Abstract
Deep brain stimulation of the subthalamic nucleus (STN DBS) in Parkinson disease (PD) improves motor function but has variable effects on mood. Little is known about the relationship between electrode contact location and mood response. We identified the anatomical location of electrode contacts and measured mood response to stimulation with the Visual Analog Scale in 24 STN DBS PD patients. Participants reported greater positive mood, decreased anxiety and apathy with bilateral and unilateral stimulation. Left DBS improved mood more than right DBS. Right DBS-induced increase in positive mood was related to more medial and dorsal contact locations. These results highlight the functional heterogeneity of the STN.
Keywords: Parkinson disease, deep brain stimulation, mood
INTRODUCTION
Although the motor benefits of deep brain stimulation in the subthalamic nucleus (STN DBS) for Parkinson disease (PD) are well documented,1 the effects on mood are highly variable and even detrimental in some instances.2–4 The psychological outcomes of STN DBS have included post-surgical reductions in depression and anxiety symptoms,5 but STN DBS also may precipitate or exacerbate symptoms of (hypo)mania, depression, anxiety, apathy, and psychosis.6 However, post-surgical changes in mood may reflect not only the effects of stimulation itself but also continued disease progression, changes in medication, pre-surgical psychiatric history,7;8 microlesion effects from electrode implantation, and psychosocial adjustment and adaptation to DBS.4 Therefore, recent studies have begun to focus on the effects of STN stimulation itself on mood.
The few systematic investigations on the effects of bilateral STN stimulation have found reductions in depression, psychiatric symptoms, and apathy.9;10 One study reported significantly reduced depression and psychiatric symptoms when bilateral stimulators were turned on, compared to OFF. 9 Another study also found reduced ratings of apathy with stimulators on;10 however, examination of within-subject effects revealed that apathy improved in some, stayed the same in others, and worsened in one patient.10 Individual case studies have reported more dramatic and variable effects of stimulation, including uncontrolled fits of laughter,11 (hypo)mania12–14 and severe transient depressive symptoms.15;16 These seemingly inconsistent findings may be due, in part, to differences in electrode contact location.13;15 The different sections of the STN are anatomically connected to different regions of cortex, supporting the hypothesis that the specific location of stimulation may be a mediating factor in the motor and non-motor behavioral responses to STN DBS.
Current models of STN circuitry propose that functionally segregated sections of the STN subserve motor, emotion, and cognitive processing.17–19 Although the surgical procedure targets the dorsal portion of the STN to provide optimal motor benefit, the span of electrode contact locations is greater than the size of the STN; thus, there is the potential for considerable variability in active contact location which may account for individual variability in mood response. Unfortunately, few studies have examined the role of contact location on motor and non-motor responses to STN DBS.
One recent study compared the effects of anatomically defined contact locations on motor and cognitive functions and demonstrated that stimulation of the ventral rather than dorsal STN region impaired response inhibition, as measured with the Go-No-Go task, yet both dorsal and ventral stimulation improved motor function, as measured by the Unified Parkinson Disease Rating Scale (UPDRS) motor score (Part III).20 These findings support the notion of functional heterogeneity of the STN in regards to motor and cognitive functioning. Similar investigations on mood have demonstrated that stimulation of the most ventral electrode contacts were more likely to affect mood.12;21–23 However, the studies on mood provided limited information on the anatomical site of stimulation, usually defining location only relative to the contact with optimal motor benefit or choosing the most ventral contact without confirming anatomical location.12;21–23 Additional limitations of these studies include focusing primarily on patients with unilateral STN DBS21;22 precluding the ability to test for hemispheric differences, small sample sizes (e.g., N = 2 and N = 5),12;22 and assessment of patients while on medication.23
The purpose of this study was to 1) examine the effects of unilateral and bilateral STN stimulation of the clinically determined optimal electrode contact on mood response and 2) investigate the relationship between the anatomically defined electrode contact location and mood responses. For this study we assessed the effects of DBS on mood in PD patients with bilateral STN DBS, after overnight withdrawal of medication, across multiple stimulation conditions: bilateral off (DBS OFF), only left stimulation (left DBS), only right stimulation (right DBS), and bilateral stimulation (bilateral DBS). Unlike previous studies on the effects of contact location on mood, we did not manipulate location of stimulation in the present study; rather, we identified the precise anatomical location of the clinically determined optimal electrode contact using a validated atlas registration procedure24.
