10 ng/ml TNFα (Sigma) in the presence of 10 μg/ml cycloheximide, 5 μg/ml anisomycin (Sigma), or water or DMSO (Burdick and Jackson, Muskegon, MI) vehicle controls for 1h or 4h at 37°C. During the last hour of drug treatment, cells were labeled with 3H-serine in the presence of cycloheximide, as described previously [23]. When the caspase inhibitor was used in the assay, cells were pre-incubated with 50μM quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methyl ketone (Q-VD-OPh, R&D Systems) for 1h and then TNFα, anisomycin or vehicle control was added for the subsequent 4h in presence of 50 μM Q-VD-OPh. Lipids were extracted by the standard Bligh and Dyer [28] method with modifications and run on high performance-thin layer chromatography silica gel plates and exposed to phosphorimaging screens, as described previously [23]. The bands were subjected to analysis using Molecular Imager FX (Bio-Rad Laboratories, Inc) and Quantity One software (Bio-Rad Laboratories, Inc). The amount of each lipid measured was normalized to the amount of protein in each sample.
Indirect immunofluorescence and confocal microscopy
HeLa cells were transiently transfected for approximately 24h at 37°C with 0.5–1 μg DNA per 3.5 cm dish with Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Cells were then treated with TNFα (10 ng/ml) in the presence of 10 μg/ml cycloheximide, anisomycin (5 μg/ml), or water or DMSO vehicle for 1h or 4h at 37°C. During the last hour of drug treatment, cells were incubated in serum-free medium containing cycloheximide to mimic the lipid labeling experiments. Immunofluorescence studies were carried out to assess the localization of proteins of interest as described previously [23]. For staining endogenous CERT, cells were permeabilized with 0.05% saponin (Sigma) instead of Triton X-100. For Figure 2, DNA was also stained using 0.1 μg/ml Hoechst 33342 (Sigma). Cells were visualized with an Axioskop microscope (Zeiss, Thornwood, NY) equipped for epifluorescence using an ORCA-03G charge-coupled device camera (Hamamatsu, Japan). iVision imaging software (BioVision Technologies, Exton, PA) was used to collect and analyze the images. Cells expressing low levels of protein were selected for imaging.
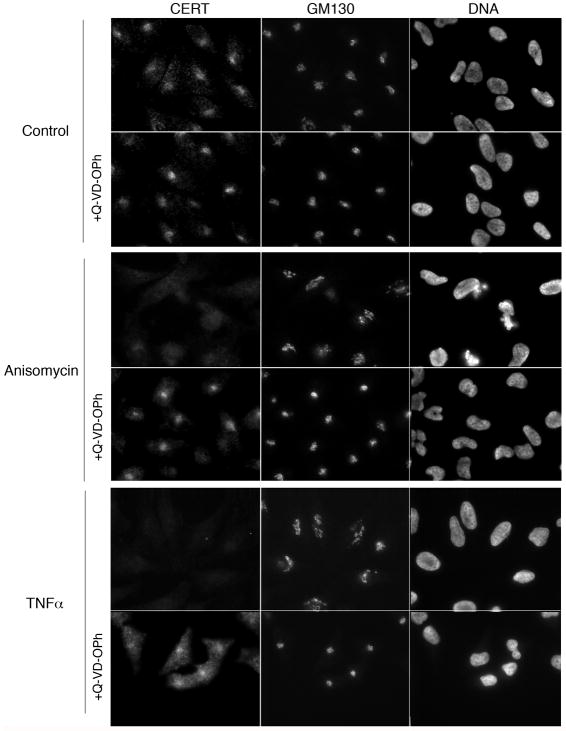
Figure 2. Disruption of CERT localization by proapoptotic drugs is caspase-dependent.
HeLa cells were pre-incubated with DMSO or Q-VD-OPh for 1h, and then subjected to treatment with vehicle control, 5 μg/ml anisomycin, or 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 4h in the presence or absence of the caspase inhibitor as indicated. Cells were stained for endogenous CERT, the peripheral Golgi membrane protein GM130, and DNA and analyzed by indirect immunofluorescence microscopy.
Confocal imaging was performed with a single point laser scanning confocal microscope (Zeiss Axiovert 200 microscope with 510-Meta confocal module from Zeiss, Thornwood, NY) as described previously [23].
SM synthase assay
SM synthase activity in cell lysates was measured as described previously [23], except that TNFα (10 ng/ml), anisomycin (5 μg/ml) or DMSO was added along with 10 nmol of nitro-2-1,3-benzoxadiazol-4-yl (NBD)-C6-ceramide (NBD-C6-Cer) (Molecular Probes Inc.) complexed to bovine serum albumin to each reaction before incubation. Alternatively, HeLa cells grown on 6 cm dishes were incubated with TNFα (10 ng/ml) in the presence of 10 μg/ml cycloheximide, anisomycin (5 μg/ml), or water or DMSO vehicle for 4h. During the last hour of drug treatment, cells were incubated in serum-free medium containing cycloheximide to mimic the lipid labeling experiments. Fluorescent NBD-SM made from the exogenously added NBD-C6-Cer substrate was analyzed by Molecular Imager FX (Bio-Rad Laboratories, Inc) and quantified by Quantity One software (Bio-Rad Laboratories, Inc). The amount of each lipid measured was normalized to the amount of protein in each sample.
Immunoblotting
HeLa cells were grown to confluence on 6 cm dishes at 37°C. The cells were treated with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide, 5 μg/ml anisomycin, or water or DMSO vehicle controls for 1h or 4h at 37°C. During the last hour of drug treatment, cells were grown in minimum essential medium (Gibco Invitrogen) in the presence of 10μg/ml cycloheximide to mimic lipid labeling experiments. When the caspase inhibitor was used in the assay, cells were pre-incubated with 50 μM Q-VD-OPh for 1h and then TNFα, anisomycin or vehicle control was added for the subsequent 4h in the presence of 50 μM Q-VD-OPh. For analysis of CERT cleavage in cells expressing V5-CERT or V5-CERT-D213A, the transfection medium was removed after 16h, and cells were allowed to recover for 24h before treating with proapoptotic drugs. Cells were resuspended in lysis buffer and analyzed by Western blotting as described previously [23].
CERT knockdown
HeLa cells were depleted of endogenous CERT by RNA interference (RNAi). Briefly, cells were transiently transfected with a pool of 4 duplexes (siGENOME SMARTpool M-012101-00-0005, Thermo Scientific, Lafayette, CO) using Oligofectamine transfection reagent (Gibco Life Technologies) for 18h, after which the medium was replaced. Cells from mock transfected and CERT RNAi plates were trypsinized after 48h and replated on 6 cm dishes. At 72h post-RNAi transfection, the cells were transfected for 12h with cDNAs encoding either full-length wild type Myc-tagged CERT or N-terminal fragment of CERT. We could not successfully express epitope-tagged C-terminal fragment of CERT, even when different tags (Myc, Flag, V5) were used at the N-terminus or C-terminus. The protein appeared unstable and was most probably subjected to rapid degradation. At 84h post-RNAi transfection, cells were labeled with 3H-serine for 1h and newly synthesized SM was quantified as described above. The experiment was carried out in parallel on 3.5 cm dishes to assess depletion of endogenous CERT and expression of full length Myc-tagged CERT or the N-terminal fragment of CERT by immunoblot and immunofluorescence microscopy.
