Abstract
The human mitochondrial genome has an exclusively maternal mode of inheritance. Mitochondrial DNA (mtDNA) is particularly vulnerable to environmental insults due in part to an underdeveloped DNA repair system, limited to base excision and homologous recombination repair. Radiation exposure to the ovaries may cause mtDNA mutations in oocytes, which may in turn be transmitted to offspring. We hypothesized that the children of female cancer survivors who received radiation therapy may have an increased rate of mtDNA heteroplasmy mutations, which conceivably could increase their risk of developing cancer and other diseases. We evaluated 44 DNA blood samples from 17 Danish and 1 Finnish families (18 mothers and 26 children). All mothers had been treated for cancer as children and radiation doses to their ovaries were determined based on medical records and computational models. DNA samples were sequenced for the entire mitochondrial genome using the Illumina GAII system. Mother’s age at sample collection was positively correlated with mtDNA heteroplasmy mutations. There was evidence of heteroplasmy inheritance in that 9 of the 18 families had at least one child who inherited at least one heteroplasmy site from his or her mother. No significant difference in single nucleotide polymorphisms between mother and offspring, however, was observed. Radiation therapy dose to ovaries also was not significantly associated with the heteroplasmy mutation rate among mothers and children. No evidence was found that radiotherapy for pediatric cancer is associated with the mitochondrial genome mutation rate in female cancer survivors and their children.
1. Introduction
Mitochondria play an important role in cellular energy metabolism, free radical generation, and apoptosis [1,2]. Mitochondrial DNA (mtDNA), located within the mitochondrial matrix, is distinct and replicates independently of nuclear DNA. mtDNA is a maternally-inherited, 16,569-bp, closed-circle, double-stranded molecule that encodes two rRNAs, 22 tRNAs, and 10 polypeptides. Although mtDNA represents less than 1% of total cellular DNA, its gene products are essential for normal cell function [3]. Mitochondrial dysfunction is one of the most prominent features of cancer cells and has been suspected of contributing to the development and progression of cancer [2,3,4,5]. Several approaches have been applied to investigate radiation exposure and subsequent mtDNA mutations. An analysis of pedigrees who reside in an area with a high level of natural radiation demonstrated a significantly increased rate of germ-line point mutations in mtDNA between mothers and their offspring [6], however this result requires confirmation. Saliva samples were collected from the radiation-exposed population and the extracted mtDNA was then sequenced using the Applied Biosystems PRISM 310 Genetic Analyzer. In another study somatic mutation levels were examined in 10 workers from a nuclear fuel facility with ionizing radiation doses >0.9 Sv cumulated over 20-37 years of employment and compared with the mutation levels in a control group of 10 individuals with negligible doses who worked at the same facility. The mtDNA was extracted from blood lymphocytes and sequenced with Beckman Coulter CEQ8000. No significant increase in mtDNA mutation was detected among the high dose radiation workers [7]. However, it is important to note that this study only examined somatic mutations, not the inheritance of germ line mutations. The exclusive maternal inheritance of mtDNA in mammals means that only maternal radiation exposure, and specifically the radiation exposure of the ovary, is significant for the possible formation of inherited mtDNA mutations.
Mammalian cells typically contain over 1,000 mitochondria, and each mitochondrion harbors 2-10 copies of mtDNA [8]. Thus, mtDNA mutations are usually heteroplasmic, with a mixture of mutant and wild-type mtDNA copies within a cell [9]. Mitochondrial DNA mutations could either arise in the female germ line and predispose to cancer or arise in the mtDNAs of the tissues and participate in the tumor progression process. Various studies have shown that mutations in mtDNA can contribute to cancer etiology [10,11] and mtDNA mutations are associated with various types of cancer, including breast cancer [12], prostate cancer [13,14], head and neck cancer [15], and bladder cancer [16,17]. It also has been shown that a mtDNA mutation does not need to reach homoplasmy, i.e., all copies of mtDNA within a cell are mutated, to promote tumor growth [18,19].
Previous studies evaluating mitochondria mutations and maternal radiation had a limited ability to detect low level heteroplasmy due to limitations in the available technology for sequencing at the time the studies were conducted. When the heteroplasmy mutation rate in mtDNA is low (less than 5%), mutations are not readily detected by conventional technologies such as Sanger Sequencing, Affymetrix’s GeneChip Human Mitochondrial Resequencing Array 2.0, or real time polymerase chain reaction (RT-PCR). In recent years, high-throughput sequencing technologies have matured in step with reduced costs for the sequencing procedures. The high coverage of a DNA sequence provides a powerful tool to study the heteroplasmy in mtDNA [20]. In our study we aim to answer the following question with the advantages of high-throughput and high-depth sequencing technology in the framework of a unique experiment design: whether the radiation received by mothers to their ovaries during treatments for childhood cancer can cause an increase in the mtDNA heteroplasmy mutation rate in their children.
2. Material and Methods
2.1. Ethical Statement
Written informed consent of the Danish Families and approval for the study was obtained from the Danish Scientific Ethical Committee and the Danish Data Protection Agency (2001-41-1113). Written informed consent of the Finnish family was obtained as was the approval from the Ethical Committee of the Hospital District of Varsinais-Suomi in Finland.