METHODS
Participants
Forty-two individuals with PD and bilateral STN DBS were recruited from the Movement Disorders Center at Washington University in St Louis. Participants met diagnostic criteria for clinically definite PD based on established criteria25;26 including benefit from levodopa, and had no evidence of dementia based on clinical history and performance on either the MMSE (≤ 24/30) or the Mattis Dementia Rating Scale (≤ 130/148) obtained as part of the pre-surgical neuropsychological evaluation. Participants had no evidence of other serious neurologic diagnoses by history or examination (e.g. stroke, head injury). DBS therapy was optimized clinically before recruitment in the study. This study was approved by the Human Research Protection Office at Washington University in St. Louis and written informed consent was obtained for all participants.
Neuroimaging
As part of standard clinical care, pre-operative magnetic resonance (MR) images were acquired with a Siemens Vision 1.5T scanner and included two T2-weighted turbo spin-echo sequences: one acquired in transverse planes (TR = 8904 ms, TE = 90 ms, flip angle = 180, 53 planes, 1 × 1 × 2 mm voxels) and one acquired in coronal planes (TR = 3700 ms, TE = 96 ms, flip angle = 180, 19 slices, 1 × 1 × 2 mm voxels). Head movement was prevented during MR imaging by a Leksell stereotactic frame anchored to the skull. Post-operative computed tomography (CT) images were acquired after removal of the stereotactic frame with one of three Siemens Somatom scanners (Definition 64, Sensation 64 or Plus 4) with 120 kV, 206–320 mAs and 0.5 × 0.5 × 1 mm or 0.5 × 0.5 × 2 mm voxels. CT images were examined for movement, recognizable by discontinuities along the skull in coronal and sagittal views. CT scans with significant movement were excluded.
Contact Localization
Image processing and atlas registration procedures were performed as described previously.24 Briefly, fiducial structures identified on the MR image and the electrode position from the CT image were used to localize the position of each contact in Mai atlas space.27 Based on the electrode configuration and stimulator settings used in our study, it is likely that current activates neurons and axons within about a 2 mm radius from the center of the contact.28 Thus, a reasonable approximation of the likely suprathreshold effect of monopolar stimulation at a given contact is within a 2 mm radius sphere centered on the contact itself. Bipolar or multipolar settings could alter the pattern and extent of current spread and thereby which structures and pathways are affected; therefore, only participants with monopolar settings were included in these analyses.
Stimulation Variables
We used the clinically determined optimal electrode contact and settings to test the effects of stimulation on mood response (see Table 1).
Table 1.
Clinical and demographic information.
| PD | |
|---|---|
| N = 24 | |
| Sex | 5 F, 19 M |
| Age (years) | 59.54 (7.79) |
| Education (years) | 15.72 (3.52) |
| Duration PD (years) | 14.44 (4.66) |
| Months since surgery | 13.32 (12.72) |
| UPDRS Motor Score (OFF/OFF) | 40.33 (11.24) |
| Stimulation Settings | Left DBS | Right DBS |
|---|---|---|
| Voltage (volts) | 2.74 (.49) | 2.75 (.47) |
| Pulse Width (microseconds) | 60 | 60 |
| Rate (Hz) | 185 | 185 |
| Impedance (ohms) | 994.5 (168.17) | 1078.5 (223.16) |
Note: Values represent means (standard deviation). UPDRS = Unified Parkinson Disease Rating Scale.
Behavioral Protocol
Participants withheld PD medications overnight and were in the “practical-defined off state” 29 at the time of testing. Participants completed motor and mood assessments in four conditions on the same day: 1) bilateral off (DBS OFF); 2) only left unilateral stimulation (left DBS); 3) only right unilateral stimulation (right DBS); and 4) bilateral stimulation (bilateral DBS). Data were collected in a counterbalanced, double-blind manner. Participants were studied at least 42 minutes after changing stimulator conditions to ensure near-steady state motor status during testing.30;31 Motor signs were measured with the Unified Parkinson Disease Rating Scale (UPDRS) motor subscale by a blinded, validated Movement Disorders specialist that had undergone training and validation requiring intraclass correlations > 0.90 compared to senior investigators trained in UPDRS. Mood response was measured with a computerized version of the Visual Analog Scale (VAS) which is a valid and reliable self-report measure of current mood state32 that has been previously used in studies on PD21;33. For each stimulation condition, participants were presented with two opposite adjectives and asked to mark how they felt at that moment, on that dimension. The result is quantified as the distance along a horizontal line between the two adjectives (range 0–100mm). Adjectives were chosen based on a Circumplex Model of emotion34, which posits that emotional states can be represented by a 2-dimensional circle in which the proximity to the circumference indicates the intensity of that emotion and the location around the circle indicates which emotions are related or opposite of each other. The two primary dimensions in this model are valence (the pleasant or unpleasantness of an emotional state; also referred to as positive and negative affect) and emotional arousal (from low arousal to high arousal). The items included in the VAS were chosen to sample the following operationally defined mood states: valence (average of responses to sad/happy and grouchy/cheerful items), emotional arousal (average of tranquil/intense, and passive/aroused items), apathy (motivated/apathetic item) and anxiety (average of responses to calm/nervous, relaxed/distressed, and calm/tense items).