In vitro transcription and translation
35S-labeled methionine substrates were generated using TNT T7-coupled reticulocyte lysate kit (Promega). 1 μg of plasmid DNA (Myc-tagged CERT wild type, Myc-tagged CERT mutants D197A and D213A, Myc-tagged caspase-2 long isoform, or Myc-tagged golgin-160 N-terminal head (amino acids 1–393) was used as template during the reaction with 10 μl of reticulocyte lysate and 10 μCi 35S methionine, and incubated at 30°C for 90 minutes.
Caspase cleavage assay
35S-labeled in vitro transcribed and translated substrates (described above) were incubated with recombinant caspases-2 (generous gift by Antony Rosen, Johns Hopkins University, originally obtained from Donald Nicholson at Merck-Frosst), -3, -6, -7, -8, 9, and -10 (Enzo) in their respective buffers (listed below) at 37°C for 1h, and the reaction was stopped by adding sample buffer (50 mM Tris-HCl, pH 6.8, 2 % sodium dodecyl sulfate (SDS), 20 % glycerol, 0.025 % bromophenol blue) containing 3.75% 2-mercaptoethanol. Samples were run on an SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel, followed by phosphorimaging. Caspase-2 cleavage reactions were carried out in buffer containing 100 mM MES-NaOH pH 5.5, 10 % PEG, 0.1 % CHAPS, and 5 mM DTT [4, 29]. Caspase-3, 8, and 10 cleavage assays were carried out in buffer containing 100 mM Hepes-NaOH, pH 7.0, 10 % PEG, 0.1 % CHAPS, and 10 mM DTT [29]. The caspase-7 reaction was carried out in buffer containing 50 mM Hepes-NaOH, pH 7.4, 100 mM NaCl, 0.1 % CHAPS, 1 mM EDTA, pH 7.5, 10 % glycerol, and 10 mM DTT as per the manufacturer’s directions. The caspase-9 assay was carried out in buffer containing 100 mM MES-NaOH, pH 6.5, 10 % PEG, 0.1 % CHAPS, and 10 mM DTT [29]. Different concentrations of recombinant capsase-2, including 4.5 nM, 17.9 nM, 71.5 nM, and 143 nM were used to determine kcat/Km for caspase-2 cleavage of myc-tagged wild type CERT, myc-tagged CERT D213A, and myc-tagged golgin-160 N-terminal head containing amino acids 1–393. Kcat/Km was determined using the equation: percent substrate cleaved = 100 × (1−e−kcat × [E]/Km × time) as described previously [4].
RESULTS
Golgi disassembly alters CERT localization after proapoptotic stimuli
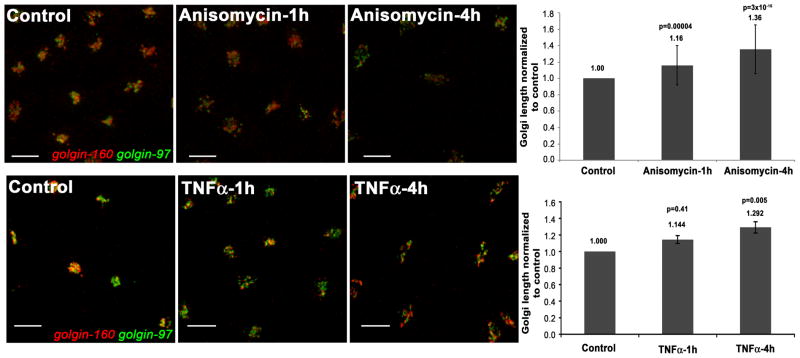
We monitored Golgi structural perturbations in HeLa cells after 1h and 4h of treatment with TNFα or anisomycin. TNFα induces the apoptotic pathway extrinsically through death receptor ligation, whereas anisomycin induces the intrinsic pathway by inhibition of protein synthesis and activation of mitogen-activated and stress kinase pathways. We assessed Golgi structure by confocal microscopy after staining for two resident Golgi proteins, golgin-160 and golgin-97. The morphological changes to the Golgi apparatus were subtle after 1h of treatment, but elongation of the Golgi structure occurred, and was dramatic by 4h (Figure 1). Further disassembly at the later time was observed as reduced overlap of the two Golgi markers. Thus, treatment with either TNFα or anisomycin induced Golgi disassembly, confirming previous studies [30, 31].
Figure 1. Golgi morphology is altered after treatment with proapoptotic stimuli.
HeLa cells were treated with 5 μg/ml anisomycin (top) or with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide (bottom) or with vehicle control for 1h and 4h, as indicated. Cells were stained for golgin-160 (red) and golgin-97 (green), and analyzed by confocal microscopy. A merge of the two staining patterns is shown. The length of the Golgi apparatus was measured from one end to the other using Volocity software through multiple z-stacks. The graphs accompanying the images represent the average length of the Golgi apparatus for each treatment after normalization to the vehicle-treated control (which was set to 1.0). The graph on the top represents Golgi length analyzed for 99–200 individual cells for each treatment, while the graph on the bottom represents Golgi length analyzed for 53–79 individual cells for each treatment. Error bars represent standard error of the mean. Student’s t-test values (p), where data from each treatment were compared to the vehicle-treated control, are indicated above each column.
CERT is predominantly confined to the Golgi region (Golgi membranes and nearby punctae), and its localization is dependent on Golgi morphology [23]. Thus, we determined if localization of CERT at the Golgi region was affected by Golgi disassembly induced by proapoptotic stress. We incubated HeLa cells in the absence or presence of TNFα or anisomycin for 4h, and stained for endogenous CERT and GM130, a peripheral Golgi membrane protein. As shown in Figure 2, CERT was predominantly present at the Golgi region in control cells. However, after 4h of TNFα and anisomycin treatments, localization of CERT at the Golgi region was markedly decreased. Given that both TNFα and anisomycin induce proapoptotic pathways [32, 33], we suspected that caspase cleavage might contribute to the decrease of CERT at the Golgi region. Pre-treating cells with the pan-caspase inhibitor Q-VD-OPh prevented the loss of CERT from the Golgi region (Figure 2). Golgi morphology was also protected in cells pre-treated with the caspase inhibitor (GM130 panels in Figure 2). These results suggest that activated caspases contribute to a reduction of CERT localization at the Golgi. To test if caspase activation impacted the Golgi pool of PI4P to which CERT binds, we assessed binding of a CERT mutant that cannot interact with the ER. Overlap of CERT lacking its ER interacting motif with a Golgi marker (galT-DsRed) was determined as described in Experimental Procedures. There was no significant difference in binding to Golgi membranes in treated cells versus control cells even though the Golgi structure was perturbed, suggesting that PI4P levels and thus PI4 kinase IIIβ at the Golgi [34] were not affected by TNFα or anisomycin treatment (Figure S1).