2.2. Study Cohort
The work is part of an ongoing international study investigating adverse reproductive outcomes in childhood and young adult cancer survivors [21] (www.gcct.org). Briefly, 44 blood samples from 17 Danish families and 1 Finnish family, a total of 26 mother-offspring pairs (some mothers have multiple children), were included in the study. The samples from the Danish families have been used in a variety of previous studies [22,23,24,25] which describe in detail the sample collection and DNA extraction protocols. All of the mothers were survivors of childhood and adolescent cancer who received radiotherapy as part of their initial treatments which resulted in scatter radiation to ovaries. The Danish families were identified from a Danish population-based cohort of 8,759 cancer survivors who had 13,894 children after their cancer diagnosis. The cohort comprised all patients notified to the Danish Cancer Registry with cancer at age < 35 years between 1943 (start of the registry) and 1996 (age < 20 years) or 2002 (age 20-34 years) who subsequently had children. Survivors had to be alive on or born after April 1, 1968, when the national Central Population Register (CPR) was established and a unique personal identification number was assigned for all citizens. All 18 participating families gave informed consent and to ensure anonymity, all family samples were coded at the time of collection. Medical records were retrieved and abstracted for chemotherapy and radiation therapy exposures. Ovarian doses for individual patients were reconstructed based on information available in radiation therapy records. The complete radiation therapy records were submitted to The University of Texas M. D. Anderson Cancer Center, Houston, Texas, USA, for data abstraction and estimation of radiation dose to the ovaries [26]. Methods used for confirmation of maternity and sample identity for the 28 Danish families are described in Tawn et al [25]. There were no exclusions based on non-maternity, though maternity was also tested in the current study by comparing mtDNA sequences. The Finnish family participated in an ongoing study of the genetic consequences of cancer treatments of similar design and following similar protocols as the Danish study [27]. The mother received radiotherapy for bone marrow transplantation to treat acute myelogenous leukemia when 14 years of age and subsequently had two children. The family was of interest because of the high ovarian dose (4.6 Gy).
2.3. Whole Mitochondrial Genome Sequencing
The entire mitochondrial genome was PCR amplified and sequenced using the Illumina GAII high throughput sequencing platform. The raw data were quality controlled, and aligned against the revised Cambridge mitochondrial reference sequence. We achieved high quality data, the median depth for all samples is 3981x. Genetic variants including SNPs, Indels, and heteroplasmy were computed.
2.4. Analysis of heteroplasmy
Examination of the Illumina high-throughput sequencing data revealed that in a few cases the information extracted from forward and reverse strand sequencing was biased. This type of strand bias is observed when the allele frequency at a given position is inconsistent between the two strands. In extreme cases, one of the strands infers a homozygous genotype while the other strand infers a heterozygous genotype, causing an entire or nearly entire heteroplasmy mutation signal coming from one of the two strands. Such signals have been studied in other sequencing data and measurement of such signals has been implemented into Genome Analysis Toolkit (GATK).
A previous study [9] showed widespread heteroplasmy in human mitochondria cells using the Illumina GAII sequencer, however the strand bias issue was not addressed. To account for possible strand bias in our study of heteroplasmy that may be generated in the oocytes following cancer treatments, we focused on heteroplasmy positions that satisfied the following three conditions in each sample: (1) the sequencing coverage of both forward and reverse strands of DNA had to have a depth of 200x or more, (2) the strand bias score had to be less than 1, and (3) the heteroplasmy mutation rate level of each strand had to be at least 1%. We selected 1% as our heteroplasmy cutoff based on previous study by He et. al. [9], in which the maximum heteroplasmy error level was estimated to be 0.82%. By also controlling for strand bias, we further reduced the effect of qPCR error in our study, and thus 1% is a conservative threshold. We calculated heteroplasmy per 1000 base pairs by dividing the number of positions that satisfy all three of the heteroplasmy conditions by the total number of qualified base pairs, i.e., all base pairs satisfy condition (1) and (2) and multiplied by 1000. When the number of reads supporting heteroplasmy mutation exceeds roughly 20% of total reads (adjusting for prior probabilities based on known SNPs), the position was also considered as a SNP.
We fit a mixed-effects model to estimate the effect of radiation therapy on heteroplasmy mutation rate change in children of childhood cancer survivors, adjusting for administered chemotherapy (yes/no) and mother’s age at sample collection. The heteroplasmic mutation rate was calculated per 1000 bps for each eligible sample. The difference in the heteroplasmy mutation rate between each pair of mother and child was used as the outcome in the model. Because some mothers had more than one child enrolled in the study and because siblings are potentially correlated with each other, a random effect was specified to address this correlation structure. This model was used to detect any significant increase of the heteroplasmic mutation rate of the child possibly caused by mother’s prior radiation therapy during childhood or adolescence.