Analyses
To determine if stimulation condition affected mood response overall, we ran a repeated measures MANOVA with stimulation condition as the repeated measure and each of the mood state variables (valence, emotional arousal, anxiety, and apathy) as the multiple measures. Based on the results of this omnibus test, univariate repeated measures ANOVAs were used to determine which mood states were affected by stimulation. One-sample t-tests were used to determine if stimulation-induced change in mood response from DBS OFF was significantly different from 0. Chi-square tests were used to assess the proportion of participants reporting DBS-induced improved mood versus those reporting no improvement (no change or a decline in mood). To determine possible laterality effects of stimulation, paired t-tests were used to compare mood ratings across unilateral stimulation conditions. Pearson correlations were used to test the relationship between mood response and contact location (Mai atlas coordinates, separately along each axis). Due to the rank order nature of the UPDRS motor ratings, non-parametric analyses (Wilcoxon signed rank tests, Spearman rank correlations) were used for UPDRS motor ratings. The threshold for statistical significance was set at p < .05.
RESULTS
Participants
Only data from participants with adequate pre-operative and post-operative MR and CT scans for atlas registration and bilateral monopolar settings were analyzed. Of the 42 participants who completed mood ratings, the following were excluded: 11 did not have contact location information due to inadequate neuroimaging data or poor atlas registration due to enlarged ventricles, 5 had bipolar or multipolar contact settings, and 2 did not complete mood ratings for all four stimulation conditions. Analyses are therefore based on 24 participants with identified contact locations, monopolar settings, and complete mood data (see Table 1 for clinical and demographic information).
Clinical Contact Locations
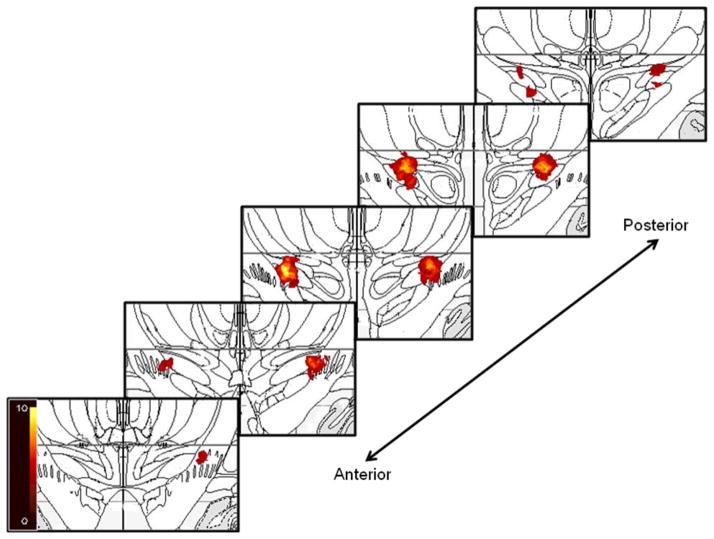
The majority of clinically optimal contacts resided in and near the dorsal portion of the STN and extended into the zona incerta. There was more variability in location along the anterior-posterior and medial-lateral axes for the right DBS than left DBS contacts (see Figure 1). Stimulation variables are summarized in Table 1.
Figure 1.
Location and distribution of the clinically chosen optimal STN DBS electrode contacts superimposed on coronal slices from the Mai atlas 27. For illustration purposes, a 2 mm radius sphere was placed on the center of each active contact as a visual estimate of current spread 28. The scale bar indicates the number of points (participants) at that location.