Full length CERT decreases after treatment with proapoptotic drugs
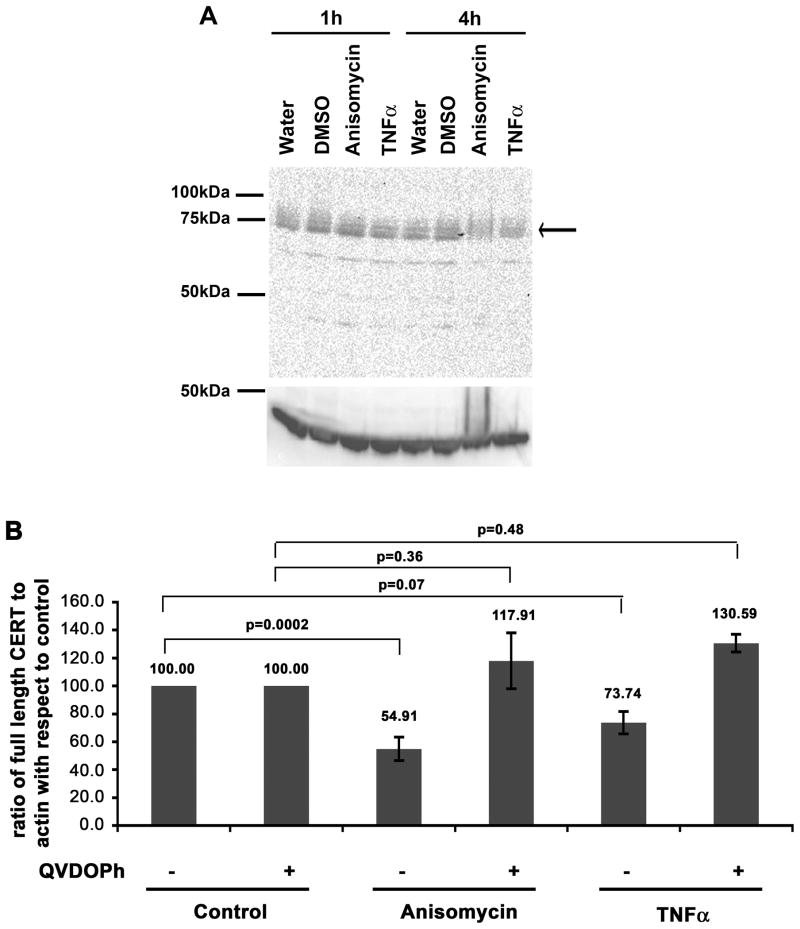
The result that PI4P levels appeared unaffected in cells treated with the proapoptotic drugs suggested that CERT localization was affected by cleavage of CERT itself. To address caspase cleavage of CERT, we analyzed the protein by immunoblotting with an antibody that recognizes an epitope near the middle of the protein (between amino acids 300–350). We found that the level of full-length CERT decreased after treatment with TNFα and anisomycin (Figure 3A). The full length protein decreased 45% and 26% in cells treated for 4h with anisomycin or TNFα, respectively (Figure 3B). A cleavage product of about 47 kDa was observed when blots were exposed for long times (Figure S2). Given that long exposures were required to detect a CERT cleavage product, we concluded that the fragment containing the antibody epitope was short-lived.
Figure 3. Endogenous full length CERT decreases after treatment with proapoptotic stimuli.
HeLa cells were treated with vehicle control, 5 μg/ml anisomycin or 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 4h as indicated. Some cells were pre-treated for 1h with 50 μM Q-VD-OPh by continued incubation for the subsequent 4h in the presence of TNFα or anisomycin. A) The top panel is representative blot probed for endogenous CERT. The full-length protein runs at about 75kDa. The bottom panel represents the same blot probed for actin (loading control). B) After quantification of the enhanced chemiluminescence signal, the intensity of the full length CERT band was normalized to the intensity of the actin band for each treatment and the ratio was then normalized to the control (set to 100). Error bars represent standard error of the mean. Student’s t-test values (p), where data from each treatment were compared to the vehicle-treated control are indicated above each column. The data represent 6 independent experiments for anisomycin and TNFα treatments, and 3 independent experiments for anisomycin and TNFα treatments in the presence of Q-VD-OPh.
We pre-treated HeLa cells with the caspase inhibitor Q-VD-OPh for 1h, and then subsequently for an additional 4h in the presence of TNFα or anisomycin. Treatment with the caspase inhibitor prevented reduction of full length CERT in TNFα and anisomycin-treated cells (Figure 3B), suggesting that the decrease in full length CERT in the presence of proapoptotic stimuli was mediated by caspase cleavage. Therefore, it is likely that reduced CERT staining at the Golgi region (Figure 2) can be partly attributed to cleavage of the full-length CERT.
CERT is a caspase substrate
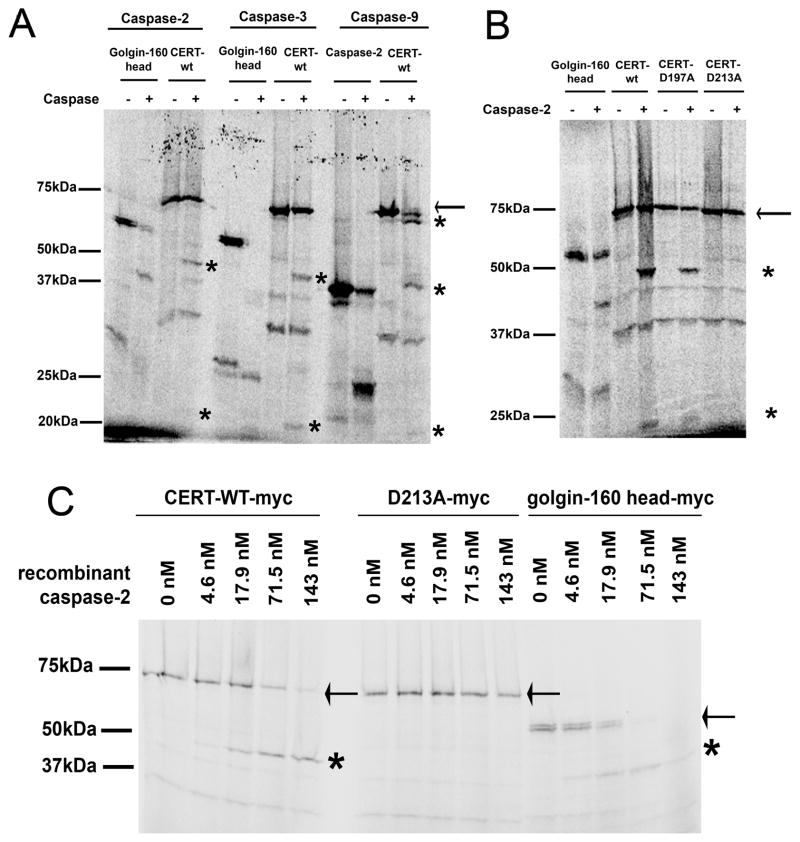
To directly test whether CERT is cleaved by caspases, we incubated in vitro transcribed and translated 35S-labeled CERT with recombinant caspases-2, -3, -6, -7, -8, -9, and -10. Incubation with caspases-2, -3 and -9 produced fragments of similar size, approximately 47 and 22 kDa (asterisks, Figure 4A). Caspase-9 also appeared to cleave CERT near one of the termini, resulting in a band slightly smaller than the full-length protein. There was no detectable cleavage with caspases-6, 7, 8 and 10 (data not shown). As both CERT and caspase-2 are partly localized at the Golgi region [4, 23], we further characterized cleavage of CERT by caspase-2. To identify the caspase-2 cleavage site in CERT, we mutated aspartates at several potential sites to alanines. Incubation of in vitro transcribed and translated CERT mutant proteins with recombinant caspase-2 identified aspartate 213 (D213) in the sequence TTRSD213 as the preferred caspase-2 cleavage site in CERT (Figure 4B). Kcat/Km values were calculated for cleavage of both wild type and D213A mutant CERT by caspase-2. The Kcat/Km for wild type CERT was 4.02 × 103 M−1s−1, while that of D213A mutant was 0.007 × 103 M−1s−1 (Figure 4C; see Experimental Procedures). However, CERT with either the D197A or D213A mutation was still cleaved by caspases-3 and -9 (Figure S3), suggesting cleavage by these caspases occurred at other sites. Caspase-3 and/or -9 could cleave CERT near D213, or at a site towards the C-terminus that would produce fragments of similar size to those produced by caspase-2 cleavage at D213. We conclude that it is possible that CERT is cleaved at D213 by caspase-2 at the Golgi during proapoptotic stress induced by TNFα and anisomycin. However, cleavage of CERT by caspases-3 and -9 is also likely to contribute to fragmentation of the protein in apoptotic cells.