In addition, we examined the linear relationship between the heteroplasmy rate both of mother and child and explanatory variables such as mother’s age at treatment, mother’s age at sample collection, children’s age at sample collection, and radiation dose to ovaries. To evaluate the effect of strand bias, the same analysis was performed on data without controlling for strand bias, and on the same data after applying a strand bias (SB) filter with SB < 1.
3. Results
3.1. Data Quality
All 44 samples sequenced had 100 percent initial coverage of the whole mitochondria genome and an average of 97.51% coverage after filtering based on mapping and base quality score. All 44 samples (18 families) were used for SNP, indel and heteroplasmy analyses. The clinical information for 18 female survivors of childhood and adolescent cancer and their 26 children who provided the 44 samples for this analysis can be found in Table 1. Fifteen out of the 17 Danish families (excluding T10 and T27) were previously studied for minisatellite mutation [25], Sixteen Danish families (excluding T16) were studied for chromosomal radiosensitivity [22,24] and 10 Danish families (T07,T10, T13, T16, T20, T21, T25,T26, T27, T28) were studied for genomic instability [23]. Out of the 18 families with sufficient information for sequencing evaluation, the maternal ovarian doses ranged between 0.03 to 9.2 Gy (mean 1.12 Gy, median 0.285 Gy). The ages of the mothers at radiotherapy ranged from 1 to 20 years and the ages at blood draw ranged from 25 to 61 years.
Table 1.
Clinical information for 18 female survivors of cancer in childhood and adolescence
| Cancer survivor (mother) |
Cancer type | Chemo therapy (yes/no) |
Radiation dose (Gy) |
Age at treatment |
Year of treatment |
Mother’s age at sample collection |
Number of Children |
|---|---|---|---|---|---|---|---|
| Fin | Leukemia | yes | 4.6 | 15 | 1985 | 39 | 1 |
| T07 | Hodgkin | yes | 0.08 | 19 | 29 | 1 | |
| T10 | Wilms’ Tumor Malignant | yes | 0.69 | 4 | 25 | 1 | |
| T13 | Lymphoma | no | 0.05 | 20 | 30 | 1 | |
| T16 | Hodgkin | yes | 0.29 | 20 | 33 | 1 | |
| T20 | Hodgkin | yes | 0.29 | 17 | 33 | 2 | |
| T21 | Hodgkin | yes | 0.09 | 19 | 35 | 1 | |
| T25 | Neuroblastoma | yes | 9.2 | 1 | 36 | 1 | |
| T26 | Hodgkin | yes | 0.08 | 19 | 36 | 2 | |
| T27 | Wilms’ Tumor | yes | 1.2 | 2 | 36 | 1 | |
| T28 | Wilms’ Tumor | yes | 1.7 | 2 | 34 | 1 | |
| T37 | Hodgkin | yes | 0.09 | 14 | 34 | 2 | |
| T38 | Hodgkin Malignant | yes | 0.09 | 15 | 41 | 2 | |
| T39 | Lymphoma | no | 0.03 | 10 | 61 | 1 | |
| T41 | Hodgkin Malignant | no | 0.48 | 61 | 2 | ||
| T45 | Lymphoma | no | 0.1 | 16 | 55 | 1 | |
| T46 | Wilms’ Tumor | yes | 0.63 | 3 | 32 | 2 | |
| T54 | Hodgkin | no | 0.24 | 16 | 54 | 3 |
Fifteen out of the 17 Danish families (excluding T10 and T27) were studied for minisatellite mutations by Tawn et al [25], Sixteen Danish families (excluding T16) were studied for chromosomal radiosensitivity by Curwen et al [22] and 10 Danish families (T07,T10, T13, T16, T20, T21, T25,T26, T27, T28) were studied for genomic instability by Tawn et al [23].
3.2. SNPs and Indels
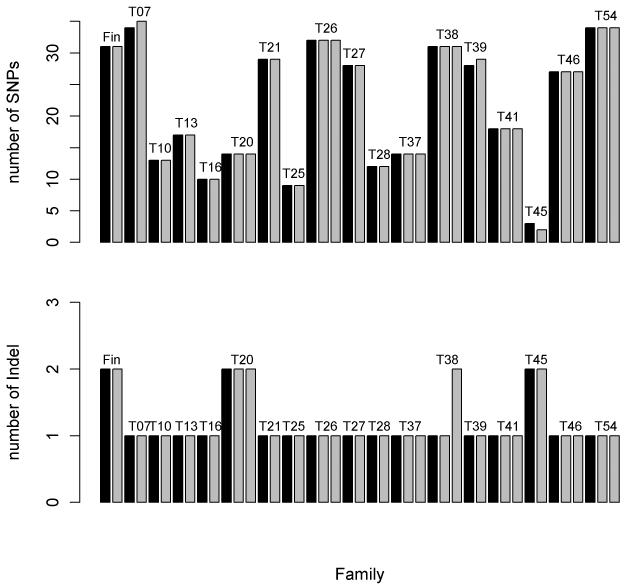
SNPs and indels were called from GATK’s The Unified Genotyper [28] developed by Broad Institute, and glfMultiple developed by University of Michigan. From the consensus SNP list generated from The Unified Genotyper and glfMultiple, we observed near perfect consistency between mother and offspring in term of SNP and indel inheritance as shown in Fig. 1. If a SNP or indel is observed in a mother, it is almost always observed in all of her offspring. A discrepancy occurred only when there were too few reads to make a SNP or indel call at a given position or when there was merely a shift in the magnitude of heteroplasmy mutation level. For example, at position 15,355 of sample 1032QC25 (mother) and sample 1032QC26 (offspring) the heteroplasmy mutation rate of the mother is at 9% which is not considered as a SNP by either variance caller; however the offspring at the same site is considered as a SNP by the both variance callers with heteroplasmy mutation rate at 27%. All of the SNPs observed have high quality confidence Phred scores. One hundred percent of the SNPs had Phred quality score greater than 100 (probability of being wrong is less than 1×10−10), 99% of the SNPs have a Phred quality score greater than 1000 (probability of being wrong is less than 1×10−100). We also obtained haplogroup information (Table S1) for each sample by checking the SNP result against phylotree.org’s mtDNA phylogeny tree [29]. The haplogroup information provided evidence for the subjects’ European ancestry and further confirmed the maternal relatedness.