Mood Response to DBS
One participant reported extreme improvements (>200%) in valence ratings with stimulation and was removed from all analyses of valence ratings as a statistical outlier. Of note, this participant’s extreme mood improvement was driven by very low mood ratings in the DBS OFF condition and elevated mood ratings in the stimulation ON conditions; the contact locations for this participant were similar to those for other participants.
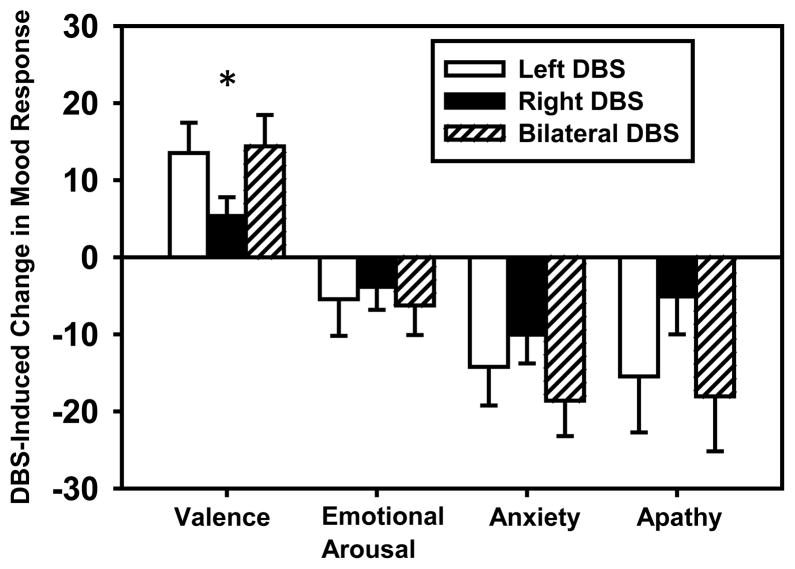
Overall, mood ratings improved with stimulation and the majority of participants reported a benefit of stimulation on mood (see Table 2). There was a significant effect of stimulation condition on mood response in general (omnibus test; Wilk’s lambda = .68, p < .02). Univariate repeated measures ANOVAs revealed a significant effect of stimulation condition on all mood ratings (see Figure 2) except emotional arousal (p = .24). Specifically, stimulation significantly increased valence (F(3,69) = 7.20, p <.01), decreased anxiety (F(3,69) = 6.81, p <.01) and decreased apathy (F(3,69) = 3.58, p =.03). In fact, DBS-induced changes (difference from DBS OFF) were significant for each stimulation condition for all of the mood ratings (one sample t-tests; all ps <.05) except emotional arousal (all ps >.11) and apathy ratings for the right DBS condition (p = .31). Furthermore, a significant proportion of participants reported improvements in mood with bilateral STN DBS (see Table 2; valence: χ2(1, n = 23) = 7.35, p <.01; anxiety: χ2 (1, n = 24) = 8.17, p <.01; apathy: χ2 (1, n = 24) = 6.0, p = .01; arousal: n.s.). Similarly, a majority of participants reported improved mood with unilateral DBS (see Table 2), but only valence (Left DBS: χ2 (1, n = 23) = 7.35, p <.01) and anxiety (Left DBS: χ2 (1, n = 24) = 4.17, p =.04; Right DBS: χ2 (1, n = 24) = 8.17, p <.01) reached statistical significance.
Table 2.
Effects of STN stimulation on motor functioning and acute mood state.
| Rating | Improvement | |
|---|---|---|
| UPDRS | ||
| DBS OFF | 40.33 (11.24) | |
| Right DBS | 31.13 (9.14) | 92% (22/24)* |
| Left DBS | 34.19 (9.90) | 79% (19/24)* |
| Bilateral DBS | 24.48 (9.48) | 100% (24/24)* |
| Valence | ||
| DBS OFF | 55.54 (12.84) | |
| Right DBS | 60.89 (14.59) | 61% (14/23) |
| Left DBS | 69.07 (17.97) | 78% (18/23)* |
| Bilateral DBS | 69.93 (19.82) | 78% (18/23)* |
| Emotional Arousal | ||
| DBS OFF | 46.40 (16.09) | |
| Right DBS | 42.56 (16.80) | 63% (15/24) |
| Left DBS | 40.96 (17.04) | 58% (14/24) |
| Bilateral DBS | 40.15 (11.43) | 63% (15/24) |
| Apathy | ||
| DBS OFF | 52.71 (22.53) | |
| Right DBS | 47.62 (21.62) | 67% (16/24) |
| Left DBS | 37.25 (23.55) | 58% (14/24) |
| Bilateral DBS | 34.67 (26.37) | 75% (18/24)* |
| Anxiety | ||
| DBS OFF | 48.74 (19.86) | |
| Right DBS | 38.69 (19.55) | 79% (19/24)* |
| Left DBS | 34.51 (19.66) | 71% (17/24)* |
| Bilateral DBS | 30.13 (15.79) | 79% (19/24)* |
Note: values represent means (standard deviation). Improvement column refers to the percentage of people with DBS-induced improvement in motor functioning (UPDRS) and mood.