Figure 4. CERT is cleaved by caspases in vitro.
A) CERT was in vitro transcribed and translated in presence of 35S methionine, and then incubated in absence or presence of recombinant caspases-2, -3, and -9. Golgin-160 (1–393), a known substrate for caspases-2, -3 and -7 [4] was used as a positive control for caspases-2 and -3, and in vitro translated procaspase-2 was used as a positive control for caspase-9 [50]. The full-length protein is indicated by an arrow and cleaved forms are indicated by asterisks. B) Wild type and mutant CERT proteins were in vitro transcribed and translated in presence of 35S methionine, and then incubated in absence or presence of recombinant caspase-2. The full-length protein is indicated by an arrow and cleaved forms are indicated by asterisks. C) Wild type CERT and the D213A mutant were in vitro transcribed and translated in presence of 35S methionine, and then incubated in absence or presence of different concentrations of recombinant caspase-2 as indicated. Golgin-160 (residues 1–393) was used as a positive control as described in A). The full length protein is indicated by an arrow and cleaved forms by asterisks.
Caspase-2 cleavage is predicted to inactivate CERT
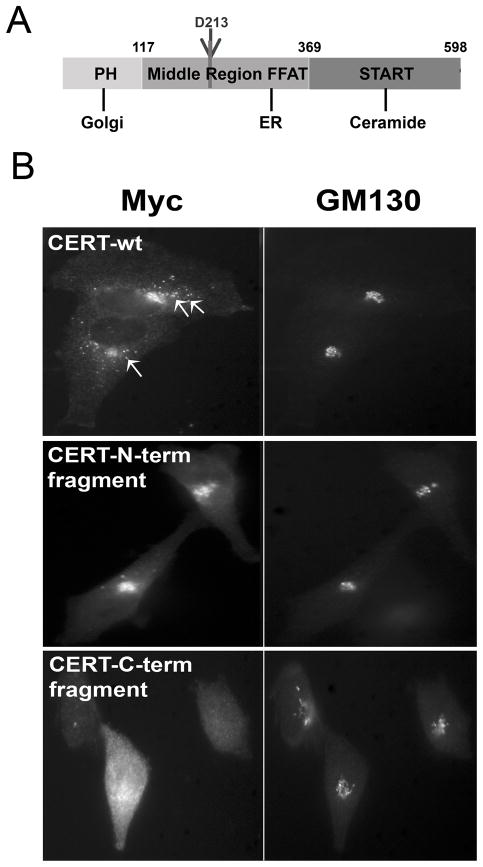
Cleavage of CERT by caspase-2 at D213 would generate two fragments of CERT, an N-terminal fragment (amino acids 1–213) and a C-terminal fragment (amino acids 214–598) (Figure 5A). The N-terminal fragment of CERT contains the Golgi-interacting PH domain of CERT and part of the middle region, including the serine repeat motif that can be phosphorylated [22, 35, 36]. The C-terminal fragment encompasses the ceramide extracting START domain of CERT as well as a part of the middle region that interacts with the ER [24]. We predicted that the two fragments of CERT would be targeted to different organelles and thus would not function efficiently in ceramide transport. We expressed epitope-tagged N-terminal and C- terminal fragments of CERT in HeLa cells and determined their subcellular localization by immunofluorescence microscopy (Figure 5B). Wild type, full length CERT localized to the Golgi, with additional punctae present around the Golgi region (Figure 5B and [23]). We previously hypothesized [23] that the punctae may represent ER-Golgi contact sites [25, 26], where efficient ceramide transport occurs. In comparison, the N-terminal fragment of CERT localized exclusively to the Golgi region without additional punctae (Figure 5B). The C-terminal fragment of CERT showed a dispersed ER-like staining pattern (Figure 5B). The C-terminal fragment was poorly expressed, but the localization pattern was similar in each of the rare cells expressing this truncation. Not surprisingly, the staining pattern of the N-terminal and C-terminal fragments of CERT resemble point mutants where the ER-binding or the Golgi-binding domains of CERT were inactivated, as described previously [23]. Thus, it is likely that the fragments of CERT produced by caspase-2 cleavage would be mislocalized and rendered nonfunctional for efficient ER to Golgi trafficking of ceramide.
Figure 5. Altered localization of CERT fragments corresponding to those generated by caspase-2 cleavage.
A) The cartoon indicates the potential caspase-2 cleavage site in CERT at D213; two fragments would be generated by cleavage. The locations of the PH domain, the middle region containing the FFAT (F321, F322AT) domain, and the START domain are also indicated. B) HeLa cells expressing Myc-tagged wild type full-length CERT, the N-terminal fragment of CERT, or the C-terminal cleavage fragment of CERT were stained for Myc and golgin-160 (Golgi marker) and analyzed by indirect immunofluorescence microscopy. The arrows in the CERT-wt panel indicate a few of the many punctae near the Golgi that are lacking in cells expressing the N-terminal fragment.