Fig. 1. Distribution of SNPs and Indel.
The SNP (upper) and indel (lower) are nearly 100 percent concordant within each family. The only exception is when the level of mutation observed is not strong enough to be called as a SNP. For example, in family T07, the offspring has one more SNP not found in mother, however heteroplasmy mutation of the same direction was observed in the mother at the same position. In this case the mutation level was not strong enough to be detected as a SNP by the variant calling programs.
We observed a total of 1031 SNPs across 44 sequenced samples; the ti/tv ratio was 38.65:1 (1005 transitions vs. 26 transversions), heavily favoring transitions. The dominance of transitions over transversions has long been documented in both human and animal mtDNA [30]. A previous study showed when considering homoplasmic polymorphisms appearing in over 0.1% of the human mtDNA sequences deposited in GenBank, the ti/tv ratio was 21.2:1 [31]. Our study shows, at least for heteroplasmy mtDNA variations within our study population, transitions are even more heavily biased than previously reported. We identified 17 SNPs that are not listed on the current MITOMAP database (www.mitomap.org). Eleven of 17 SNPs were reported by previous studies but in a very small number of individuals [31], while 6 are novel (Table 2). A majority of the 17 are unique to each pedigree.
Table 2.
Novel SNPs Observeda
| rCRS | |||
|---|---|---|---|
| Family | positionb | Reference | Alternative |
| T41 | 4135 | T | C |
| T27 | 6434 | C | T |
| T13 | 7313 | C | T |
| T26 | 7749 | T | C |
| T37 | 9629 | A | C |
| T10 | 9631 | T | G |
The SNPs were called using GATK’s Unified Genotyper and glfMultiple. A consensus of list of SNPs was produced by taking the consensus of results from the 2 programs.
Revised Cambridge Reference Sequence
3.3. Heteroplasmy and Inheritance
Our findings of heteroplasmy mutation for each subject are summarized in Table 3. Out of the 18 families, 9 families (56%) showed evidence of heteroplasmy inheritance by having at least one offspring inheriting at least one heteroplasmy site from his/her mother. Furthermore, we calculated Spearman’s correlation coefficient for mutation levels at all positions of the mtDNA sequence of all possible pairs. The median correlation of mother-offspring pairs was 0.6474 while the median correlation of non-related pairs was −0.0456.
Table 3.
Heteroplasmy Positions1
| Family ID |
Mother | Offspring | Mother heteroplasmy rCRS Positions2 |
Offspring Heteroplasmy Positions2 |
Inheritance |
|---|---|---|---|---|---|
| Fin | 1032QC50 | 1032QC51 | 574, 10599, 10677 |
15692 | N |
| T07 | 1032QC25 | 1032QC26 | 195, 15355 | 195, 234, 15355 | Y |
| T10 | 1032QC27 | 1032QC28 | 316 | 1329, 3542, 5036, 7615, 11664, 15871 |
N |
| T13 | 1032QC31 | 1032QC32 | 3649,13928, 16092 |
72, 13928, 16519 | Y |
| T16 | 1032QC01 | 1032QC02 | 15734 | 24,013,102,346,715,700 | |
| T20 | 1032QC03 | 1032QC04 | NA | 16360 | N |
| T21 | 1032QC06 | 1032QC07 | NA | NA | N |
| T25 | 1032QC11 | 1032QC12 | 16234 | NA | N |
| T26 | 1032QC13 | 1032QC14 | 7762, 12973 | 12973 | Y |
| 1032QC13 | 1032QC15 | 7762, 12973 | 6131, 7279, 7346, 7762, 9173, 9762, 12973, 14554 |
||
| T27 | 1032QC16 | 1032QC17 | 316, 8301 | 316, 6182, 16192 | Y |
| T28 | 1032QC19 | 1032QC20 | NA | NA | N |
| T37 | 1032QC22 | 1032QC23 | 310 | 310, 316 | Y |
| 1032QC22 | 1032QC24 | 310 | 3243 | N | |
| T38 | 1032QC37 | 1032QC38 | 608 | 234, 16189 | N |
| 1032QC37 | 1032QC39 | 608 | NA | N | |
| T39 | 1032QC40 | 1032QC41 | 4906, 12092, 14148, 14971 |
12092 | Y |
| T41 | 1032QC42 | 1032QC43 | 4674, 4959 | 316, 16129 | N |
| 1032QC42 | 1032QC44 | 4674, 4959 | 5823 | N | |
| T45 | 1032QC45 | 1032QC46 | 146, 316 2107, 6293, 7161, 14666 |
146, 6293, 14178, 14299 |
Y |
| T46 | 1032QC47 | 1032QC48 | 16399 | 15218, 16256, 16270, 16399 |
Y |
| 1032QC47 | 1032QC49 | 16399 | 3645, 16399 | Y | |
| T54 | 1032QC33 | 1032QC34 | 4227, 6876, 9967, 15872 |
4227 | Y |
| 1032QC33 | 1032QC35 | 4227, 6876, 9967, 15872 |
195, 4227 | Y | |
| 1032QC33 | 1032QC36 | 4227, 6876, 9967, 15872 |
4227, 8430 | Y |