indicates a significant proportion of participants with DBS-induced improvement (p<.05).
Figure 2.
Stimulation induced changes in mood response (mean ± SEM) for unilateral and bilateral DBS as compared to DBS OFF condition. * indicates a significant difference between left and right DBS-induced mood improvement (p<.05).
To evaluate hemispheric differences in mood response, change in self-reported mood from the DBS OFF condition was computed for both unilateral stimulation conditions. Left DBS induced significantly greater changes than did right DBS in valence (t(22) = 2.38, p = .03) and trend level for apathy ratings (t(23) = 1.83, p = .08) although there were no significant differences for emotional arousal or anxiety ratings (paired t-tests; both ps > .19).
Contact Location and Mood Response
Right DBS-induced improvements in valence ratings were related to the “Z” coordinates (r = .44, p = .04), such that more dorsal contacts were associated with greater improvements in valence ratings. There was a non-significant trend for right DBS-induced improvement in valence to relate to the “X” coordinate of the contact location (r = −.39, p = .07) such that more medial contacts were associated with greater improvements in valence ratings. There were no significant relationships between left active contact location (X, Y, Z coordinates) and change in mood response to left DBS (all ps >.16).
UPDRS
Unilateral and bilateral stimulation improved motor function (see Table 2). There was a significant improvement in UPDRS motor score with stimulation, compared to DBS OFF (Wilcoxon signed rank tests; left DBS: z = −3.48, p <.01; right DBS: z = −4.02, p <.01; bilateral DBS: z = −4.29, p <.01). Contralateral motor responses to left DBS were not different from right DBS (p =.67).
Contact Location and UPDRS
There were no significant relationships between contact location (X, Y, and Z coordinates) and stimulation-induced changes in contralateral UPDRS motor ratings (both left DBS and right DBS; all ps > .28).
Relationship Between Mood Responses, Motor Responses and Clinical Characteristics
The magnitude of UPDRS motor score improvement (i.e., change from DBS OFF) did not correlate with the magnitude of improvement in mood ratings for either unilateral stimulation condition (all ps > .33). For the bilateral DBS condition, the magnitude of UPDRS motor score change correlated with the magnitude of the change in apathy ratings (rs = .51, p = .01) but not to other mood ratings (ps > .17). Furthermore, the only significant relationship between baseline clinical characteristics (age, duration of PD, months since DBS surgery and OFF/OFF UPDRS motor score) and DBS-induced mood response was between change in arousal ratings and months since DBS surgery (r = −0.44, p = .04). Thus, there were no consistent relationships between mood response and motor function or other clinical characteristics.
DISCUSSION
The present study investigated the effects of unilateral and bilateral STN stimulation on mood response in PD patients and the relationship between contact location and mood response. The most important and interesting finding was that both unilateral and bilateral STN stimulation improved mood for the majority of participants. Furthermore, left DBS produced greater mood improvement than right DBS. The degree of DBS-induced mood improvement was not related to the degree of DBS-induced motor improvement. Finally, for these clinically optimal STN DBS contacts, mood response to right STN DBS was related to contact location.
A major strength of our study is that we focused on the effects of STN stimulation itself, by comparing mood responses across stimulation conditions within each participant after overnight withdrawal of PD medications. In this study, we were able to make within-subject comparisons of the effects of left versus right unilateral stimulation as compared to the DBS OFF state. Previous studies have either only examined effects of bilateral stimulation or have compared unilateral stimulation across participants. Furthermore, we were able to precisely identify the anatomical location of each active contact to more explicitly test the relationship between contact location and mood response. We found that stimulation of the clinically optimal contact improves mood for the majority of participants. Although others have reported that mood state does not change with stimulation, differences in study design may account for these different findings.35 Most previous reports of adverse mood effects of STN DBS focused primarily on post-surgical changes or were limited to case studies.