Cleavage of CERT at the D213 site in transfected cells
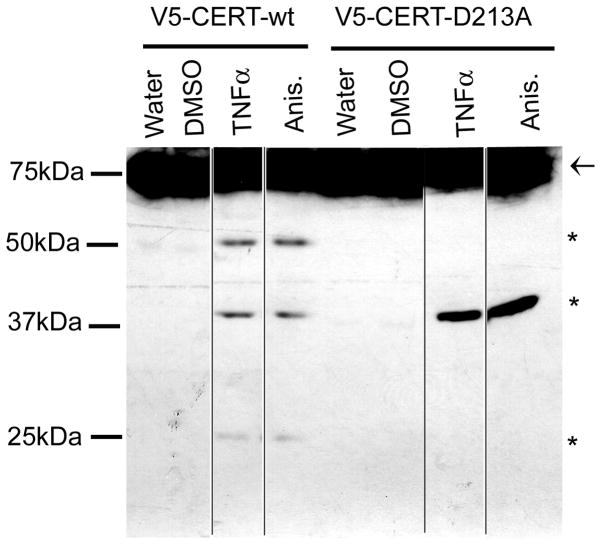
To analyze cleavage of CERT at D213, we expressed epitope-tagged wild-type CERT or the D213A mutant in HeLa cells. We chose to use an N-terminal epitope tag (V5), since the C-terminal CERT fragment appeared to be extremely unstable. After 4h of anisomycin or TNFα treatment, very low levels of CERT cleavage products were produced in transfected cells (data not shown). However, after 8h or 16h of treatment, N-terminal fragments could be detected after blotting with the anti-V5 antibody. In cells expressing wild-type CERT, bands at 25 kDa, 39 kDa, and 52 kDa were present along with the full-length V5-CERT (shown at 16h of treatment in Figure 6). The 25 kDa form is the size predicted for the V5-tagged N-terminus after cleavage by caspase-2 at D213. In cells expressing the D213A mutant, the 25 kDa band was absent (as was the 52 kDa band), but the 39 kDa form was still produced (Figure 6). These results indicate that CERT is cleaved at D213 during proapoptotic stress, but also at other sites as well. Since we did not observe a fragment corresponding to the 39 kDa form in the in vitro cleavage assays with any of the caspases we tested, it is possible that post-translational modifications (not present in the in vitro synthesized protein) are required for caspase cleavage at this site, or that other proteases are responsible. The 52kDa form containing the N-terminal tag could be produced by cleavage by caspase-3 or -9 at a site towards the C-terminus of the protein. Interestingly, this form was only produced when the D213 site was intact. Perhaps this downstream site is poorly accessible in the D213A mutant. Regardless of the order of cleavage and the caspase responsible, CERT cleavage at any of these potential sites would be predicted to reduce the efficiency of CERT-mediated ceramide transport.
Figure 6. Cleavage of N-terminally tagged CERT in apoptotic cells.
HeLa cells expressing V5-CERT or V5-CERT-D213A were treated for 16h with vehicle control, 5 μg/ml anisomycin (anis.), or 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide. Lysates were immunoblotted with anti-V5 antibody to detect the N-terminal fragments. These fragments were also detected after 8h of treatment (not shown), but were not visible after 4h. The full-length protein is indicated by an arrow, and the cleaved forms by asterisks. The samples were all run on the same gel, but irrelevant lanes were removed (spliced positions marked by black lines). We have noted that transiently transfected cells are more resistant to apoptosis than non-transfected cells, perhaps explaining the low level of cleavage in this experiment.
Treatment with proapoptotic stimuli impairs CERT function
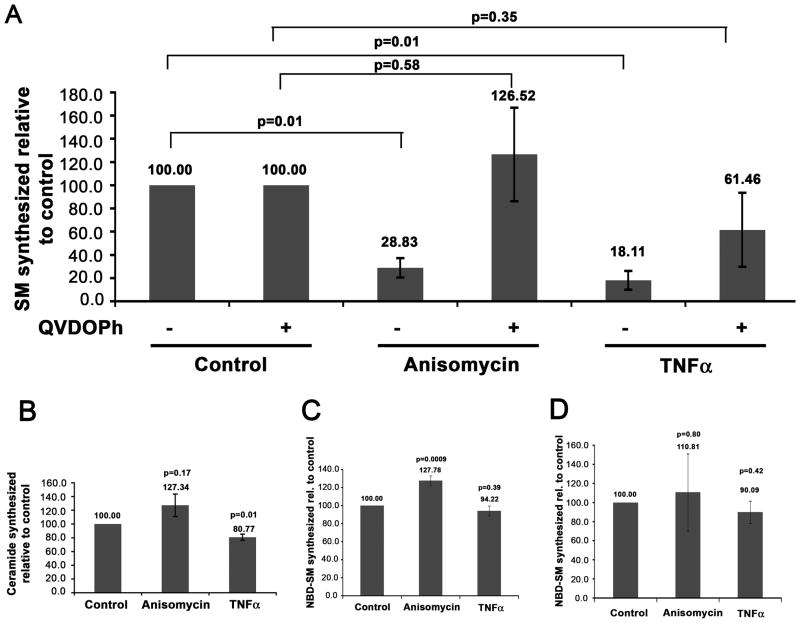
Morphological alteration of the Golgi complex, reduced localization of CERT at the Golgi region, and cleavage of CERT after proapoptotic stimuli could each lead to reduction of SM synthesis at the trans-Golgi. We thus analyzed de novo SM synthesis in treated cells. HeLa cells were treated with anisomycin, TNFα, or vehicle control for 4h in the absence or presence of the caspase inhibitor Q-VD-OPh, and labeled with 3H-serine for the last 1h of treatment. Lipids were extracted and analyzed by thin layer chromatography and phosphorimaging. SM production was significantly reduced in cells treated with anisomycin or TNFα (Figure 7A). This decrease was completely (anisomycin) or partially (TNFα) abrogated by the caspase inhibitor. The decrease in SM synthesis induced by anisomycin and TNFα was not due to a block in ceramide synthesis (Figure 7B). Anisomycin treatment resulted in a small increase in 3H-labeled ceramide, whereas newly synthesized ceramide was decreased about 19% in cells treated with TNFα. However, the decrease in de novo ceramide in TNFα-treated cells cannot completely account for the much larger decrease (about 82%) in SM synthesis. We performed several control experiments to rule out effects of the drugs on SM synthase. The proapoptotic drugs did not directly inhibit SM synthase, as shown by using an in vitro assay in cell lysates (Figure 7C). We also found that SM synthase activity in lysates from anisomycin and TNFα treated cells was not significantly altered from the control, suggesting that SM synthase is not inhibited through signaling events caused by proapoptotic treatments (Figure 7D). Thus, disassembly of the Golgi complex, reduction of CERT at the Golgi region, and cleavage of CERT induced by proapoptotic drugs are accompanied by a decrease in SM synthesis, all of which are largely caspase dependent.
Figure 7. Reduction in de novo SM synthesis in cells treated with proapoptotic stimuli.
A) HeLa cells were untreated or pre-incubated with the general caspase inhibitor Q-VD-OPh for 1h, and then subjected to treatment with vehicle control, 5 μg/ml anisomycin, or 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 4h in the presence or absence of the caspase inhibitor as indicated. Cells were labeled with 3H-serine and lipids were extracted and analyzed. The graph represents the level of newly synthesized SM as a percentage of the control. The data represent 2 independent experiments. B) HeLa cells were subjected to proapoptotic treatments as in A) but in the absence of the caspase inhibitor. The level of newly synthesized ceramide is indicated as a percentage of the level in the control. The data represent 3 independent experiments. C) HeLa cell lysates were incubated with vehicle control, 5 μg/ml anisomycin, or 10 ng/ml TNFα in addition to 10 nmol NBD-C6-Cer. Lipids were extracted and fluorescent SM was quantified. The data represent 3 independent experiments. D) HeLa cells were treated with vehicle control, 5 μg/ml anisomycin, or with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 4h, as indicated. Lysates from treated cells were incubated with 10 nmol NBD-C6-Cer. Lipids were extracted and fluorescent SM was quantified as in C). The graph represents the percent of fluorescent SM made for each treatment when compared to the control. The data represent 4 independent experiments. Error bars represent the standard error of the mean, and Student’s t-test values (p) are represented on the graph.