The heteroplamy are filtered based on following criteria: a. Strand Bias score ≤1; b. Each strand has reads ≥ 200; c. Heteroplasmy Level is ≥1%
Revised Cambridge Reference Sequence
While there is strong evidence showing heteroplasmy inheritance within the pedigrees, our data also suggests that heteroplasmy sites are usually unique to the pedigree. However, there were some exceptions where a heteroplasmy site is shared by more than one family. For example, the heteroplasmy site at position 316 appeared in 5 different families (6 individuals). One possible explanation is that position 316 is located right after a large poly-c tract (301-aaccccccctcccccgcttc-320). Poly-c tract has been reported to be unstable and to cause mutations that extend the poly-c tract[32,33]. All individuals observed with heteroplasmy at position 316 have the minor allele C. We summarized the heteroplasmy positions shared between at least 2 families in Table 4. The position 316 is clearly affected by neighboring poly-c tracts, the other three were either located on other repetitive sequence or caused by low GC content. This leads us to believe that heteroplasmy in mtDNA is highly related to repetitive sequence features such as poly-c tracts. We also computed Ti/Tv ratio for all heteroplasmy sites and found that transitions were still highly favored over transversion with a transition transversion ratio of 14.56:1.
Table 4.
Common heteroplasmy sites observeda
| rCRS positionb |
Families | Individuals | Sequence | Function | Occurence in 5140 GenBank sequencesc |
|---|---|---|---|---|---|
| 195 | 2 | 3 | gcgaacatacT/Ctactaaagtg | D loop, HVS21 |
973 |
| 234 | 2 | 2 | tgtaggacatA/Tataataacaa | D loop, HVS2 |
19 |
| 316 | 5 | 6 | cccctcccccG/Ccttctggcca | D loop, HVS2 |
73 |
| 16256 | 1 | 1 | aactccaaagC/Tcacccctcac | D loop, HVS1 |
0 |
Heteroplamy positions that appeared in 2 different families.
Revised Cambridge Reference Sequence
5140 Mitochondrial sequences were studied by Pereira et al [8], denoting the number of appearances of each SNP in the GenBank database.
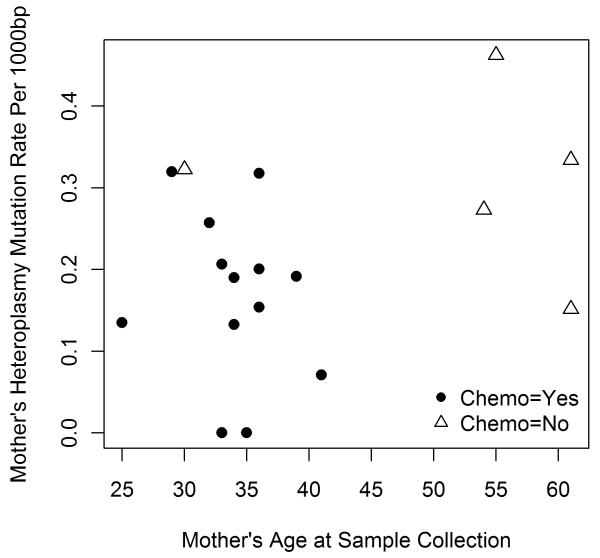
Analysis using data without controlling for strand bias did not detect any significant difference between mother and child on heteroplasmy rate or any of the explanatory variables (Table 5). After controlling for strand bias (SB<1), we found a marginal statistically significant association between mother’s age at sample collection and the heteroplasmy mutation rate difference between mother and child (p=0.0712) (Table 5). No significant association was detected between heteroplasmy mutation rate difference and radiation dose among mother-child pairs where mother received childhood radiation therapy (p= 0.2705). Neither was a significant association found between chemotherapy (yes/no) and heteroplasmy mutation rate difference (p=0.8492). We also performed the analyses restricted to children’s heteroplasmy mutation rate and mother’s heteroplasmy mutation rate as outcomes. No significant associations were detected between radiation dose and outcomes (Table 5). Chemotherapy was not associated with heteroplasmy mutation rate when the analysis was restricted to children only. A significant p-value, however, was observed between chemotherapy and mother’s heteroplasmy mutation rate using a linear model (Table 5). Fig. 2 shows the relationship between mother’s age at sample collection and mother’s heteroplasmy mutation rate. Among the total of 18 cancer survivors, 4 patients were above the age of 50 and 14 patients were below the age of 44 at the time of sample collection. Interestingly, all four older patients were not treated with chemotherapy and 13 out of the 14 younger (age<45) patients received chemotherapy. The results of multivariate analysis confirms that the significant association between chemotherapy and mother’s heteroplasmy mutation rate is a result of the confounding effect of age at sample collection, rather than the effect of chemotherapy itself. This different age distribution in chemotherapy treatment may be due to the rapid development of chemotherapeutical drugs in the 1950s as well as differences in the types of malignancies being treated.