Left STN DBS significantly differed from right in mood response. Although right hemisphere lesions are associated with increased positive mood, even euphoria, and left lesions are more commonly associated with depression,36;37 left DBS had a more positive impact on mood than right DBS. At first this might seem contradictory; however, it may be entirely consistent if DBS reduces abnormal firing patterns rather than blocking normal function as a lesion does.38 Additional data on the physiological effects of unilateral DBS would help to further clarify this distinction.
Left STN DBS produced greater stimulation-induced effects on mood, but right DBS mood response depended significantly on contact location within the STN region. Specifically, more medial and dorsal contacts produced greater mood improvement with right DBS. However, as revealed in Figure 1, left DBS contacts were more tightly concentrated in the medial-dorsal STN area; the lack of a relationship between left DBS location and mood response may simply reflect less variability in contact location. Greater improvement in mood with more medial contact locations is consistent with recent research utilizing diffusion tensor imaging to demonstrate that the medial STN receives input from the medial forebrain bundle.13 This highlights the importance of considering not only the section of STN that is stimulated, but also the surrounding structures and fiber pathways.
Case studies12;22 and other reports indicate that stimulation of more ventral STN DBS contacts produces greater mood effects.21;23 However, it should be noted that these previous studies only used the relative location (either the contact ventral to the clinically optimal contact or the most ventral contact), whereas we had precise anatomically defined contact locations. The clinically chosen contacts in this study were located primarily in the dorsal STN region, limiting our ability to confirm these findings. Additional studies manipulating the active contact locations, beyond just the clinically optimal contact will be required to more definitively determine the role of contact location on mood response to STN DBS.
One interpretation of our finding that STN stimulation improves mood would be that participants are merely responding emotionally to improvements in motor function. However, our data and prior observations argue against this interpretation. Most tellingly, mood response varied with stimulation location within the right STN, whereas motor response did not. Furthermore, mood improved more with left DBS despite similar motor benefit with right DBS. Additionally, the unilateral DBS-induced mood responses did not correlate with motor improvements. Similarly, prior cross-sectional studies of PD found no correlation of motor disability with depressive symptoms across subjects.39
There are a few limitations of this study that should be considered when interpreting these findings. Mainly, caution should be taken when attempting to generalize these findings. We only used monopolar stimulation; bipolar or multipolar stimulation could potentially produce different responses by activating other nearby tissues. This study does not address the temporal aspect of stimulation-induced mood changes and therefore we cannot comment on the duration of these mood effects. The VAS was used as a brief assessment of current mood state and is not as comprehensive as individual scales designed to assess particular mood states, such as the Hamilton Depression Rating Scale or the Apathy Evaluation Scale. Additionally, a more thorough assessment of mood would be required to determine if the self-reported changes in mood found with acute stimulation represent clinically meaningful changes.
These data suggest that unilateral and bilateral STN stimulation affect mood, with an overall improvement in mood ratings. For these clinically optimal contacts, mostly within and around dorsal STN, right DBS contact location correlated with mood response. These results highlight the functionally heterogeneous nature of the STN region, particularly for non-motor functions, even within the more restricted regions targeted for optimal motor responses in STN DBS. It remains possible that stimulation of other portions of the STN may produce different mood responses.
Acknowledgments
This study was supported by a NARSAD Young Investigator Award (MCC), the Greater St. Louis Chapter of the American Parkinson Disease Association (APDA), NIH (NS41509; NS54353; NS058797; K24 MH087913), NCRR (UL1 RR024992), the Neuroscience Blueprint Grant at Washington University (P30 NS057105), APDA Advanced Center for PD Research at Washington University, McDonnell Center for Higher Brain Function and the Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund, the Handelman Fund and Jack Buck Fund for PD Research).
Footnotes
Dr. Karimi previously received partial fellowship funding from Medtronic, Inc., the manufacturer of the implanted stimulators; no other authors have any conflicts of interest related to this research.
FINANCIAL DISCLOSURES
Dr. Campbell receives salary and research support from NIH, NARSAD, McDonnell Foundation, American Parkinson Disease Association (APDA), and the Greater St. Louis Chapter of the APDA.
Dr. Black receives salary and research support from NIH and Tourette Syndrome Association. He has consulted for Gerson Lehman Group, Corregidor, and Merck. He is an inventor on a patent in prosecution (#11/583,896), U. S. patent pending, “Novel methods for medicinal dosage determination and diagnosis,” inventors Kevin J. Black and Jonathan M. Koller.