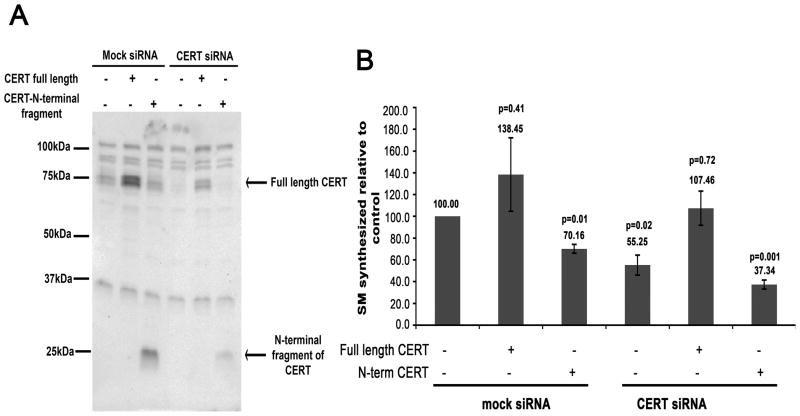
Since the fragments corresponding to caspase-2 cleavage of CERT lacked either the N-terminal Golgi-targeting PH domain or the C-terminal ceramide extraction domain (Figure 5A), it is likely that these fragments also lacked efficient ER to Golgi ceramide transport activity. Although overexpression of the START domain can support ceramide transport [22, 35], this activity may not be efficient for fragments expressed at endogenous levels [24]. To examine the function of cleaved CERT, we depleted HeLa cells of endogenous CERT by RNAi, expressed either full-length CERT or the N-terminal fragment of CERT, and monitored SM synthesis. We were unable to successfully express sufficient levels of the C-terminal fragment of CERT to include it in this assay. As shown in Figure 8A, depletion of CERT by RNAi resulted in a 74% decrease in endogenous protein by Western blot at 84h post-transfection. Expression of epitope-tagged full length CERT or the N-terminal fragment of CERT corresponding to caspase-2 cleavage resulted in an approximate four-fold increase in protein compared to the endogenous level (Figure 8A). Analysis of SM synthesis in cells treated with CERT RNAi revealed a 45% reduction in newly synthesized SM (Figure 8B). This decrease was rescued upon expression of Myc-tagged wild type CERT to a similar level as the control (Figure 8B). Given that only about 25% of the depleted cells expressed the Myc-tagged CERT, it is not unexpected that SM synthesis was not higher in the rescued cells. However, expression of the Myc-tagged N-terminal fragment of CERT did not rescue de novo SM synthesis (Figure 8B). Surprisingly, expression of the N-terminal fragment of CERT in mock depleted control cells showed a statistically significant decrease in the level of newly synthesized SM by about 30% (p=0.01) when compared to mock depleted control cells alone. This suggests that the N-terminal fragment of CERT inhibits endogenous CERT activity, possibly by competing for binding sites on Golgi membranes and may thus act in a dominant negative manner. Although we could not test it, we predict that the C-terminal fragment of CERT would also be inefficient for transporting ceramide from the ER to the Golgi, given its mislocalization and instability.
Figure 8. The N-terminal fragment of CERT cannot rescue de novo SM synthesis in CERT-depleted cells.
A) HeLa cells were depleted of endogenous CERT by RNAi for 84h. Lysates were resolved by SDS-PAGE and immunoblotted for CERT. Mock RNAi treatment was used as a control. At 66h of depletion, cells were transiently transfected with cDNAs encoding Myc-tagged full-length CERT or the N-terminal fragment of CERT corresponding to caspase-2 cleavage as indicated. Lysates were run on an SDS-PAGE and immunoblotted for CERT using anti-rabbit CERT antibody. The full-length and N-terminal CERT fragment proteins are indicated. B) Cells prepared as in A) were labeled with 3H-serine and lipids were extracted and analyzed. The graph represents percent of newly synthesized SM for each rescue with respect to the control (which is set to 100). Standard error of the mean and Student’s t-test (p) values are indicated on the graph. The data represent 3 independent experiments for rescue with full-length CERT, and 2 independent experiments for rescue with the N-terminal fragment of CERT.
DISCUSSION
The mammalian Golgi apparatus plays a central role in the secretory pathway and in sphingolipid synthesis. The complex architecture of the mammalian Golgi ribbon with ER-trans Golgi contact sites is thought to facilitate delivery of ceramide from the ER for synthesis of SM at the trans-Golgi [25, 26]. We previously showed that certain Golgi morphologies (induced by drugs that perturb Golgi structure) promote efficient ceramide trafficking and SM synthesis, while others do not [23]. Efficient SM synthesis correlated with CERT localization at Golgi membranes. A recent study also found a dramatic decrease in SM synthesis in cells deficient in conserved oligomeric Golgi complex-2 (Cog2), a component of a retrograde trafficking complex that is required for proper localization of certain glycosyltransferases [27]. The morphology of the Golgi complex in Cog2 null cells is disrupted, which may interfere with proper CERT localization. Thus, Golgi architecture is important for efficient SM synthesis. Here, we showed that induction of proapoptotic pathways by TNFα and anisomycin results in disassembly of the Golgi complex, probably due to cleavage of Golgi structural proteins including golgin-160, giantin, p115 and GM130 by activated caspases [4–7]. In addition, to Golgi disassembly, treatment with proapoptotic drugs resulted in a decrease of CERT at the Golgi region, caspase cleavage of CERT, and a large reduction in SM synthesis.
CERT was cleaved by caspases-2, 3, and 9 in vitro, and we mapped the caspase-2 cleavage site to D213 in the sequence TTRSD. Although this sequence is not predicted to be a good caspase-2 target [37], the best-characterized caspase-2 cleavage sequence (GESPD in golgin-160) does not fit the prediction either. The Kcat/Km for cleavage of CERT by caspase-2 was 4.02 × 103 M−1s−1, and the Kcat/Km for cleavage of golgin-160 was 4.38 × 103 M−1s−1. Thus, CERT is almost as good a substrate for caspase-2 as golgin-160. The Kcat/Km values measured by us for cleavage of golgin-160 by caspase-2 was less than the Kcat/Km value (33 × 103M−1s−1) reported previously [4]. This discrepancy could result from our use of the N-terminal portion instead of the full-length golgin-160 substrate, or a different preparation of recombinant caspase-2. Although we did not map the sites of cleavage for caspases-3 and -9, we found that CERT-D213A was still cleaved in cells treated with proapoptotic drugs at sites downstream of D213. Thus, even though caspase-2 may contribute to inactivation of CERT, other caspases can also perform this role if the caspase-2 site is absent or if activation of procaspase-2 does not occur.
Interestingly, although anisomycin and TNFα treatments resulted in about 45% and 26% decrease in full-length endogenous CERT, newly synthesized SM levels decreased by about 71% and 82%, respectively. Thus, it is likely that Golgi disassembly and CERT mislocalization also contribute to the impairment of SM synthesis, thereby causing a greater reduction in newly synthesized SM than would be expected with just CERT cleavage alone.