Table 5.
P-values for associations of heteroplasmy rate with radiation, chemotherapy, and age
| SB cutoff | |||
|---|---|---|---|
| Mixed model 1c | NAa | 1b | |
| Outcome | Rate difference between mother and children | ||
| Covariates | Received Chemotherapy | 0.5220 | 0.8492 |
| Radiation dose | 0.5838 | 0.2705 | |
| Mother’s age at sample collection | 0.6323 | 0.0712 | |
| Mixed model 2 | |||
| Outcome | Child’s heteroplasmy mutation rate | ||
| Covariates | Received Chemotherapy | 0.2113 | 0.4197 |
| Radiation dose | 0.9636 | 0.3349 | |
| Mother’s age at sample collection | 0.1247 | 0.1514 | |
| Linear model 1d | |||
| Outcome | Mother’s heteroplasmy mutation rate | ||
| Covariates | Received Chemotherapy | 0.0215e | 0.0292e |
| Radiation dose | 0.4717 | 0.8962 | |
| Mother’s age at sample collection | 0.2047 | 0.3731 | |
Not controlling for strand bias
Filtering by strand bias ≤ 1
Mixed model was used when adjusting for correlation within family
Linear model was used when there was no need to adjust for correlation within family
Mother’s heteroplasmy rate is strongly associated with chemo status. However, it is due to a confounding effect of age at sample collection. The detail is shown in Fig. 2, and detailed explanation is provided in section 3.3 paragraph 3.
Fig. 2. Mother’s heteroplasmy mutation per 1000bp VS mother’s age at sample collection.
Chemo is a confounder of age. For patients who did not receive chemo therapy 4 out of 5 were over age 50, yet all patients who received chemo therapy were under age 45 at sample collection.
We calculated Spearman’s correlation coefficients between outcome variables (mother’s heteroplasmy mutation rate, child’s heteroplasmy mutation rate, and their rate difference) and explanatory factors (mother’s age at sample collection, children’s age at sample collection, mother’s treatment age, and radiation dose) (Table 6). Using data without controlling for strand bias, no significant correlation was detected. However, for data filtered for strand bias, mother’s age at sample collection was significantly correlated with mother’s heteroplasmy mutation rate (r=0.5179 p=0.0277) There was also a significant correlation between child’s age at sample collection and mother’s heteroplasmy mutation rate (r=0.5656 p=0.0026).
Table 6.
Association between heteroplasmy rates and explanatory factorsa
| SB cutoff |
Mother’s age at treatment |
Mother’s age at sample collection |
Children age at sample collection |
Radiation dose | |
|---|---|---|---|---|---|
|
Mother’s heteroplasmy rate |
NAb | 0.1850 (0.4623) |
0.0725 (0.7750) |
0.2424 (0.2327) |
−0.0641 (−0.8003) |
| 1c15 | 0.2388 (0.3405) |
0.5179 (0.0277) |
0.5656 (0.0026) |
−0.3340 (0.1755) |
|
| Child’s heteroplasmy rate | NAb | 0.0228 (0.9120) |
−0.1453 (0.4788) |
0.0466 (0.8210) |
0.0540 (0.7934) |
| 1c | 0.1749 (0.3929) |
−0.3585 (0.0721) |
−0.2202 (0.2798) |
−0.0804 (0.6962) |
|
Data presented are r (p values)
Not controlling for strand bias
Filtering b□ strand bias ≤ 1
4. Discussion
The health and life style of childhood cancer survivors and their offspring has been a genuine concern in society and has been studied intensely over the past few decades. According to a review article published in Journal of Clinical Oncology in 2009, numerous articles have been published in the scientific literatures by researchers devoted to the study of pediatric cancer treatment and its subsequent effect on health, including behavioral, socio-demographic outcomes, and the risk of developing cancer and other disease in offspring [34]. Among all cancer patients, half receive some type of radiation therapy during the course of their treatment (www.nci.com). Radiation can both cure malignancies and also induce second primary tumors and chromosome aberrations. Inherited radiation-induced germline effects, whilst evident in animal studies, have yet to be confirmed in human studies [35]. Minisatellite mutations have been reported in offspring of paternally irradiated populations in some but not all human studies [36]. The samples used in this study were taken as part of an ongoing international study investigating adverse reproductive outcomes in childhood and young adult cancer survivors [21]. Analysis of eight hypervariable minisatellite loci in 100 families where one parent was a survivor of childhood and young adult cancer who had received radiotherapy revealed no evidence that preconceptional radiation exposure increased the germline minisatellite mutation rate [25]. Moreover, preliminary findings on approximately 25,000 of cancer survivors and 6,500 offspring indicated that cancer treatments including radiation therapy did not carry a significant risk for inherited genetic disease in offspring born to a cancer survivor after therapy [21].