Mr. Weaver receives salary support from NIH.
Ms. Lugar receives salary support from NIH.
Dr. Videen receives salary and research support from NIH and the Michael J. Fox Foundation.
Dr. Tabbal receives salary and research support from NIH.
Dr. Karimi receives salary and research support from NIH. Dr. Karimi previously received partial fellowship funding from Medtronic, Inc., the manufacturer of the implanted stimulators.
Dr. Perlmutter receives salary and research support from NIH, Cure Huntington Disease Initiative, American Parkinson Disease Association, Greater St. Louis Chapter of the APDA, McDonnell Foundation, Barnes-Jewish Hospital Foundation, Washington University, Huntington’s disease Society of America, Michael J. Fox Foundation, Express Scripts and the Bander Foundation for Medical Business Ethics; NIH subcontracts via Emory and the University of Rochester. Honoraria: Toronto Western Hospital, University of Maryland, University of Saskatoon, Parkinson Study Group (University of Rochester), Society of Nuclear Medicine, Movement Disorders Society, Bachmann Strauss Foundation and American Academy of Neurology. He is on the Dystonia Medical Research Foundation and APDA advisory boards.
Dr. Hershey receives salary and research support from NIH. She has received honoraria from NIH and from the journal Diabetic Hypoglycemia. She is on the Tourette Syndrome Association scientific advisory board.
Reference List
- 1.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Broussolle E, Perret JE, Benabid AL. Bilateral subthalamic nucleus stimulation for severe Parkinson’s disease. Mov Disord. 1995;10:672–4. doi: 10.1002/mds.870100523. [DOI] [PubMed] [Google Scholar]
- 2.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism. Relat Disord. 2006;12:265–72. doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Burn DJ, Troster AI. Neuropsychiatric complications of medical and surgical therapies for Parkinson’s disease. J Geriatr Psychiatry Neurol. 2004;17:172–80. doi: 10.1177/0891988704267466. [DOI] [PubMed] [Google Scholar]
- 4.Troster AI. Neuropsychology of deep brain stimulation in neurology and psychiatry. Front Biosci. 2009;14:1857–79. doi: 10.2741/3347. [DOI] [PubMed] [Google Scholar]
- 5.Daniele A, Albanese A, Contarino MF, Zinzi P, Barbier A, Gasparini F, Romito LM, Bentivoglio AR, Scerrati M. Cognitive and behavioural effects of chronic stimulation of the subthalamic nucleus in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:175–82. doi: 10.1136/jnnp.74.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, Chabardes S, Foote K, Benabid AL, Pollak P. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, du Moncel ST, Bonnet AM, Pidoux B, Dormont D, Cornu P, Agid Y. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2002;72:701–7. doi: 10.1136/jnnp.72.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider F, Reske M, Finkelmeyer A, Wojtecki L, Timmermann L, Brosig T, Backes V, Amir-Manavi A, Sturm V, Habel U, Schnitzler A. Predicting acute affective symptoms after deep brain stimulation surgery in Parkinson’s disease. Stereotact Funct Neurosurg. 2010;88:367–73. doi: 10.1159/000319046. [DOI] [PubMed] [Google Scholar]
- 9.Schneider F, Habel U, Volkmann J, Regel S, Kornischka J, Sturm V, Freund HJ. Deep brain stimulation of the subthalamic nucleus enhances emotional processing in Parkinson disease. Arch Gen Psychiatry. 2003;60:296–302. doi: 10.1001/archpsyc.60.3.296. [DOI] [PubMed] [Google Scholar]
- 10.Czernecki V, Pillon B, Houeto JL, Welter ML, Mesnage V, Agid Y, Dubois B. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2005;76:775–9. doi: 10.1136/jnnp.2003.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krack P, Kumar R, Ardouin C, Dowsey PL, McVicker JM, Benabid AL, Pollak P. Mirthful laughter induced by subthalamic nucleus stimulation. Mov Disord. 2001;16:867–75. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- 12.Mallet L, Schupbach M, N’diaye K, Remy P, Bardinet E, Czernecki V, Welter ML, Pelissolo A, Ruberg M, Agid Y, Yelnik J. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci USA. 2007;104:10661–6. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenen VA, Honey CR, Hurwitz T, Rahman AA, McMaster J, Burgel U, Madler B. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery. 2009;64:1106–14. doi: 10.1227/01.NEU.0000345631.54446.06. [DOI] [PubMed] [Google Scholar]