Changes in Golgi morphology during early times (1h) of proapoptotic treatment were subtle, and we observed CERT mislocalization, reduction in full-length protein, and impairment of SM synthesis only after 4h of proapoptotic treatment when Golgi alterations were more significant. Activation of initiator caspases and cleavage of a subset of Golgi substrates at early times may lead to small changes in Golgi organization. Subsequent activation of effector caspases may then result in gross morphological changes of the Golgi apparatus, including generation of dispersed Golgi membranes seen at later times. It is also possible that gross morphological changes at later time points correlate with dysregulation of cellular ceramide levels, thus amplifying the proapoptotic signaling pathway. Ceramide is also thought to play a role in maintenance of structural integrity of the ER and the Golgi [38], as well as in apoptotic signaling pathways [16]. Cells experiencing mild stress may activate survival responses (e.g. the unfolded protein response). However, cells exposed to prolonged or severe stress may activate caspases and commit to the apoptotic pathway [39]. One route for this could be inhibition of SM synthesis and an increase in the ceramide pool. However, at 4h of TNFα and anisomycin treatments we did not observe a significant increase in newly synthesized ceramide. Since we were only analyzing de novo synthesis of ceramide during the proapoptotic treatments, we cannot rule out generation of unlabeled ceramide from SM, sphingosine and other sphingolipids in different regions of the cell. Previous studies have indicated that TNFα treatment in particular, is associated with a rapid increase in ceramide levels at the plasma membrane by the activation of sphingomyelinase [40, 41]. Thus, understanding how cells sense ceramide levels at the ER and the Golgi [12], and integrate proapoptotic signaling is imperative. This would help us examine the possibility that the Golgi apparatus acts as a platform to integrate proapoptotic and sphingolipid signaling pathways via caspases and CERT.
Inactivation of CERT and inhibition of SM synthesis results in decreased utilization of substrates ceramide and phosphatidylcholine (PC). While the consequence of increased ceramide levels in cells has been studied (discussed above), it is not clear how cells may handle PC when SM synthesis is inhibited during proapoptotic treatments [42]. When SM is synthesized in the trans Golgi from substrates ceramide and PC, diacylglycerol (DAG) is produced [43, 44]. DAG at Golgi membranes serves to recruit protein kinase D (PKD), which regulates vesicle production and thus plays a role in modulating cargo trafficking through the organelle [45–47]. If SM synthesis decreases due to inactivation of CERT, DAG and consequently PKD recruitment to Golgi membranes would also presumably decrease, suggesting that CERT may function indirectly in cargo trafficking through the Golgi apparatus [48]. Additionally, altering SM synthesis in the Golgi and accordingly SM levels along the secretory pathway may also alter the SM/cholesterol ratio [49], thereby affecting partitioning and trafficking of lipids and proteins to their target destination (e.g. proteins targeted to lipid rafts in the plasma membrane). Thus, CERT may play an important role in regulating both lipids and proteins when cells are under severe stress.
In this study we have shown that both Golgi disassembly and inactivation of CERT contribute to decreased SM synthesis during proapoptotic stress. A more comprehensive approach for determining the order in which apoptotic signaling, ceramide accumulation, and Golgi disassembly occur will be required to understand how the Golgi apparatus contributes to cellular stress sensing and integration of apoptosis signaling.
Supplementary Material
HeLa cells expressing the Golgi marker galT-DsRed and Myc-tagged CERT-FFATmut [23] were treated with vehicle control, 5 μg/ml anisomycin or with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 4h. CERT-FFATmut was identified by indirect immunofluorescence microscopy using anti-Myc antibody and Alexa-488 conjugated donkey anti-mouse IgG. The graph represents the overlap coefficient of CERT with the Golgi marker for 22–28 individual cells for each treatment. Error bars represent standard error of the mean. Student’s t-test values (p), where data from each treatment were compared to the vehicle-treated control are indicated above each column.
HeLa cells were treated with vehicle control, 5 μg/ml anisomycin, or with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 1h or 4h, as indicated. The blot was probed for endogenous CERT. A long exposure to visualize potentially unstable cleavage products is shown. The full-length protein runs as a doublet at about 75kDa (arrow). A cleavage band at about 47 kDa is indicated by an asterisk. Due to the long exposure, decrease of full-length CERT is not evident, but was reduced similarly to that in Figure 3 when quantified.
CERT or CERT mutants were in vitro transcribed and translated in presence of 35S methionine and incubated with caspases-3 and -9 as described in Experimental Procedures. Arrows mark the full-length protein, and asterisks mark cleavage products. Both caspases cleave the mutant that is resistant to caspase-2 (D213A). The site of caspase-3 and -9 cleavage could be near D213, or could be at a site towards the C-terminus of the protein that would produce cleavage products of similar size.
Acknowledgments
We thank Winny Yun for contributing to the construction of the plasmid encoding V5-tagged CERT, the Johns Hopkins University School of Medicine Microscope Facility for assistance with confocal microscopy, and Antony Rosen for purified caspase-2. We also thank Travis Ruch and David Zuckerman for useful comments on the manuscript.
FUNDING
This work was supported by National Institutes of Health Grant GM42522 (to C. E. M).
Abbreviations used
- CERT
ceramide transport protein
- COG
conserved oligomeric Golgi complex
- DAG
diacylglycerol
- DMSO
dimethyl sulfoxide
- ER
endoplasmic reticulum
- NBD
nitro-2-1,3-benzoxadiazol-4-yl
- NBD-C6-Cer
NBD-C6-ceramide
- PC
phosphatidylcholine
- PH
pleckstrin homology
- PI4P
phosphatidylinositol 4-phosphate
- PKD
protein kinase D
- Q-VD-OPh
quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methyl ketone
- RNAi
RNA interference
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel elcetrophoresis
- SM
sphingomyelin
- START
steroidogenic acute response protein related lipid transfer
- TNFα
tumor necrosis factor-alpha
Footnotes
AUTHOR CONTRIBUTION
Suchismita Chandran performed the experiments, analyzed the data, and wrote the paper. Carolyn Machamer analyzed the data and edited the paper.