Combined with the power of high depth sequencing technology and our unique experimental design we were able to study the heteroplasmy mutation in mtDNA to an extent not possible in years past. We found that mtDNA mutation rate is positively correlated with age of mother at sample collection which is consistent with previous finding by Sondheimer et al. [37]. This result indirectly supports the quality of our sequence data. Our study did not find SNP disagreement between mother and child, though a few heteroplasmy mutation rate level shifts were detected. There was no significant association found between the radiation therapy dose for the mother and the mtDNA heteroplasmy mutation rate in the child. It is possible that mtDNA heteroplasmy mutation rates differ among mature oocytes after radiation exposure to the ovaries when the primordial germ cell differentiates to oocytes [38]. Therefore, the level of mtDNA mutations in offspring may not highly correlate to the level of radiation exposure to the ovaries of the mother. Our analysis suggests childhood radiation therapy that results in ovarian exposures among female patients does not increase SNP or heteroplasmy mutation rate in mtDNA.
The high-throughput sequencing technology is currently the best tool to study general mtDNA heteroplasmy (as opposed to targeting specific known heteroplasmic sites). However, it is not flawless. There are two major areas we believe can be improved with high depth sequencing technology to enhance accuracy. First, the variances of raw read count of pooled and barcoded samples are high, which can cause uneven coverage between samples and subsequently result in inconsistent heteroplasmy call between samples. The large variance of raw read count is partially related to the variation of sample quality, but the major cause is the uneven amplification between pooled samples. A more sophisticated library preparation protocol is required to amplify each sample individually before barcoding and pooling, and then individually amplified samples should produce more evenly distributed read count. Second, because of strand bias produced from either sequencing errors or alignment errors, we cannot fully trust the allele frequency information when the forward strand and reverse strand are reporting inconsistent variance calls. Therefore, we were forced to define filter criteria in order to discard positions with high strand bias scores. We have observed similar strand biases in other types of high-throughput sequencing data such as exome sequencing, RNA-seq.
Highlights.
We sequenced mitochondrial genome of 18 female childhood cancer survivors who received radiation therapy and their children.
No SNP difference was observed between mother and their children.
No insertion or deletion difference was observed between mother and their children.
No increase of heteroplasmy mutation rate was observed in the offspring comparing their mother.
Age is positively correlated with heteroplasmy mutation rate.
Acknowledgements
We thank the families for participating in this study and Brian Møllgren, Rigshospitalet, for collection of blood samples. Radiation dosimetry estimates were provided by Rita Weathers, Catherine Kasper and Susan Smith, University of Texas M.D. Anderson Cancer Center. We would like to thank Regina Courtney and Melanie Robinson for their excellent work for preparing the samples. DNA sample preparation was conducted at Survey and Biospecimen Shared Resource.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None.
References
- 1.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 2.Verma M, Kumar D. Application of mitochondrial genome information in cancer epidemiology. Clin Chim Acta. 2007;383:41–50. doi: 10.1016/j.cca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Modica-Napolitano JS, Kulawiec M, Singh KK. Mitochondria and human cancer. Curr Mol Med. 2007;7:121–131. doi: 10.2174/156652407779940495. [DOI] [PubMed] [Google Scholar]
- 4.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 6.Forster L, Forster P, Lutz-Bonengel S, Willkomm H, Brinkmann B. Natural radioactivity and human mitochondrial DNA mutations. Proc Natl Acad Sci U S A. 2002;99:13950–13954. doi: 10.1073/pnas.202400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilding CS, Cadwell K, Tawn EJ, Relton CL, Taylor GA, et al. Mitochondrial DNA mutations in individuals occupationally exposed to ionizing radiation. Radiat Res. 2006;165:202–207. doi: 10.1667/rr3494.1. [DOI] [PubMed] [Google Scholar]
- 8.Robin ED, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol. 1988;136:507–513. doi: 10.1002/jcp.1041360316. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Wu J, Dressman DC, Iacobuzio-Donahue C, Markowitz SD, et al. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature. 464:610–614. doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 11.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canter JA, Kallianpur AR, Parl FF, Millikan RC. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005;65:8028–8033. doi: 10.1158/0008-5472.CAN-05-1428. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann PC, Gillespie JW, Charboneau L, Bichsel VE, Paweletz CP, et al. Mitochondrial proteome: altered cytochrome c oxidase subunit levels in prostate cancer. Proteomics. 2003;3:1801–1810. doi: 10.1002/pmic.200300461. [DOI] [PubMed] [Google Scholar]