- 14.Mandat TS, Hurwitz T, Honey CR. Hypomania as an adverse effect of subthalamic nucleus stimulation: report of two cases. Acta Neurochir (Wien) 2006;148:895–7. doi: 10.1007/s00701-006-0795-4. [DOI] [PubMed] [Google Scholar]
- 15.Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, Cornu P, Pidoux B, Samson Y, Agid Y. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–80. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 16.Doshi PK, Chhaya N, Bhatt MH. Depression leading to attempted suicide after bilateral subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002;17:1084–5. doi: 10.1002/mds.10198. [DOI] [PubMed] [Google Scholar]
- 17.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 18.Alexander GE, Crutcher M, DeLong M. Basal ganglia-thalamo-cortical circuits: Parallel substrates for motor, oculomotor, “prefrontal,” and “limbic” functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- 19.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- 20.Hershey T, Campbell MC, Videen TO, Lugar HM, Weaver PM, Hartlein J, Karimi M, Tabbal SD, Perlmutter JS. Mapping Go-No-Go performance within the subthalamic nucleus region. Brain. 2010 doi: 10.1093/brain/awq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, Suelter M, Jacobson C, Wang X, Gordon CW, Zeilman P, Romrell J, Martin P, Ward H, Rodriguez R, Foote KD. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE Trial. Ann Neurol. 2009;65:586–94. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okun MS, Green J, Saben R, Gross R, Foote KD, Vitek JL. Mood changes with deep brain stimulation of STN and GPi: results of a pilot study. J Neurol Neurosurg Psychiatry. 2003;74:1584–6. doi: 10.1136/jnnp.74.11.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenhouse I, Gould S, Houser M, Hicks G, Gross J, Aron AR. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson’s disease. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Videen TO, Campbell MC, Tabbal SD, Karimi M, Hershey T, Perlmutter JS. Validation of a fiducial-based atlas localization method for deep brain stimulation contacts in the area of the subthalamic nucleus. J Neurosci Methods. 2008;168:275–81. doi: 10.1016/j.jneumeth.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88:539–43. [PubMed] [Google Scholar]
- 26.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 27.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 28.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–70. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 31.Sturman MM, Vaillancourt DE, Shapiro MB, Metman LV, Bakay RA, Corcos DM. Effect of short and long term STN stimulation periods on parkinsonian signs. Mov Disord. 2008;23:866–74. doi: 10.1002/mds.21979. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Luria R. Reliabiliy, validity, and clinical application of the visual analogue mood scale. Psychol Med. 1973;3:479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 33.Black KJ, Hershey T, Hartlein JM, Carl JL, Perlmutter JS. Levodopa challenge neuroimaging of levodopa-related mood fluctuations in Parkinson’s disease. Neuropsychopharmacology. 2005;30:590–601. doi: 10.1038/sj.npp.1300632. [DOI] [PubMed] [Google Scholar]
- 34.Larsen RJ, Diener E. Promises and problems with the circumplex model of emotion. In: Clarck MS, editor. Emotion: Review of personality and social psychology. Thousand Oaks, CA: Sage Publications, INC; 1992. pp. 25–59. [Google Scholar]
- 35.Berney A, Panisset M, Sadikot AF, Ptito A, Dagher A, Fraraccio M, Savard G, Pell M, Benkelfat C. Mood stability during acute stimulator challenge in Parkinson’s disease patients under long-term treatment with subthalamic deep brain stimulation. Mov Disord. 2007;22:1093–6. doi: 10.1002/mds.21245. [DOI] [PubMed] [Google Scholar]
- 36.Starkstein SE, Robinson RG. Mechanism of disinhibition after brain lesions. J Nerv Ment Dis. 1997;185:108–14. doi: 10.1097/00005053-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Starkstein SE, Robinson RG. Cerebral lateralization in depression. Am J Psychiatry. 1986;143:1631–2. doi: 10.1176/ajp.143.12.1631. [DOI] [PubMed] [Google Scholar]
- 38.Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–21. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- 39.Black KJ, Pandya A. Depression in Parkinson disease. In: Gilliam F, Kanner AM, Sheline YI, editors. Depression and Brain Dysfunction. New York: Taylor and Francis; 2006. pp. 199–237. [Google Scholar]