References
- 1.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 2.Maag RS, Hicks SW, Machamer CE. Death from within: apoptosis and the secretory pathway. Curr Opin Cell Biol. 2003;15:456–461. doi: 10.1016/s0955-0674(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 3.Bouchier-Hayes L, Oberst A, McStay GP, Connell S, Tait SW, Dillon CP, Flanagan JM, Beere HM, Green DR. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol Cell. 2009;35:830–840. doi: 10.1016/j.molcel.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, Rosen A. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149:603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe M, Lane JD, Woodman PG, Allan VJ. Caspase-mediated cleavage of syntaxin 5 and giantin accompanies inhibition of secretory traffic during apoptosis. J Cell Sci. 2004;117:1139–1150. doi: 10.1242/jcs.00950. [DOI] [PubMed] [Google Scholar]
- 6.Chiu R, Novikov L, Mukherjee S, Shields D. A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol. 2002;159:637–648. doi: 10.1083/jcb.200208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker A, Ward C, Sheldrake TA, Dransfield I, Rossi AG, Pryde JG, Haslett C. Golgi fragmentation during Fas-mediated apoptosis is associated with the rapid loss of GM130. Biochem Biophys Res Commun. 2004;316:6–11. doi: 10.1016/j.bbrc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Short B, Preisinger C, Korner R, Kopajtich R, Byron O, Barr FA. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol. 2001;155:877–883. doi: 10.1083/jcb.200108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L, Tai G, Hong W. Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network. Mol Biol Cell. 2004;15:4426–4443. doi: 10.1091/mbc.E03-12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Z, Joseph D, Bugnard E, Zaal KJ, Ralston E. Golgi complex reorganization during muscle differentiation: visualization in living cells and mechanism. Mol Biol Cell. 2001;12:795–808. doi: 10.1091/mbc.12.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diao A, Rahman D, Pappin DJ, Lucocq J, Lowe M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol. 2003;160:201–212. doi: 10.1083/jcb.200207045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vacaru AM, Tafesse FG, Ternes P, Kondylis V, Hermansson M, Brouwers JF, Somerharju P, Rabouille C, Holthuis JC. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J Cell Biol. 2009;185:1013–1027. doi: 10.1083/jcb.200903152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam D, Heinrich M, Kabelitz D, Schutze S. Ceramide: does it matter for T cells? Trends Immunol. 2002;23:1–4. doi: 10.1016/s1471-4906(01)02091-9. [DOI] [PubMed] [Google Scholar]
- 14.Futerman AH, Pagano RE. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991;280 (Pt 2):295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Maziere AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004;20:301–317. doi: 10.1023/B:GLYC.0000033627.02765.cc. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki T, Bielawska A, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 19.Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, Stephens DJ. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetz CA, Hunn M, Rojas P, Torres V, Leyton L, Quest AF. Caspase-dependent initiation of apoptosis and necrosis by the Fas receptor in lymphoid cells: onset of necrosis is associated with delayed ceramide increase. J Cell Sci. 2002;115:4671–4683. doi: 10.1242/jcs.00153. [DOI] [PubMed] [Google Scholar]
- 21.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 22.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 23.Chandran S, Machamer CE. Acute perturbations in Golgi organization impact de novo sphingomyelin synthesis. Traffic. 2008;9:1894–1904. doi: 10.1111/j.1600-0854.2008.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- 25.Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci USA. 2001;98:2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE. Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic. 2004;5:338–345. doi: 10.1111/j.1398-9219.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 27.Spessott WUA, Maccioni HJ. Cog2 null mutant CHO cells show defective sphingomyelin synthesis. J Biol Chem. 2010;285:41472–41482. doi: 10.1074/jbc.M110.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Calvo MPE, Rasper DM, Vaillancourt JP, Zamboni R, Nicholson DW, Thornberry NA. Purification and catalytic properties of human caspase family members. Cell Death Differ. 1999;6:362–369. doi: 10.1038/sj.cdd.4400497. [DOI] [PubMed] [Google Scholar]
- 30.Maag RS, Mancini M, Rosen A, Machamer CE. Caspase-resistant Golgin-160 disrupts apoptosis induced by secretory pathway stress and ligation of death receptors. Mol Biol Cell. 2005;16:3019–3027. doi: 10.1091/mbc.E04-11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Shields D. Nuclear Import Is Required for the Pro-apoptotic Function of the Golgi Protein p115. J Biol Chem. 2009;284:1709–1717. doi: 10.1074/jbc.M807263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croons VMW, Herman AG, Timmermans JP, De Meyer GR. The Protein Synthesis Inhibitor Anisomycin Induces Macrophage Apoptosis in Rabbit Atherosclerotic Plaques through p38 Mitogen-Activated Protein Kinase. J Pharmacol Exp Ther. 2009;329:856–864. doi: 10.1124/jpet.108.149948. [DOI] [PubMed] [Google Scholar]
- 33.Van Herreweghe FFN, Declercq W, Vandenabeele P. Tumor necrosis factor-mediated cell death: to break or to burst, that’s the question. Cell Mol Life Sci. 2010;67:1567–1579. doi: 10.1007/s00018-010-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth B, Balla A, Ma H, Knight ZA, Shokat KM, Balla T. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J Biol Chem. 2006;281:36369–36377. doi: 10.1074/jbc.M604935200. [DOI] [PubMed] [Google Scholar]
- 35.Kumagai K, Kawano M, Shinkai-Ouchi F, Nishijima M, Hanada K. Interorganelle trafficking of ceramide is regulated by phosphorylation-dependent cooperativity between the PH and START domains of CERT. J Biol Chem. 2007;282:17758–17766. doi: 10.1074/jbc.M702291200. [DOI] [PubMed] [Google Scholar]
- 36.Saito S, Matsui H, Kawano M, Kumagai K, Tomishige N, Hanada K, Echigo S, Tamura S, Kobayashi T. Protein phosphatase 2Cepsilon is an endoplasmic reticulum integral membrane protein that dephosphorylates the ceramide transport protein CERT to enhance its association with organelle membranes. J Biol Chem. 2008;283:6584–6593. doi: 10.1074/jbc.M707691200. [DOI] [PubMed] [Google Scholar]
- 37.Thornberry NART, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 38.Hu W, Xu R, Zhang G, Jin J, Szulc ZM, Bielawski J, Hannun YA, Obeid LM, Mao C. Golgi fragmentation is associated with ceramide-induced cellular effects. Mol Biol Cell. 2005;16:1555–1567. doi: 10.1091/mbc.E04-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 41.van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZVD. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Ullman MD, Radin NS. The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J Biol Chem. 1974;249:1506–1512. [PubMed] [Google Scholar]
- 44.Voelker DR, Kennedy EP. Cellular and enzymic synthesis of sphingomyelin. Biochemistry. 1982;21:2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- 45.Maeda Y, Beznoussenko GV, Van Lint J, Mironov AA, Malhotra V. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J. 2001;20:5982–5990. doi: 10.1093/emboj/20.21.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 47.Bankaitis VA. Cell biology. Slick recruitment to the Golgi. Science. 2002;295:290–291. doi: 10.1126/science.1068446. [DOI] [PubMed] [Google Scholar]
- 48.Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry RJ, Ridgway ND. Molecular mechanisms and regulation of ceramide transport. Biochim Biophys Acta. 2005;1734:220–234. doi: 10.1016/j.bbalip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Samraj AKSD, Schulze-Osthoff K, Schmitz I. Loss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarization. Mol Biol Cell. 2007;18:84–93. doi: 10.1091/mbc.E06-04-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HeLa cells expressing the Golgi marker galT-DsRed and Myc-tagged CERT-FFATmut [23] were treated with vehicle control, 5 μg/ml anisomycin or with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 4h. CERT-FFATmut was identified by indirect immunofluorescence microscopy using anti-Myc antibody and Alexa-488 conjugated donkey anti-mouse IgG. The graph represents the overlap coefficient of CERT with the Golgi marker for 22–28 individual cells for each treatment. Error bars represent standard error of the mean. Student’s t-test values (p), where data from each treatment were compared to the vehicle-treated control are indicated above each column.
HeLa cells were treated with vehicle control, 5 μg/ml anisomycin, or with 10 ng/ml TNFα in the presence of 10 μg/ml cycloheximide for 1h or 4h, as indicated. The blot was probed for endogenous CERT. A long exposure to visualize potentially unstable cleavage products is shown. The full-length protein runs as a doublet at about 75kDa (arrow). A cleavage band at about 47 kDa is indicated by an asterisk. Due to the long exposure, decrease of full-length CERT is not evident, but was reduced similarly to that in Figure 3 when quantified.
CERT or CERT mutants were in vitro transcribed and translated in presence of 35S methionine and incubated with caspases-3 and -9 as described in Experimental Procedures. Arrows mark the full-length protein, and asterisks mark cleavage products. Both caspases cleave the mutant that is resistant to caspase-2 (D213A). The site of caspase-3 and -9 cleavage could be near D213, or could be at a site towards the C-terminus of the protein that would produce cleavage products of similar size.