- 14.Petrosillo G, Di Venosa N, Ruggiero FM, Pistolese M, D’Agostino D, et al. Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim Biophys Acta. 2005;1710:78–86. doi: 10.1016/j.bbabio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Zhou S, Chang SS, McFate T, Verma A, et al. Mitochondrial mutations contribute to HIF1alpha accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenease kinase 2 in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15:476–484. doi: 10.1158/1078-0432.CCR-08-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- 17.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 18.Lewis PD, Baxter P, Paul Griffiths A, Parry JM, Skibinski DO. Detection of damage to the mitochondrial genome in the oncocytic cells of Warthin’s tumour. J Pathol. 2000;191:274–281. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH634>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Sharma LK, Li H, Xiang R, Holstein D, et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. 2009;18:1578–1589. doi: 10.1093/hmg/ddp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang S, Huang T. Characterization of mitochondrial DNA heteroplasmy using a parallel sequencing system. Biotechniques. 2010;48:287–296. doi: 10.2144/000113389. [DOI] [PubMed] [Google Scholar]
- 21.Boice JD, Jr., Tawn EJ, Winther JF, Donaldson SS, Green DM, et al. Genetic effects of radiotherapy for childhood cancer. Health Phys. 2003;85:65–80. doi: 10.1097/00004032-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Curwen GB, Winther JF, Tawn EJ, Smart V, Whitehouse CA, et al. G(2) chromosomal radiosensitivity in Danish survivors of childhood and adolescent cancer and their offspring. Br J Cancer. 2005;93:1038–1045. doi: 10.1038/sj.bjc.6602807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawn EJ, Whitehouse CA, Winther JF, Curwen GB, Rees GS, et al. Chromosome analysis in childhood cancer survivors and their offspring--no evidence for radiotherapy-induced persistent genomic instability. Mutat Res. 2005;583:198–206. doi: 10.1016/j.mrgentox.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curwen GB, Cadwell KK, Winther JF, Tawn EJ, Rees GS, et al. The heritability of G2 chromosomal radiosensitivity and its association with cancer in Danish cancer survivors and their offspring. Int J Radiat Biol. 2010;86:986–995. doi: 10.3109/09553002.2010.496027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tawn EJ, Rees GS, Leith C, Winther JF, Curwen GB, et al. Germline minisatellite mutations in survivors of childhood and young adult cancer treated with radiation. Int J Radiat Biol. 2011;87:330–340. doi: 10.3109/09553002.2011.530338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stovall M, Donaldson SS, Weathers RE, Robison LL, Mertens AC, et al. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–552. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Madanat-Harjuoja LM, Malila N, Lahteenmaki PM, Boice JD, Jr., Gissler M, et al. Preterm delivery among female survivors of childhood, adolescent and young adulthood cancer. Int J Cancer. 2010;127:1669–1679. doi: 10.1002/ijc.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 2009;30:E386–394. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 30.Lanave C, Tommasi S, Preparata G, Saccone C. Transition and transversion rate in the evolution of animal mitochondrial DNA. Biosystems. 1986;19:273–283. doi: 10.1016/0303-2647(86)90004-3. [DOI] [PubMed] [Google Scholar]
- 31.Pereira L, Freitas F, Fernandes V, Pereira JB, Costa MD, et al. The diversity present in 5140 human mitochondrial genomes. Am J Hum Genet. 2009;84:628–640. doi: 10.1016/j.ajhg.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malyarchuk BA. The role of nucleotide context in the induction of mutations in human mitochondrial DNA genes. Genetika. 2005;41:385–390. [PubMed] [Google Scholar]
- 33.Kieleczawa J. Fundamentals of sequencing of difficult templates--an overview. J Biomol Tech. 2006;17:207–217. [PMC free article] [PubMed] [Google Scholar]
- 34.Leisenring WM, Mertens AC, Armstrong GT, Stovall MA, Neglia JP, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyrobek AJ, Mulvihill JJ, Wassom JS, Malling HV, Shelby MD, et al. Assessing human germ-cell mutagenesis in the Postgenome Era: a celebration of the legacy of William Lawson (Bill) Russell. Environ Mol Mutagen. 2007;48:71–95. doi: 10.1002/em.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouffler SD, Bridges BA, Cooper DN, Dubrova Y, McMillan TJ, et al. Assessing radiation-associated mutational risk to the germline: repetitive DNA sequences as mutational targets and biomarkers. Radiat Res. 2006;165:249–268. doi: 10.1667/rr3506.1. [DOI] [PubMed] [Google Scholar]
- 37.Sondheimer N, Glatz CE, Tirone JE, Deardorff MA, Krieger AM, et al. Neutral mitochondrial heteroplasmy and the influence of aging. Human molecular genetics. 2011;20:1653–1659. doi: 10.1093/hmg/ddr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease--its impact, etiology, and pathology. Curr Top Dev Biol. 2007;77:113–155. doi: 10.1016/S0070-2153(06)77005-3. [DOI] [PubMed] [Google Scholar]